Introduction
Congenital bicuspid aortic valve (CBAV) is a common
congenital heart malformation that occurs in ~2% of the population
(1). Compared with normal
tricuspid aortic valves (TAVs), CBAV is more likely to progress to
valve stenosis, regurgitation or endocarditis (2). Stenosis is the most frequent
complication of CBAV, often necessitating surgical intervention
relatively early in life compared with TAVs (3). The early onset of severe stenosis
reflects the more rapid progression of valve stenosis that is
frequently observed in patients with CBAV. For this reason, CBAV
has been a particular focus of study. Valve calcification and
atherosclerosis have pathological similarities, including the
destruction and apoptosis of endothelial cells, infiltration of
inflammatory cells, new blood vessel formation, lipid plaque
deposition and eventual calcification and ossification. Therefore,
macrophages have an important role in the process of inflammatory
factor infiltration (4–6).
Macrophages within the lesion are heterogeneous in
nature, and are able to differentiate into either a
pro-inflammatory (M1) subtype, also known as a classically
activated subtype, or an anti-inflammatory alternatively activated
subtype (M2) according to their microenvironment (7). Classically-activated pro-inflammatory
macrophages may be induced by incubation with the Th1 cytokines
interferon γ (IFN-γ) (for M1) and tumour necrosis factor (TNF-α)
which work synergistically together and are both required to induce
maximal macrophage activation. These macrophages are characterized
by their secretion of pro-inflammatory cytokines, nitric oxide and
high capacity to present antigen. By contrast,
alternatively-activated anti-inflammatory macrophages may be
induced with the Th2 cytokines interleukin 4 (IL-4) (for M2) and
IL-13 and are characterized by their secretion of anti-inflammatory
cytokines such as IL-10 and TGF-β (8). IL-4 stimulates the activity of the
nuclear receptor peroxisome proliferator-activated receptor (PPARγ)
which has been shown to mediate alternative macrophage activation
as well as the transcriptional repression of several
pro-inflammatory transcription factors. Therefore, macrophages may
be categorized into the classically activated type (M1) and the
alternatively activated type (M2). M1 macrophages promote
inflammation and remove pathogenic microorganisms, while M2
macrophages inhibit inflammation and heal wounds (9,10).
Numerous studies have investigated the distribution and pathogenic
mechanisms of macrophage subtypes in atherosclerosis and cancer
(11–13). The present study aimed to determine
whether inflammation, defined by inflammatory cell infiltration and
neovascularization, is increased in stenotic CBAV, and to explore
the classification and possible mechanisms of differentiation of
M1/M2 macrophages during the process of CBAV stenosis.
Materials and methods
Study population and data collection
From January 2011 to December 2012, a total of 30
patients with severely stenotic CBAVs underwent successful aortic
valve replacement at Nanjing Hospital Affiliated to Nanjing Medical
University (Nanjing, China). A group comprising 30 patients with
severely stenotic TAVs was enrolled in parallel, and all CABVs and
TAVs were included for further analyses. There were no
statistically significant differences between the CABV and the TAV
group in preoperative echocardiography, cardiovascular risk
factors, other underlying diseases or the use of relevant
cardiovascular medications (P>0.05; Table I). All cases were diagnosed using
clinical and pathological evidence. This study conformed to the
principles outlined in the Declaration of Helsinki. All of the
retrospective review of the clinical data involving human samples
were approved by the Human Research Ethics Committee of Nanjing
Medical University, and written informed consent was obtained from
each patient and the families of the prospective heart donors.
 | Table IClinical and demographic
characteristics of the patients. |
Table I
Clinical and demographic
characteristics of the patients.
| CBAV (n=30) | TAV (n=30) | P-value |
|---|
| Age (years) | 61±9 | 59±11 | 0.44 |
| Female (n) | 13 | 11 | 0.60 |
| Risk factors |
| Hypertension
(n) | 11 | 9 | 0.58 |
| Hypercholesterolemia
(n) | 10 | 10 | 1.00 |
| Diabetes mellitus
(n) | 4 | 6 | 0.49 |
| Chronic kidney
disease (n) | 1 | 0 | 0.31 |
| Smoking (n) | 11 | 14 | 0.43 |
| Medical therapy |
| Diuresis (n) | 27 | 29 | 0.30 |
| ARB (n) | 4 | 5 | 0.72 |
| ACEI (n) | 6 | 5 | 0.74 |
| UCG parameters |
| LVEF (%) | 52±6 | 54±4 | 0.13 |
| AVA (cm) | 0.88±0.2 | 0.81±0.3 | 0.29 |
| Mean gradient
(mmHg) | 55±16 | 59±14 | 0.31 |
| Aortic annulus
diameter (cm) | 23±2.3 | 24±1.9 | 0.07 |
Specimen collection and sectioning
All aortic valve specimens were fixed with 4%
formaldehyde, followed by gradient alcohol dehydration, paraffin
embedding and preparation of 3-μm sections.
Hematoxylin-eosin (HE) staining
For HE staining, sections were deparaffinized,
hydrated, stained with hematoxylin for 5 min and rinsed with water
for 10 min. The sections were then decolorized using 1% HCl, rinsed
with water for 10 min, stained with eosin for 1 min, dehydrated in
gradient alcohols, and cleared in xylene prior to being sealed with
neutral gum (Beijing Zhongshan Golden Bridge Biotechnology,
Beijing, China).
Immunohistochemical staining
Sections were deparaffinized, hydrated and rinsed
twice in phosphate-buffered saline (PBS) for 5 min. The sections
were then boiled to retrieve antigens and incubated with sheep
serum to block nonspecific staining. Primary antibodies to cluster
of differentiation (CD)68 (Beijing Zhongshan Golden Bridge
Biotechnology), CD163 (Beijing Zhongshan Golden Bridge
Biotechnology), inducible nitric oxide synthase (iNOS, 1:400;
Beijing Zhongshan Golden Bridge Biotechnology), endothelial nitric
oxide synthase (eNOS, 1:200; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA), interleukin-10 (IL-10, 1:100; Santa Cruz
Biotechnology, Inc.), arginase-1 (Arg-1, 1:2,000; Santa Cruz
Biotechnology, Inc.) and macrophage colony-stimulating factor
(M-CSF, 1:50; Santa Cruz Biotechnology, Inc.) were added. A diluted
primary antibody was used as a reference and the sections were
incubated at 4°C overnight.
The sections were subsequently brought to room
temperature for 30 min and rinsed with PBS four times for 5 min,
followed by the addition of anti-rabbit, -goat or -rat secondary
antibodies (Santa Cruz Biotechnology, Inc.). The sections were
incubated at room temperature for 15–60 min (according to the
secondary antibody) and rinsed with PBS four times for 5 min. The
sections were then stained with hematoxylin while being observed
under a microscope, dehydrated, cleared and sealed.
Specimens were scored as positive when the cytoplasm
and nucleus contained yellow or brown particles. Five samples were
selected at random and viewed under high magnification (x400;
Binocular Biological Microscope XSZ-PW150; Nikon, Tokyo, Japan).
Positive cells were classified using the Fromowitz
semi-quantitative method, which considers staining intensity and
the percentage of positive cells (14). The staining intensity was scored as
follows: No staining or similar to the background, 0 points;
lightly stained or slightly higher than the background, 1 point;
moderately stained or significantly higher than the background, 2
points; strongly or deeply stained, 3 points. The percentage of
positive cells was scored as follows: <5%, 0 points; 5–25%, 1
point; 26–50%, 2 points; 51–75%, 3 points; >75%, 4 points. The
scores for the two items were combined to derive a final score that
was divided into four grades: 0–1 point, (−); 2–3 points, (+); 4–5
points, (++); 6–7 points, (+++).
Real-time polymerase chain reaction
(qPCR)
The TRIzol® method was used to extract
total RNA. Fluorescence qPCR was performed according to the
instructions of the 2X One-Step qRT-PCR SYBR-Green kit (Tiangen
Biotech (Beijing) Co., Ltd., Beijing, China), using primers
designed by Shanghai Biological Engineering (Shanghai, China). Each
reaction included 10 μl 2X One-Step SYBR-Green Master Mix, 300 ng
template RNA, 0.4 μl upstream primer (10 μmol/l), 0.4 μl downstream
primer (10 μmol/l) and 1 μl RT-PCR mix, and was adjusted to 20 μl
with RNase-free water. RT-PCR was performed as follows: 42°C for 20
min; 95°C for 5 min; 40 cycles at 95°C for 15 sec, 55°C for 15 sec
and 72°C for 20 sec; 95°C for 1 min, 55°C for 30 sec and 95°C for
30 sec. Each sample was evaluated in triplicate. The amplification
and melting curves were plotted and the β-actin gene was used as an
internal control. qPCR was performed using a 3.0×10−4 to
3.0×10−5 dilution series and five temperature gradients,
the temperature of 55°C five additional cycles were carried
out.
Statistical analysis
All data are presented as the mean ± standard
deviation (SD). For two-group comparisons, Gaussian samples were
compared using the two-tailed t-test, while non-Gaussian samples
were compared using the Mann-Whitney nonparametric test.
Statistical analyses were performed using SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to denote a
statistically significant difference.
Results
HE staining
Histological morphology revealed calcification in
all valves; however, more severe calcification was observed in
CBAVs. HE staining revealed a higher cell density in CBAVs
(Fig. 1A) than TAVs (Fig. 1B), and more neovascularization was
exhibited in the CBAV (Fig. 1A)
compared with the TAV group (Fig.
1B).
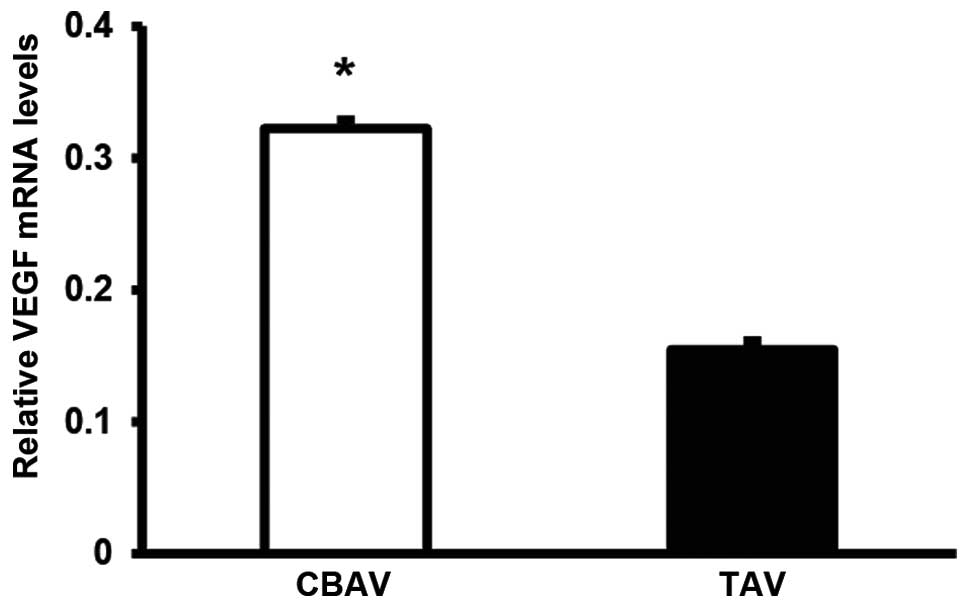
VEGF expression
Despite the severe calcification and formalin
fixative, expression levels of VEGF were evaluable in 19 and 22
specimens in the TAV and CBAV groups, respectively. mRNA expression
of VEGF in the CBAV specimens was two-fold that observed in the TAV
specimens (P=0.02; Fig. 2). These
results indicate that a positive correlation may exist between
valve inflammation and neovascularization.
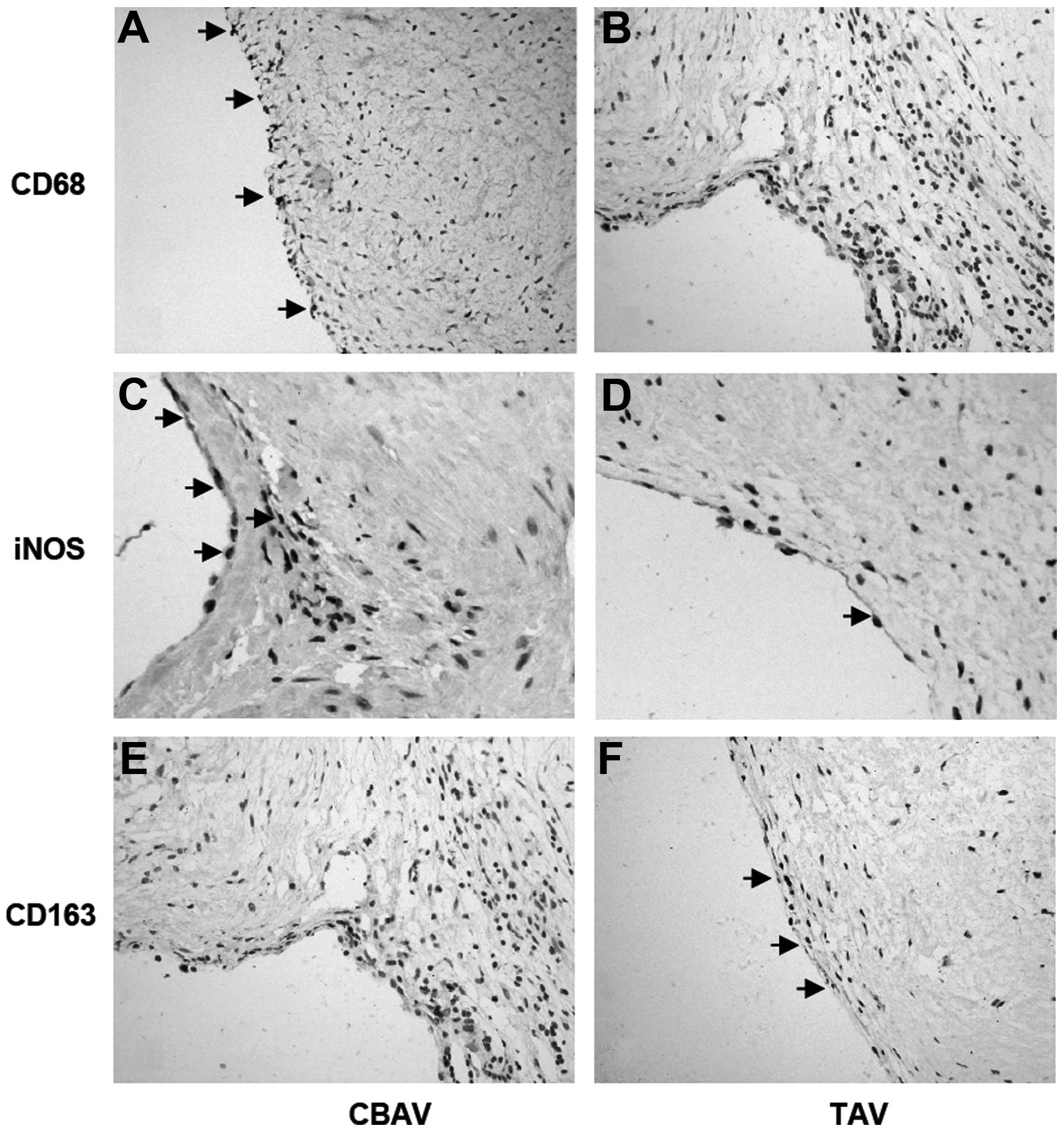
Total and M1/M2 macrophage levels
CD68, iNOS and CD163 staining was used to evaluate
total, M1 and M2 macrophages, respectively. In the CBAV group, the
density and distribution of macrophages (CD68) was significantly
higher and wider than in the TAV group (4.53±1.92 versus 3.00±1.41,
P=0.029; Fig. 3A and B), spanning
the lining layer of the aortic valve. This was consistent with the
results for the iNOS-labeled M1 macrophages in CBAVs compared with
TAVs (3.20±2.27 versus 1.40±0.99, P=0.021; Fig. 3C and D). However, significantly
fewer CD163-labeled M2 macrophages were observed in CBAVs than in
TAVs (1.13±0.92 versus 2.27±1.58, P=0.033; Fig. 3E and F). Collectively, these data
suggest that iNOS-labeled M1 macrophages may participate in CBAV
stenosis.
Expression of M-CSF and other
inflammatory factors associated with macrophage transformation
In CBAVs, eNOS expression was significantly higher
than in TAVs (4.93±2.25 versus 2.00±1.41, P=0.0003; Fig. 4A and B). However, Arg-1 expression
was slightly lower in CBAVs than TAVs (1.40±1.06 versus 3.53±1.51,
P=0.0009; Fig. 4C and D), as
evidenced by the expression of IL-10 (2.07±0.88 versus 4.67±1.54,
P=0.0007; Fig. 4E and F) and M-CSF
(2.40±1.06 versus 4.47±1.23, P=0.0006; Fig. 4G and H). Thus, inflammatory
responses in CBAV stenosis may result from the generation of other
inflammatory factors and inhibition of M1/M2 macrophage
transformation.
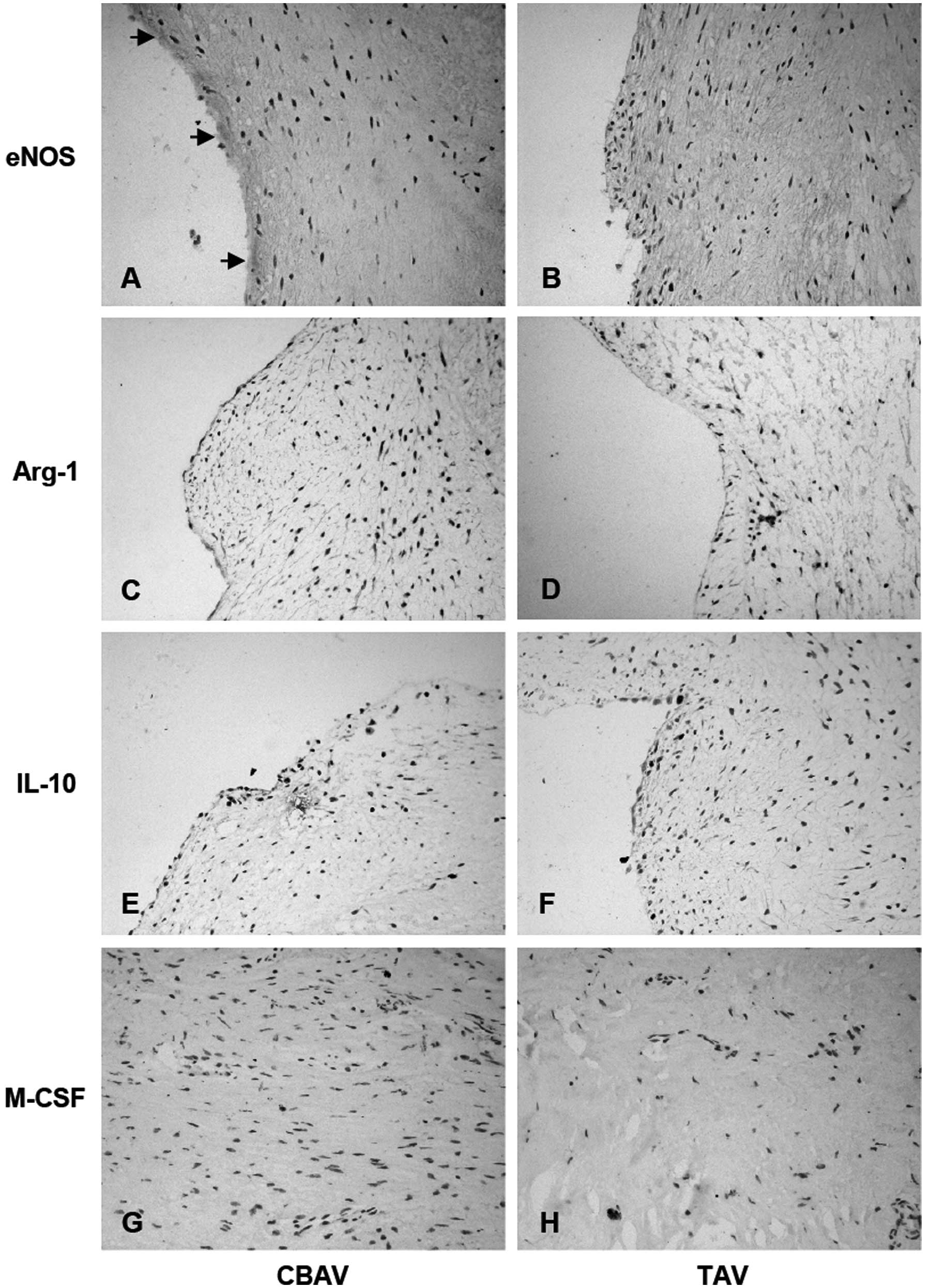 | Figure 4Immunohistochemical staining of eNOS,
Arg-1, IL-10 and M-CSF in aortic tissue. eNOS expression was
significantly higher in the (A) CBAV specimen than the (B) TAV
specimen. Arg-1, IL-10 and M-CSF expression was slightly lower in
the CBAV specimen (C, E and G, respectively) than the TAV specimen
(D, F and H, respectively) (black arrows indicate the expression of
eNOS/Arg-1/IL-10/M-CSF; magnification, ×400). eNOS, endothelial
nitric oxide synthase; Arg-1, arginase-1; IL-10, interleukin-10;
M-CSF, macrophage colony-stimulating factor; CBAV, congenital
bicuspid aortic valve; TAV, tricupsid aortic valve. |
Discussion
CBAV is a common congenital heart malformation.
Individuals with CBAV are more susceptible to aortic valve disease
at a young age, under certain circumstances even progressing to
aortic aneurysm and aortic dissection. Over the past decades,
studies have demonstrated that aortic valve calcification and
atherosclerosis are characterized by similar pathological
processes, with the two conditions being initiated by disruption of
the basement membrane and lipid deposition, and mediated by
inflammatory cell infiltration and neovascularization (15–17).
Experimental and clinical studies have also shown that the
structure and geometric shape of CBAVs differ from that of normal
TAVs (1,18,19).
CBAV leaflets experience higher shear stress, which leads to
inflammatory cell infiltration and the damage and apoptosis of
endothelial cells (20). In
addition, CBAV calcification has been associated with VEGF gene
expression (21).
Macrophages are one of the most important components
of the innate immune system. The M1 and M2 macrophage subtypes
participate in the regulation of numerous biological processes. M1
macrophages are involved in inflammation and bacterial clearance,
and are stimulated by lipopolysaccharide and/or interferon γ, which
stimulates iNOS expression. M2 macrophages are stimulated by IL-4
and IL-13, and inhibit the inflammatory reaction and promote the
repair of damaged tissue (22,23).
However, little is known about the functions of M1/M2 macrophages
during the pathogenesis of CBAV stenosis.
The present study indicates that CD68 expression was
higher in CBAVs than in TAVs, as demonstrated by iNOS expression.
However, CD163 expression was lower in CBAVs than TAVs. These
results suggest that M1 macrophage infiltration may contribute to
calcification of CBAVs. Of note, CBAVs develop earlier and more
easily than TAVs, and exhibit more severe calcification. This is in
concurrence with the hypothesis that inflammatory cell infiltration
is one of the most important aspects of calcification. Numerous
studies have demonstrated that M1 macrophages are mainly involved
in the inflammatory response, whereas M2 macrophages participate in
the inhibition of the inflammatory response (22,23).
Therefore, it may be suggested that M1 macrophages actively
participate in promoting inflammation in CBAV, thus aggravating
valvular lesions.
With regard to the function of macrophages during
the inflammatory process, with the exception of direct
phagocytosis, macrophages may participate and modify reactions
through cytokine secretion and regulation. In addition, different
macrophage subtypes may secrete and regulate different inflammatory
factors; for example, M1 macrophages express high levels of iNOS
and secrete the inflammatory cytokines tumor necrosis factor-α,
IL-1, IL-6 and IL-12, whereas M2 macrophages express high levels of
IL-10 and tumor growth factor-β (22). In the present study, eNOS
expression in CBAVs was high, while that of IL-10 was relatively
low. M1 macrophages perpetuate the inflammatory response by
upregulating eNOS expression and inhibiting IL 10 expression. The
relatively low expression of IL-10 in CBAVs could not effectively
inhibit cytokine production by activated macrophages, leading to
the perpetuation of the inflammatory response. By contrast, the low
expression of IL-10 in TAVs may led to the suppression of the
inflammatory response.
Studies in vivo have demonstrated that iNOS
and Arg-1, expressed by M1 and M2 macrophages, respectively,
competitively bind with L-arginine and exert pro- and
anti-inflammatory effects, respectively, through their metabolites
(24). iNOS degrades L-arginine
into NO and reactive oxygen species, which may promote inflammation
and the removal of invading microorganisms. However, Arg-1 degrades
L-arginine into L-ornithine, which may promote collagen synthesis
and the restoration of damage. In the present study, Arg-1
expression was low in CBAVs but slightly higher in TAVs. From these
results, it may be concluded that Arg-1 inhibits the inflammatory
response in TAVs. Conversely, the dominant M1 macrophages in CBAVs
may lead to a long-term valve inflammatory formation, involving
competitive combine with L-arginine degradation and Arg-1
suppression, respectively.
Macrophages exhibit plasticity, and studies have
shown that peroxisome proliferators are able to downregulate the
proportion of M1 to M2 macrophages, promote differentiation of M1
macrophages into M2 macrophages (25), exert an anti-inflammatory effect
and weaken the inflammatory responses of M1 macrophages (26). Randolph et al (27) demonstrated that CD163 expression
was enhanced following stimulation with M-CSF, IL-10 and
dexamethasone; therefore, M-CSF may stimulate transformation of
mature macrophages into M2 macrophages. In the present study, M-CSF
expression was significantly lower in CBAVs than in TAVs, which
indicates that, during CBAV calcification, M-CSF may decrease the
transformation of M1 macrophages to M2 macrophages and maintain the
valvular injury caused by M1 macrophages.
Notably, stenotic CBAVs were found to be associated
with increased neovascularization when compared with TAVs.
Consistent with this observation, another histopathological study
revealed an association between neovascularization and inflammatory
changes during the valvular calcification process, and the
formation of new blood vessels occurred significantly faster in
stenotic valves than in normal valves (28). Thus, these data indicate that a
positive correlation exists between valve inflammation and
neovascularization. Endothelial injury is involved in the
initiation of valve stenosis. The VEGF family has a vital role in
physiological and pathological conditions, due to the ability to
promote endothelial cell migration and proliferation, and increase
vascular permeability and thrombosis. Macrophages secrete a variety
of matrix proteins that activate VEGF and trigger the osteoblastic
transdifferentiation of vascular smooth muscle cells. This
partially explains the VEGF data from the present study, with
inflammatory cells and neovessels in close proximity to valve
calcification, and suggests that valve inflammation is responsible
for subsequent calcification and stenosis.
In conclusion, the present study demonstrates that
severe aortic stenosis in CBAVs is associated with a more
aggressive inflammatory process that involves greater inflammatory
cell infiltration and neovascularization when compared with TAVs.
Of note, the results show the importance of M1/M2 macrophage
distribution, inflammatory cytokine expression and the role of M1
macrophages in the development of CBAV stenosis. These results may
help further elucidate the more frequent onset and rapid
progression of atherosclerosis and severe aortic valve
calcification observed in patients with CBAV.
References
|
1
|
Ward C: Clinical significance of the
bicuspid aortic valve. Heart. 83:81–85. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Michelena HI, Desjardins VA, Avierinos JF,
Russo A, Nkomo VT, Sundt TM, Pellikka PA, Tajik AJ and
Enriquez-Sarano M: Natural history of asymptomatic patients with
normally functioning or minimally dysfunctional bicuspid aortic
valve in the community. Circulation. 117:2776–2784. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Otto CM: Calcification of bicuspid aortic
valves. Heart. 88:321–322. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nigam V and Srivastava D: Notch1 represses
osteogenic pathways in aortic valve cells. J Mol Cell Cardiol.
47:828–834. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weinberg EJ, Kaazempur and Mofrad MR: A
multiscale computational comparison of the bicuspid and tricuspid
aortic valves in relation to calcific aortic stenosis. J Biomech.
41:3482–3487. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McKellar SH, Tester DJ, Yagubyan M,
Majumdar R, Ackerman MJ and Sundt TM III: Novel NOTCH1 mutations in
patients with bicuspid aortic valve disease and thoracic aortic
aneurysms. J Thorac Cardiovasc Surg. 134:290–296. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jaguin M, Houlbert N, Fardel O and
Lecureur V: Polarization profiles of human M-CSF-generated
macrophages and comparison of M1-markers in classically activated
macrophages from GM-CSF and M-CSF origin. Cell Immunol. 281:51–61.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chu EM, Tai DC, Beer JL and Hill JS:
Macrophage heterogeneity and cholesterol homeostasis:
classically-activated macrophages are associated with reduced
cholesterol accumulation following treatment with oxidized LDL.
Biochim Biophys Acta. 1831:378–386. 2013. View Article : Google Scholar
|
|
9
|
Gordon S and Taylor PR: Monocyte and
macrophage heterogeneity. Nat Rev Immunol. 5:953–964. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma J, Liu L, Che G, Yu N, Dai F and You Z:
The M1 form of tumor-associated macrophages in non-small cell lung
cancer is positively associated with survival time. BMC Cancer.
10:1122010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Johnson JL and Newby AC: Macrophage
heterogeneity in atherosclerotic plaques. Curr Opin Lipidol.
20:370–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mosser DM and Edwards JP: Exploring the
full spectrum of macrophage activation. Nat Rev Immunol. 8:958–969.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Edin S, Wikberg ML, Dahlin AM, Rutegård J,
Öberg Å, Oldenborg PA and Palmqvist R: The distribution of
macrophages with a M1 or M2 phenotype in relation to prognosis and
the molecular characteristics of colorectal cancer. PLoS One.
7:e470452012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao M, Fu XL and Lv H: The expression of
EGFR in oral lichen planus, squamous cell papilloma and squamous
cell carcinoma. Shanghai Kou Qiang Yi Xue. 21:673–676. 2012.(In
Chinese).
|
|
15
|
Goldbarg SH, Elmariah S, Miller MA and
Fuster V: Insights into degenerative aortic valve disease. J Am
Coll Cardiol. 50:1205–1213. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu PJ, Skolnick A, Ferrari G, et al:
Correlation between plasma osteopontin levels and aortic valve
calcification: potential insights into the pathogenesis of aortic
valve calcification and stenosis. J Thorac Cardiovasc Surg.
138:196–199. 2009. View Article : Google Scholar
|
|
17
|
Soini Y, Salo T and Satta J: Angiogenesis
is involved in the pathogenesis of nonrheumatic aortic valve
stenosis. Hum Pathol. 34:756–763. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roberts WC: The congenitally bicuspid
aortic valve. A study of 85 autopsy cases. Am J Cardiol. 26:72–83.
1970. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Falcone MW, Roberts WC, Morrow AG and
Perloff JK: Congenital aortic stenosis resulting from a
unicommisssural valve. Clinical and anatomic features in twenty-one
adult patients. Circulation. 44:272–280. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moreno PR, Astudillo L, Elmariah S,
Purushothaman KR, Purushothaman M, Lento PA, Sharma SK, Fuster V
and Adams DH: Increased macrophage infiltration and
neovascularization in congenital bicuspid aortic valve stenosis. J
Thorac Cardiovasc Surg. 142:895–901. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Winchester R, Wiesendanger M, O’Brien W,
Zhang HZ, Maurer MS, Gillam LD, Schwartz A, Marboe C and Stewart
AS: Circulating activated and effector memory T cells are
associated with calcification and clonal expansions in bicuspid and
tricuspid valves of calcific aortic stenosis. J Immunol.
187:1006–1014. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mantovani A, Sica A, Sozzani S, Allavena
P, Vecchi A and Locati M: The chemokine system in diverse forms of
macrophage activation and polarization. Trends Immunol. 25:677–686.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tugal D, Liao X and Jain MK:
Transcriptional control of macrophage polarization. Arterioscler
Thromb Vasc Biol. 33:1135–1144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bronte V and Zanovello P: Regulation of
immune responses by L-arginine metabolism. Nat Rev Immunol.
5:641–654. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martinez FO, Gordon S, Locati M and
Mantovani A: Transcriptional profiling of the human
monocyte-to-macrophage differentiation and polarization: new
molecules and patterns of gene expression. J Immunol.
177:7303–7311. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bouhlel MA, Derudas B, Rigamonti E, et al:
PPARgamma activation primes human monocytes into alternative M2
macrophages with anti-inflammatory properties. Cell Metab.
6:137–143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Randolph GJ, Jakubzick C and Qu C: Antigen
presentation by monocytes and monocyte-derived cells. Curr Opin
Immunol. 20:52–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dorfmüller P, Bazin D, Aubert S, Weil R,
Brisset F, Daudon M, Capron F and Brochériou I: Crystalline
ultrastructures, inflammatory elements, and neoangiogenesis are
present in inconspicuous aortic valve tissue. Cardiol Res Pract.
2010:6859262010.PubMed/NCBI
|