Introduction
Pancreatic cancer is ranked as the fourth leading
cause of cancer-related mortalities in the United States (1,2) and
the sixth leading cause of mortality in China (3). Pancreatic cancer is characterized by
a highly malignant phenotype that is associated with early
metastasis and chemoresistance. Although resection of the tumor is
considered the primary option for a successful cure (4), the five-year survival rates remain at
<5%. Therefore, understanding the underlying molecular
mechanisms of invasion and metastasis in pancreatic cancer is
central to identifying effective therapeutic targets.
L1 cell adhesion molecule (L1CAM), a 200–220 kDa
transmembrane glycoprotein, is a member of the neuronal
immunoglobulin superfamily of cell adhesion molecules. L1CAM was
originally considered to be a stimulator of neurite outgrowth in
the peripheral nervous system due to its involvement in
establishing central nervous structures. Mutations in the gene that
encodes for L1CAM may result in a variety of developmental defects
in humans, including corpus callosum hypoplasia, mental retardation
and spastic paraplegia (5). In
addition, studies have found that L1CAM is aberrantly expressed in
a variety of tumor types, including human gliomas, non-small cell
lung cancer, and ovarian, colorectal and pancreatic cancer
(6–9).
The presence of L1CAM in tumor tissue and cultured
cells has been correlated with poor prognosis and advanced-stage
pancreatic cancer (10,11). Positive L1CAM expression has been
reported in ~80% of analyses of pancreatic tumor samples and cell
lines. Furthermore, L1CAM has been associated with metastasis and
angiogenesis during tumor progression by promoting cancer cell
adhesion to endothelial cell monolayers, and via transendothelial
migration (12). A combined
treatment with an L1CAM antibody and gemcitabine or paclitaxel in
severe combined immunodeficiency mouse models reduced the growth of
subcutaneous pancreatic Colo357 tumors more efficiently than
treatment with the cytotoxic agent alone (7). These data demonstrated the central
role of L1CAM in the tumorigenesis of pancreatic cancer.
In the present study it was hypothesized that the
suppression of L1CAM expression in human pancreatic cancer cells
may inhibit tumor progression. To investigate this hypothesis,
L1CAM was silenced in Capan-2 pancreatic cancer cells and the
effect on proliferation, apoptosis, cell cycle progression and
invasion was examined. In addition, the potential role of LICAM in
the activation of intracellular signaling pathways was
investigated.
Materials and methods
Cell culture
Human pancreatic cancer cell lines, Capan-2, PANC-1,
AsPC-1, BxPC-3, SW-1990, Patu-8988 and CFPAC-1, were purchased from
the Cell Bank of Type Culture Collection of Chinese Academy of
Sciences (Shanghai, China). Cells were maintained in RPMI-1640
medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal
bovine serum (FBS; Invitrogen), 100 U/ml penicillin and 100 μg/ml
streptomycin (Invitrogen). Cells were incubated at 37°C in a
humidified atmosphere of 5% CO2 and used for the assays
during the exponential phase of growth. The study was approved by
the Ethics Committee of Ruijin hospital, Shanghai Jiaotong
University, Shanghai, China.
Construction of recombinant lentivirus
and cell infection
Small-interfering RNA (siRNA;
5′-AGGGAUGGUGUCCACUUCAAATT-3) was used to downregulate the L1CAM
expression and a non-silencing fragment (5′-TTCTCCGAACGTGTCACGT-3′)
served as the negative control. The synthesis of siRNA was
conducted by Shanghai GenePharma Co., Ltd (Shanghai, China). Short
hairpin RNA (shRNA) fragments were hybridized with synthesized
sense and antisense oligonucleotides. The hybridized shRNA
fragments were cloned into the pGLV-U6-EGFP plasmid to yield
pGLV-Sh-L1CAM plasmid. The correct insertions of the shRNA
cassettes were confirmed using direct sequencing.
The shRNA-containing plasmid, pGLV-sh-L1CAM, as well
as two imperative elements for virus packaging (VSVG and
Gag/pol/rev plasmid), were co-transfected into 293T cells with
Lipofectamine 2000. After filtering the collected medium through
0.45 μm-filters, the virus was concentrated by centrifugation at
4,000 × g (Eppendorf, Hamburg, Germany) for 15 min followed by 2
min at 1,000 × g. The concentrated virus was stored at −80°C and
the titers of the lentiviral vectors were determined via dilution
using fluorescence microscopy (IX71; Olympus, Tokyo, Japan).
Lentivirus infection in Capan-2
cells
Capan-2 cells were plated at a density of
5×104 cells/well in six-well plates and incubated for 24
h at 37°C and 5% CO2. A recombinant lentivirus encoding
for shRNA against L1CAM in serum-free growth medium was added at a
multiplicity of infection of 50 and, after incubation for a further
2 h, the serum-containing growth medium was added to the cells. The
reporter gene expression was assessed by fluorescence microscopy 96
h after infection.
Polymerase chain reaction (PCR)
The cDNA template was synthesized according to the
manufacturer’s instructions (Takara, Tokyo, Japan). PCR was
performed in a 25-μl reaction mixture containing 2 μl RT products
and β-actin served as the internal control. The amplification
products were separated by electrophoresis on a 1.5% agarose gel
using the following primers: L1CAM sense,
5′-GACTACGAGATCCACTTGTTTAAGGA-3′ and antisense,
5′-CTCACAAAGCCGATGAACCA-3; β-actin sense, 5′-GCTCCTCCTGAGCGCAAG-3′
and antisense, 5′-CATCTGCTGGAAGGTGGACA-3′.
Western blot analysis
Total protein was isolated from the cells at the
exponential growth phase and the protein concentration was measured
using a Bio-Rad assay (Hercules CA, USA). The proteins were
transferred to polyvinylidene fluoride membranes following SDS-PAGE
and probed with their corresponding primary antibodies (LICAM;
Abcam, Cambridge, UK) in a blocking buffer (5% non-fat milk) at
4°C. The immunoreactive bands were detected by chemiluminescence
(Pierce Biotechnology, Inc., Rockford, IL, USA) according to the
manufacturer’s instructions. GAPDH served as the control to verify
that there was equal protein loading.
Proliferation assay
Cell proliferation assays were performed using cell
counting kit-8 (CCK-8; Beyotime Biotechnology, Shanghai, China).
The Capan-2 cells were seeded in 96-well plates at a density of
5×103 cells/well, and collected at 24, 48, 72, 96, 120
and 144 h after infection. The absorbance was read at 450 nm using
a microplate ELISA reader (SpectroMax 190; Molecular Devices,
Sunnyvale, CA, USA).
Detection of apoptosis
Cells were harvested 48 h after infection, washed in
phosphate buffered saline (PBS) and resuspended in 0.1 M PBS. A
total of 1×106 cells was collected, washed in PBS and
resuspended in Annexin V-fluorescein isothiocyanate (Biouniquer
Technology Co., Hangzhou, China) for 10 min. The level of apoptosis
was determined using an Annexin V/APC kit and propidium iodide (PI;
Biouniquer Technology Co.) according to the manufacturer’s
instructions. Following the addition of 50 mg/ml PI and 20 mg/ml
RNase A, the cells were incubated in the dark for 20 min at 4°C and
analyzed by flow cytometry (FACS420; BD Biosciences, San Jose, CA,
USA).
Cell cycle assay
Cells were harvested 48 h after infection, washed
and resuspended in 0.1 M PBS. The cells at a concentration of
1.0×106 cells/ml were fixed in 1 ml pre-cooled 70%
alcohol overnight at 4°C. Following incubation with 50 mg/ml PI and
20 mg/ml RNase A for 30 min at 4°C in the dark, the cell cycle
distribution was analyzed using flow cytometry.
Cell invasion assay
Cell invasion assays were conducted using an 8.0-μm
Millicell-24 cell culture insert plate (Millipore, Bedford, MA,
USA). Cells at a concentration of 1.0×106 cells/ml were
added to the upper compartment of the transwell insert and 500 μl
medium, with 10% FBS as a chemoattractant, was added to the lower
chamber and the plates were incubated at 37°C for 24 h. The
noninvasive cells and Matrigel were removed by scraping using
sterile cotton swabs. The cells that had invaded were stained with
hematoxylin (Biouniquer Technology Co.) and counted under a
microscope (magnification, ×100; Nikon, Tokyo, Japan). Cells were
counted in five randomly selected fields and the assays were
performed in triplicate.
Statistical analysis
Data were analyzed using SPSS 19.0 software (IBM,
Armonk, NY, USA) and are expressed as mean ± standard deviation.
Differences were determined using Student’s two-tailed t-tests and
P<0.05 was considered to indicate a statistically significant
difference.
Results
L1CAM mRNA expression in pancreatic
cancer cell lines
PCR analysis showed that L1CAM mRNA was present in
the seven pancreatic cancer cell lines, Capan-2, PANC-1, AsPC-1,
BxPC-3, SW-1990, Patu-8988 and CFPAC-1. The Capan-2 cells
demonstrated the highest levels of L1CAM mRNA and the Patu-8988
cells displayed the lowest (Fig.
1A). Therefore, the Capan-2 cell line was used for the
subsequent experiments.
 | Figure 1Lentivirus-mediated RNA interference
decreased L1CAM expression in the pancreatic cell lines. (A)
Polymerase chain reaction showed that human L1CAM mRNA was present
in seven pancreatic cancer cell lines, Capan-2, PANC-1, AsPC-1,
BxPC-3, SW-1990, Patu-8988 and CFPAC-1. L1CAM mRNA expression
levels were highest in the Capan-2 cells and lowest in the
Patu-8988 cells. (B) Light micrograph (top) and fluorescence
micrograph (bottom) images (magnification, ×200). The transduction
efficiency in the Capan-2 cells was estimated 96 h after infection
and the intensity of fluorescence indicated a high transfection
efficiency. (C) Western blot analysis of total cellular proteins
extracted from Capan-2 cells at 0, 24, 48, 72 and 96 h after
infection and targeted by antibodies against L1CAM, showed that the
L1CAM protein levels decreased in a time-dependent manner. GAPDH
served as the internal control. Data represents one of three
independent experiments. L1CAM, L1 cell adhesion molecule; NC,
negative control; Sh, short hairpin; RQ, relative quantity. |
Infection efficiency in Capan-2
pancreatic cancer cells
The infection efficiency of Capan-2 cells with
lentivirus-mediated sh-L1CAM was detected 96 h after infection by
fluorescence microscopy. A high intensity of green fluorescence was
observed, indicating a high infection efficiency (Fig. 1B). Western blot analysis indicated
that the interfering efficiency was greatest in the Capan-2 cells
96 h after infection with ~75% reduction in L1CAM protein levels.
Furthermore, the western blot analysis demonstrated that the L1CAM
protein levels decreased in a time-dependent manner following
infection (Fig. 1C).
L1CAM silencing inhibits tumor cell
proliferation and cell cycle entry in pancreatic cancer Capan-2
cells
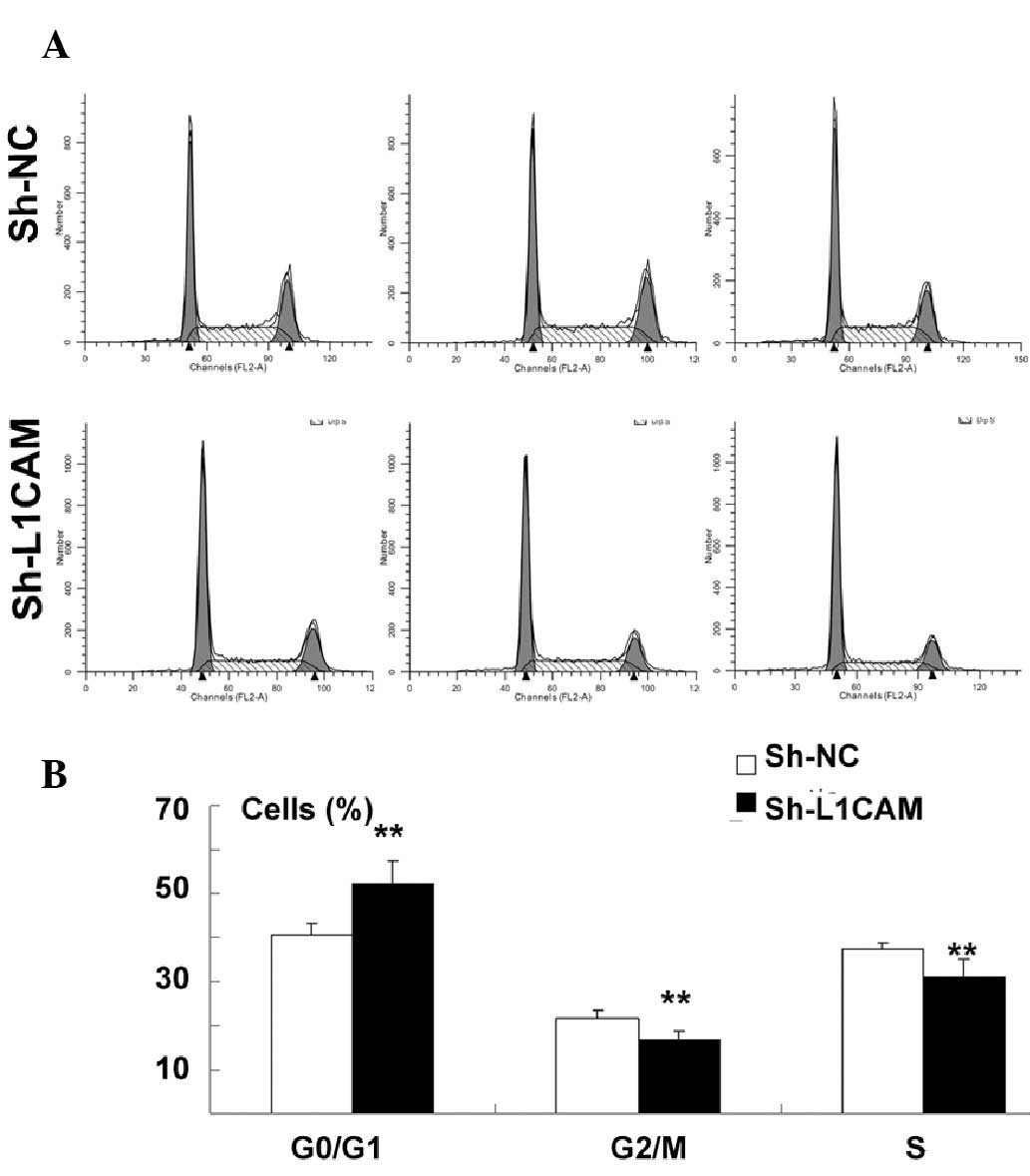
The effect of L1CAM silencing on proliferation and
cell cycle distribution in Capan-2 pancreatic cancer cells was
determined by CCK-8 and flow cytometric assays, respectively.
Proliferation was identified to be significantly inhibited by
sh-L1CAM silencing when compared with the negative control
(P<0.01; Fig. 2). In addition,
there was a significant increase in the cell population at the
G0/G1 phase, with a concurrent decrease in the cell population at
the G2/M and S phases following L1CAM knockdown (P<0.01;
Fig. 3A and B).
L1CAM silencing inhibits tumor cell
invasion but does not induce cell apoptosis in Capan-2 pancreatic
cancer cells
Next, the ability of L1CAM silencing to induce cell
apoptosis was investigated. The results of Annexin V and PI flow
cytometric analyses showed that lentivirus-mediated inhibition of
L1CAM did not significantly increase apoptosis in the Capan-2 cells
(P>0.05; Fig. 4A and B). By
contrast, the number of invasive Capan-2 cells after sh-L1CAM
silencing was identified to be significantly lower 96 h after
infection compared with the negative control (P<0.01; Fig. 5).
L1CAM silencing inhibits activation of
extracellular signal-regulated kinase (ERK) in pancreatic cancer
cells
p38 mitogen-activated protein kinase (MAPK) and ERK
have been implicated in cancer metastasis signaling pathways and
may be involved in regulating L1CAM activation (9,13).
As shown in Fig. 6, it was
identified that the downregulation of L1CAM inhibited the intrinsic
activation of ERK1/2 in Capan-2 cells, indicating that L1CAM may be
involved in cancer cell progression via the p38/ERK1/2 signaling
pathway.
Discussion
An understanding of the molecular mechanisms
underlying cancer progression is essential for the development of
optimal therapeutic modalities. Pancreatic cancer is a
heterogeneous disease involving multiple cellular components and
gene expression patterns. An increasing number of studies have
identified the molecular markers that correlate with the
development and progression of cancer.
RNA interference has been used as a therapeutic tool
in cancer treatments (14),
however, strategies to improve the methodology and efficiency of
siRNA delivery are required. Although L1CAM mRNA was observed in
the seven human pancreatic cancer cell lines that were investigated
in the present study, the level was highest in the Capan-2 cells.
Therefore, in order to assess the impact of L1CAM knockdown on the
development of pancreatic cancer in vitro, Capan-2 cells
were infected with lentivirus-mediated L1CAM-specific shRNA.
Silencing of endogenous L1CAM significantly inhibited cell
proliferation and invasion in the Capan-2 cells (P<0.01). In
addition, L1CAM silencing induced cell cycle arrest at the G0/G1
phase, however, there was no significant effect observed on
apoptosis. These results indicated that L1CAM is overexpressed in
tumors and may be significant in cancer cell invasion.
Previous studies have indicated that L1CAM, a cell
surface molecule, promotes tumor cell proliferation. Zecchini et
al (15) showed that the
upregulation of L1CAM in OVCAR3 ovarian cancer cells significantly
enhanced cell proliferation in comparison to the parental cells.
Conversely, the downregulation of L1CAM in IGROV1 ovarian cancer
cells significantly inhibited cell proliferation (15). Furthermore, when tumor cells,
including SKOV3 ovarian (16) and
HCT116 colon cancer cells (17),
were treated with an L1CAM monoclonal antibody, cell proliferation
was reduced by 40–60% (18). In
vivo studies demonstrated that cancer cells overexpressing
L1CAM significantly promoted tumor formation, with tumor volumes
that were 3–5 times greater than those with low levels of L1CAM
expression (8). Kiefel et
al (19) observed that the
upregulation of L1CAM in pancreatic PT45-P1 cells promoted cell
proliferation and xenograft growth.
L1CAM was initially hypothesized to be specific to
the nervous system and has been implicated in numerous neurological
disorders. However, L1CAM has recently been shown to be expressed
in human tumors and correlate with tumor progression, poor
prognosis and the advanced stages of cancer (9,10,20).
Investigations in a variety of tumor types demonstrated that
increased expression of L1CAM significantly increases the migration
capacity of cancer cells in vitro (17,21–25).
In addition, the upregulation of L1CAM was found to enhance cell
invasion in the SW707 human colon cancer cell line (17). In vivo experiments have
demonstrated that L1CAM significantly increases liver metastases in
mice inoculated with LS174T colon cancer cells (26). In addition, multiple clinical
pathology studies have indicated that L1CAM may promote cancer cell
invasion and metastasis (10,27–31).
Although the mechanism by which L1CAM promotes
proliferation and cell invasion in pancreatic ductal adenocarcinoma
remains to be determined, studies have investigated the role of
L1CAM-dependent activation in the MAPK-ERK signaling pathway.
Schaefer et al (32)
demonstrated that L1CAM induces ERK activity and ERK-regulated gene
expression, which contributes to cell motility and invasion.
Furthermore, L1CAM has been identified to interact with various
components of the ERK pathway, including Src protein tyrosine
kinases (33) and Ran-binding
protein M (34), indicating that
L1CAM may serve as an adaptor protein in L1CAM-induced ERK
activation. Furthermore, L1CAM-signaling has been implicated in
integrin-binding and the nuclear factor-κB pathways (35).
Epithelial-mesenchymal transition (EMT) is
characterized by morphological and phenotypical alterations in
cancer invasion and metastasis. Epithelial carcinoma cells acquire
a motile phenotype via EMT, enabling the gain of metastatic
potentials, whereas metastatic tumor cells often display a
mesenchymal phenotype with a loss of epithelial markers, such as
E-cadherin. A connection between L1CAM and EMT was initially
identified by Shtutman et al (36) and it was observed that the
expression of L1CAM in MCF7 mammary carcinoma cell lines disrupted
E-cadherin-containing adheren junctions and increased the
transcriptional activity of β-catenin. As L1CAM is a target gene of
β-catenin (17), this event
resulted in increased cell motility. In addition, treatment of
pancreatic cancer cell lines with the EMT inducer, transforming
growth factor (TGF)-β1, was found to upregulate L1CAM, leading to
increased cell migration and invasion (35,37,38).
These events have been implicated in the early stages of tumor
development in pancreatic cancer (39). Geismann et al (39) observed that treatment with TGF-β1
caused H6c7 pancreatic ductal cells to acquire a spindle-shaped
morphology, elevated cell migration potential and increased L1CAM
expression. These effects may be abolished by the interference of
TGF-β1 signaling or suppression of Slug (39). Conversely, L1CAM-mediated
metastasis in colon cancer cells was shown to be independent of EMT
induction and altered the expression of epithelial and mesenchymal
marker proteins (40). Therefore,
the impact of L1CAM on EMT requires further investigation.
In conclusion, the results of this study indicate
that downregulation of L1CAM inhibits cell proliferation, induces
cell cycle quiescence and reduces cell invasion in the Capan-2
pancreatic cell line. Thus, these effects may be associated with an
observed decrease in p38/ERK expression. These findings indicate
that L1CAM may be involved in metastatic potential and may,
therefore, be a molecular target in anti-metastatic therapies for
pancreatic cancer.
Acknowledgements
This study was supported by grants from the National
Science Foundation of China (nos. 81072025 and 81170347).
References
|
1
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar
|
|
2
|
Neoptolemos JP, Stocken DD, Friess H, et
al; European Study Group for Pancreatic Cancer. A randomized trial
of chemoradiotherapy and chemotherapy after resection of pancreatic
cancer. N Engl J Med. 350:1200–1210. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo X and Cui Z: Current diagnosis and
treatment of pancreatic cancer in China. Pancreas. 31:13–22. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schneider G, Siveke JT, Eckel F and Schmid
RM: Pancreatic cancer: basic and clinical aspects.
Gastroenterology. 128:1606–1625. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fransen E, Lemmon V, Van Camp G, Vits L,
Coucke P and Willems PJ: CRASH syndrome: clinical spectrum of
corpus callosum hypoplasia, retardation, adducted thumbs,
spastic paraparesis and hydrocephalus due to mutations in one
single gene, L1. Eur J Hum Genet. 3:273–284. 1995.PubMed/NCBI
|
|
6
|
Kajiwara Y, Ueno H, Hashiguchi Y, et al:
Expression of l1 cell adhesion molecule and morphologic features at
the invasive front of colorectal cancer. Am J Clin Pathol.
136:138–144. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schäfer H, Dieckmann C, Korniienko O, et
al: Combined treatment of L1CAM antibodies and cytostatic drugs
improve the therapeutic response of pancreatic and ovarian
carcinoma. Cancer Lett. 319:66–82. 2012.PubMed/NCBI
|
|
8
|
Bao S, Wu Q, Li Z, et al: Targeting cancer
stem cells through L1CAM suppresses glioma growth. Cancer Res.
68:6043–6048. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hai J, Zhu CQ, Bandarchi B, et al: L1 cell
adhesion molecule promotes tumorigenicity and metastatic potential
in non-small cell lung cancer. Clin Cancer Res. 18:1914–1924. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li S, Jo YS, Lee JH, et al: L1 cell
adhesion molecule is a novel independent poor prognostic factor of
extrahepatic cholangiocarcinoma. Clin Cancer Res. 15:7345–7351.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ben QW, Wang JC, Liu J, et al: Positive
expression of L1-CAM is associated with perineural invasion and
poor outcome in pancreatic ductal adenocarcinoma. Ann Surg Oncol.
17:2213–2221. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Issa Y, Nummer D, Seibel T, et al:
Enhanced L1CAM expression on pancreatic tumor endothelium mediates
selective tumor cell transmigration. J Mol Med (Berl). 87:99–112.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Silletti S, Yebra M, Perez B, Cirulli V,
McMahon M and Montgomery AM: Extracellular signal-regulated kinase
(ERK)-dependent gene expression contributes to L1 cell adhesion
molecule-dependent motility and invasion. J Biol Chem.
279:28880–28888. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Elbashir SM, Harborth J, Weber K and
Tuschl T: Analysis of gene function in somatic mammalian cells
using small interfering RNAs. Methods. 26:199–213. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zecchini S, Bianchi M, Colombo N, et al:
The differential role of L1 in ovarian carcinoma and normal ovarian
surface epithelium. Cancer Res. 68:1110–1118. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gast D, Riedle S, Riedle S, et al: L1
augments cell migration and tumor growth but not beta3 integrin
expression in ovarian carcinomas. Int J Cancer. 115:658–665. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gavert N, Conacci-Sorrell M, Gast D, et
al: L1, a novel target of beta-catenin signaling, transforms cells
and is expressed at the invasive front of colon cancers. J Cell
Biol. 168:633–642. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arlt MJ, Novak-Hofer I, Gast D, et al:
Efficient inhibition of intra-peritoneal tumor growth and
dissemination of human ovarian carcinoma cells in nude mice by
anti-L1-cell adhesion molecule monoclonal antibody treatment.
Cancer Res. 66:936–943. 2006. View Article : Google Scholar
|
|
19
|
Kiefel H, Bondong S, Erbe-Hoffmann N, et
al: L1CAM-integrin interaction induces constitutive NF-kappaB
activation in pancreatic adenocarcinoma cells by enhancing IL-1beta
expression. Oncogene. 29:4766–4778. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bondong S, Kiefel H, Hielscher T, et al:
Prognostic significance of L1CAM in ovarian cancer and its role in
constitutive NF-κB activation. Ann Oncol. 23:1795–1802.
2012.PubMed/NCBI
|
|
21
|
Izumoto S and Yoshimine T: Role of neural
cell adhesion molecule L1 in glioma invasion. Nihon Rinsho.
63(Suppl 9): 74–78. 2005.(In Japanese).
|
|
22
|
Voura EB, Ramjeesingh RA, Montgomery AM
and Siu CH: Involvement of integrin alpha(v)beta(3) and cell
adhesion molecule L1 in transendothelial migration of melanoma
cells. Mol Biol Cell. 12:2699–2710. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Min JK, Kim JM, Li S, et al: L1 cell
adhesion molecule is a novel therapeutic target in intrahepatic
cholangiocarcinoma. Clin Cancer Res. 16:3571–3580. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gavert N, Ben-Shmuel A, Lemmon V, Brabletz
T and Ben-Ze’ev A: Nuclear factor-kappaB signaling and ezrin are
essential for L1-mediated metastasis of colon cancer cells. J Cell
Sci. 123:2135–2143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mechtersheimer S, Gutwein P, Agmon-Levin
N, et al: Ectodomain shedding of L1 adhesion molecule promotes cell
migration by autocrine binding to integrins. J Cell Biol.
155:661–673. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gavert N, Sheffer M, Raveh S, et al:
Expression of L1-CAM and ADAM10 in human colon cancer cells induces
metastasis. Cancer Res. 67:7703–7712. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sebens Müerköster S, Werbing V, Sipos B,
et al: Drug-induced expression of the cellular adhesion molecule
L1CAM confers anti-apoptotic protection and chemoresistance in
pancreatic ductal adenocarcinoma cells. Oncogene. 26:2759–2768.
2007.PubMed/NCBI
|
|
28
|
Boo YJ, Park JM, Kim J, et al: L1
expression as a marker for poor prognosis, tumor progression, and
short survival in patients with colorectal cancer. Ann Surg Oncol.
14:1703–1711. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kodera Y, Nakanishi H, Ito S, et al:
Expression of L1 cell adhesion molecule is a significant prognostic
factor in pT3-stage gastric cancer. Anticancer Res. 29:4033–4039.
2009.PubMed/NCBI
|
|
30
|
Rawnaq T, Kleinhans H, Uto M, et al:
Subset of esophageal adenocarcinoma expresses adhesion molecule l1
in contrast to squamous cell carcinoma. Anticancer Res.
29:1195–1199. 2009.PubMed/NCBI
|
|
31
|
Schröder C, Schumacher U, Fogel M, et al:
Expression and prognostic value of L1-CAM in breast cancer. Oncol
Rep. 22:1109–1117. 2009.PubMed/NCBI
|
|
32
|
Schaefer AW, Kamiguchi H, Wong EV, Beach
CM, Landreth G and Lemmon V: Activation of the MAPK signal cascade
by the neural cell adhesion molecule L1 requires L1
internalization. J Biol Chem. 274:37965–37973. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gast D, Riedle S, Issa Y, et al: The
cytoplasmic part of L1-CAM controls growth and gene expression in
human tumors that is reversed by therapeutic antibodies. Oncogene.
27:1281–1289. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheng L, Lemmon S and Lemmon V: RanBPM is
an L1-interacting protein that regulates L1-mediated
mitogen-activated protein kinase activation. J Neurochem.
94:1102–1110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kiefel H, Bondong S, Pfeifer M, et al:
EMT-associated up-regulation of L1CAM provides insights into
L1CAM-mediated integrin signalling and NF-κB activation.
Carcinogenesis. 33:1919–1929. 2012.PubMed/NCBI
|
|
36
|
Shtutman M, Levina E, Ohouo P, Baig M and
Roninson IB: Cell adhesion molecule L1 disrupts
E-cadherin-containing adherens junctions and increases scattering
and motility of MCF7 breast carcinoma cells. Cancer Res.
66:11370–11380. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huszar M, Pfeifer M, Schirmer U, et al:
Up-regulation of L1CAM is linked to loss of hormone receptors and
E-cadherin in aggressive subtypes of endometrial carcinomas. J
Pathol. 220:551–561. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tischler V, Pfeifer M, Hausladen S, et al:
L1CAM protein expression is associated with poor prognosis in
non-small cell lung cancer. Mol Cancer. 10:1272011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Geismann C, Morscheck M, Koch D, et al:
Up-regulation of L1CAM in pancreatic duct cells is transforming
growth factor beta1- and slug-dependent: role in malignant
transformation of pancreatic cancer. Cancer Res. 69:4517–4526.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gavert N, Vivanti A, Hazin J, Brabletz T
and Ben-Ze’ev A: L1-mediated colon cancer cell metastasis does not
require changes in EMT and cancer stem cell markers. Mol Cancer
Res. 9:14–24. 2011. View Article : Google Scholar : PubMed/NCBI
|