Introduction
Chronic kidney disease (CKD) is characterized by the
presence of kidney damage, such as albuminuria and/or decreased
kidney function, for example, a glomerular filtration rate (GFR)
<60 ml/min/1.73 m2 for ≥3 months (1–3).
Furthermore, CKD is a life-threatening disorder, affecting a small
number of people who subsequently require care from a nephrologist.
In the USA, the prevalence to incidence ratio of CKD is currently
~200:1 (4). When symptoms of CKD
are severe, it is termed end-stage renal disease (ESRD), the
treatment of which, is via renal replacement therapy (RRT) or
transplantation. The complications of ESRD include increased risk
of cardiovascular disease, infection, cognitive impairment and
impaired physical function; however, CKD and metabolic bone
disorders (CKD-MBD) are the predominant complications (5–8). The
clinical manifestations of CKD-MBD include imbalances in blood
calcium (Ca) and phosphorus (Pi), vitamin D deficiency, increased
levels of parathyroid hormone (PTH) and serum fibroblast growth
factor-23 (FGF-23) (9). Moreover,
CKD-MBD results in bone damage, vascular calcification and an
increased risk of cardiovascular events, which consequently, may be
associated with dialysis quality, quality of life and mortality
(10).
A previous study by Slatopolsky and Moe (11) demonstrated that hyperphosphatemia
was critical in the progression of CKD-MBD, as well as identifying
that hyperphosphatemia was closely associated with the initiation,
progression and deterioration of CKD-MBD. A recent study by
Shigematsu et al (12)
demonstrated that various methods of treatment were required in the
clinical management of hyperphosphatemia, as dietary Pi restriction
and Pi removal via hemodialysis alone were insufficient.
FGF-23, an important regulatory cytokine, is a
hormone found in the blood that controls phosphate metabolism,
which ultimately influences the prognosis of patients exhibiting
CKD. FGF-23 is predominantly produced and secreted by osteogenic
cells and osteoblasts, with the kidney as the primary target organ.
During the early stages of CKD, FGF-23 may contribute to
maintaining the serum Pi levels within the normal range by
increasing the renal excretion of Pi (13). However, Bia et al (14) demonstrated that when kidney
function declined, the serum levels of FGF-23 increased, whereas
the Pi values were not considered to be abnormal until the
estimated (e)GFR in the maintenance hemodialysis (MHD) patients
decreased to <30 ml/min/1.73 m2. In addition, a study
has demonstrated that there may be a negative feedback loop, which
exists between FGF-23 levels and Pi disorders (9).
However, effective removal of FGF-23 in MHD patients
exhibiting hyperphosphatemia by blood purification is a complex
issue and currently there are only a small number of studies
regarding it. In the present study, three different blood
purification methods; hemodialysis (HD), hemodiafiltration (HDF),
and hemodialysis and hemoperfusion (HD+HP), were adopted to compare
the clearance efficacy of FGF-23 in MHD patients exhibiting
hyperphosphatemia.
Patients and methods
Patients
Sixty-five MHD patients (37 males and 28 females)
with hyperphosphatemia who had received RRT in the Blood
Purification Center of The Third Affiliated Hospital of Soochow
University (Changzhou, China) were enrolled in the present
prospective, randomized controlled study. Written informed consent
was obtained from all participants and the present study was
approved by the Ethics Commission of Soochow University (Changzhou,
China).
Patients with severe heart, liver and infectious
diseases were excluded from the study and the participants with the
following primary diseases were enrolled: Chronic
glomerulonephritis (n=35), hypertensive nephropathy (n=7), diabetic
nephropathy (n=9), obstructive nephropathy (n=2), polycystic kidney
disease (n=5), systemic lupus erythematosus (n=2) and others (n=5).
The diagnostic criteria of hyperphosphatemia were established
according to the National Kidney Disease Foundation-Kidney Disease
Outcomes Quality Initiative (NKF-K/DOQI); a pre-dialysis serum
phosphorus level ≥1.78 mmol/l indicated hyperphosphatemia (15).
The 65 participants were randomly divided into three
groups: The HD group, n=23 patients; the HDF group, n=21 patients
and the HD+HP group, n=21 patients.
Blood purification methods
The patients in the HD group were treated using low
flux synthetic filters (Diacap LOPS 15; B. Braun, Melsungen,
Germany) and high flux synthetic filters (Diacap HIPS 15; B. Braun)
were utilized on the patients in the HDF group. The duration of the
HD and HDF treatments for the two groups was 4 h and a blood flow
rate of 200–250 ml/min was used. HDF was conducted using a
post-dilution replacement fluid with a volume of 30% of the
ultrafiltration blood flow. The procedure in the HD+HP group was
conducted over 4 h, which was divided into two parts; initially,
the HA130-type resin HP (Jafron Biomedical Co., Ltd, Zhuhai, China)
was connected in series prior to the Diacap LOPS 15 for the first 2
h. This was followed by the exclusive use of Diacap LOPS 15 for the
subsequent 2 h; the blood flow rate was 180–250 ml/min. Bicarbonate
dialysate was administered to the three groups with a 500 ml/min
flow rate and heparin served as the anticoagulant.
Blood samples and biochemical
analysis
Blood samples were collected from the arterial blood
line immediately prior to and following the RRT sessions. The
levels of blood urea nitrogen (BUN), serum creatinine (SCr), serum
Ca and Pi of the patients were assessed using an automatic
biochemical analyzer (Hitachi, Ltd., Tokyo, Japan). FGF-23 was
analyzed via enzyme-linked immunosorbent assay (ELISA) and the
reagents were provided by Shanghai BlueGene Biotech Co., Ltd.
(Shanghai, China). To measure the serum FGF-23 concentration, the
blood samples were immediately centrifuged (1,000 × g for 15 min)
and the serum was stored at −80°C until all of the samples had been
collected. The FGF-23 was analyzed in accordance with the following
instructions.
Analysis of FGF-23 levels
All of the reagents and samples were heated to room
temperature prior to use. The standards or samples (100 μl of each)
were added to the appropriate wells in the polyclonal and sheep
anti-FGF-23 antibody pre-coated microtiter plate (Shanghai BlueGene
Biotech Co., Ltd., Shanghai, China). Conjugate (50 μl) was added to
each well and mixed and the plate was covered and incubated for 1 h
at 37°C. The microtiter plate was washed five times using a washing
machine and blotted dry using absorbent paper. Substrate A (50 μl)
and 50 μl substrate B were subsequently added to each well, the
plate was covered and incubated for 10–15 min at 20–25°C (exposure
to sunlight was avoided). A stop solution (50 μl) was added to each
well and mixed and the optical density was determined at a
wavelength of 450 nm using a microplate reader.
Calculations
Serum Pi and FGF-23 reduction ratios were calculated
using: i) The urea reduction ratio (URR) equation:
URR=(Cpre-Cpost)/Cpre; where,
Cpre and Cpost are pre- and post-treatment
BUN concentrations, respectively, and ii) the urea clearance index
(Kt/V): Kt/V=−ln(R-0.008xt)+(4-3.5xR)xUF/W where; ln, natural
logarithm; R, post-/pre-BUN ratio; t, dialysis session length
(hours); UF, ultrafiltrate volume (liters); W, post-dialysis weight
(kg) (16).
Statistical analysis
The data were expressed as means ± standard
deviation. Statistical analysis was performed with SPSS 13.0 (SPSS
Inc., Chicago, IL, USA). The statistical significance of pre- and
post-treatment differences was analyzed using paired t-tests.
One-way analysis of variance was applied to compare the three
groups and correlation analysis between FGF-23 and Pi levels was
conducted using Pearson’s product-moment coefficient. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
A total of 65 patients that exhibited
hyperphosphatemia and were undergoing MHD were enrolled for the
present study. The patients were randomly divided into three
groups: HD, n=23 patients; HDF, n=21 patients and HD+HP, n=21
patients. No significant differences regarding clinical and
biological variables, including gender, age, dialysis vintage, URR,
Kt/V, pre-treatment BUN levels, Scr, Pi or FGF-23 were observed in
the three groups prior to treatment (Table I).
 | Table IClinical and biological variables of
the three groups prior to treatment. |
Table I
Clinical and biological variables of
the three groups prior to treatment.
| Variable | HD (n=23) | HDF (n=21) | HD+HP (n=21) | Statistical
significance |
|---|
| Males, n (%) | 12 (52.2) | 13 (61.9) | 12 (57.1) | #P |
| Age (years) | 42.5±12.7 | 42.2±17.5 | 48.1±15.8 | *P |
| Dialysis vintage
(months) | 41.2±22.6 | 48.1±27.1 | 54.4±25.3 | *P |
| Urea reduction ratio
(%) | 70.27±2.55 | 73.31±3.813 | 70.38±2.908 | *P |
| Kt/V | 1.473±0.099 | 1.61±0.18 | 1.49±0.103 | *P |
| Pre-treatment BUN
(mmol/l) | 25.2±6.2 | 27.3±4.8 | 25.1±4.1 | *P |
| Pre-treatment SCr
(μmol/l) | 1024±199.6 | 1103±230.7 | 1030±180.6 | *P |
| Pre-treatment Pi
(mmol/l) | 1.63±0.42 | 2.10±0.54 | 2.18±0.59 | *P |
| Pre-treatment FGF-23
(pg/ml) | 764.3±109.8 | 785.5±125.5 | 850.9±108.6 | *P |
Serum Pi levels
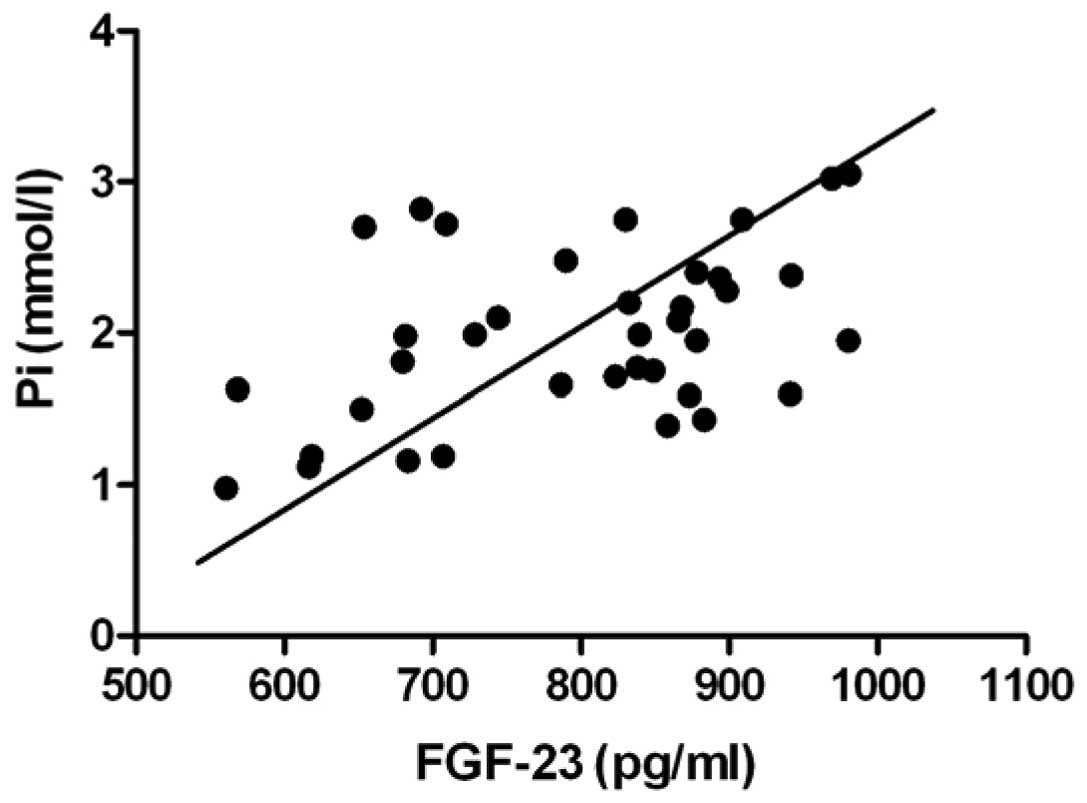
In all of the specimens prior to treatment, the
combined correlation analysis of serum FGF-23 levels indicated a
positive correlation with serum Pi levels; the Pearson
product-moment correlation coefficient was 0.45 and the difference
was statistically significant (Fig.
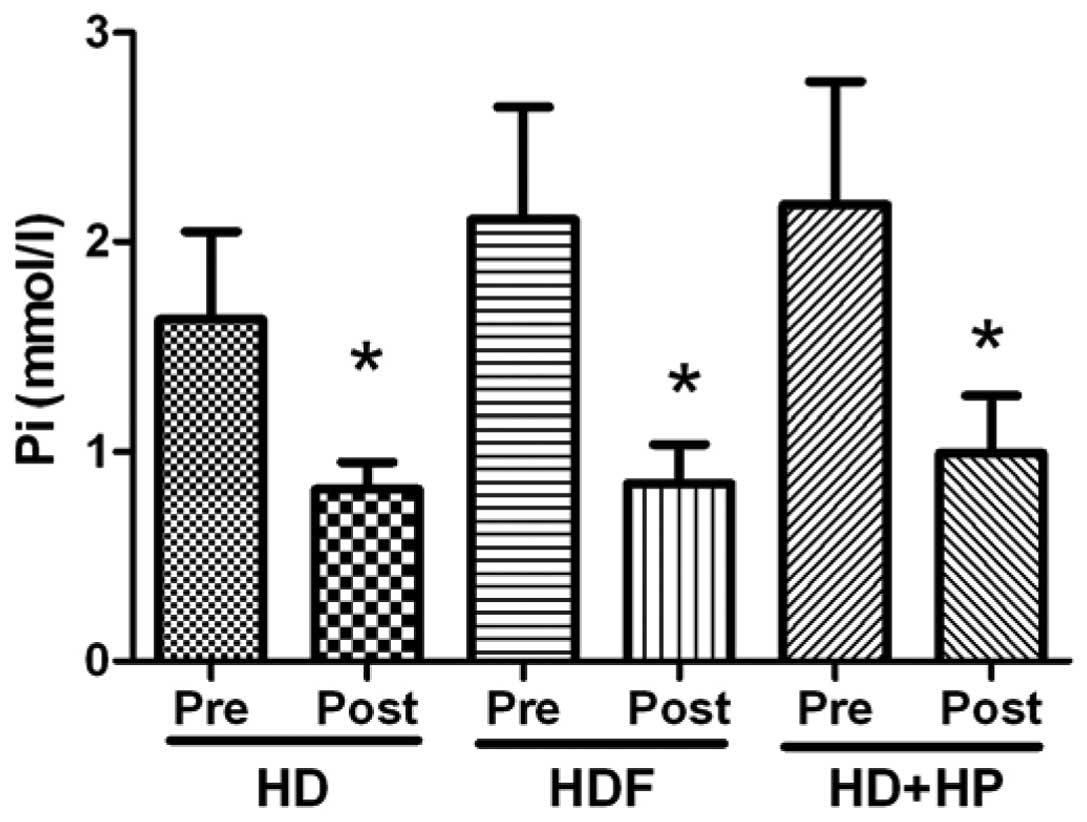
1; P<0.01). The serum Pi levels of the HD group decreased
from 1.63±0.42 mmol/l pre-hemodialysis to 0.82±0.13 mmol/l
post-hemodialysis, the difference was identified to be
statistically significant (Fig. 2;
P<0.05). The HDF and HD+HP groups demonstrated significantly
decreased serum Pi levels from 2.10±0.54 (pre-treatment) to
0.85±0.19 mmol/l (post-treatment; P<0.05) and from 2.18±0.59
(pre-treatment) to 0.99±0.27 mmol/l (post-treatment), respectively
(Fig. 2; P<0.05).
Pi reduction rate
No statistically significant difference was
identified between any of the groups regarding the Pi reduction
rate in the three types of blood purification method (Fig. 3; P>0.05).
Serum FGF-23 clearance
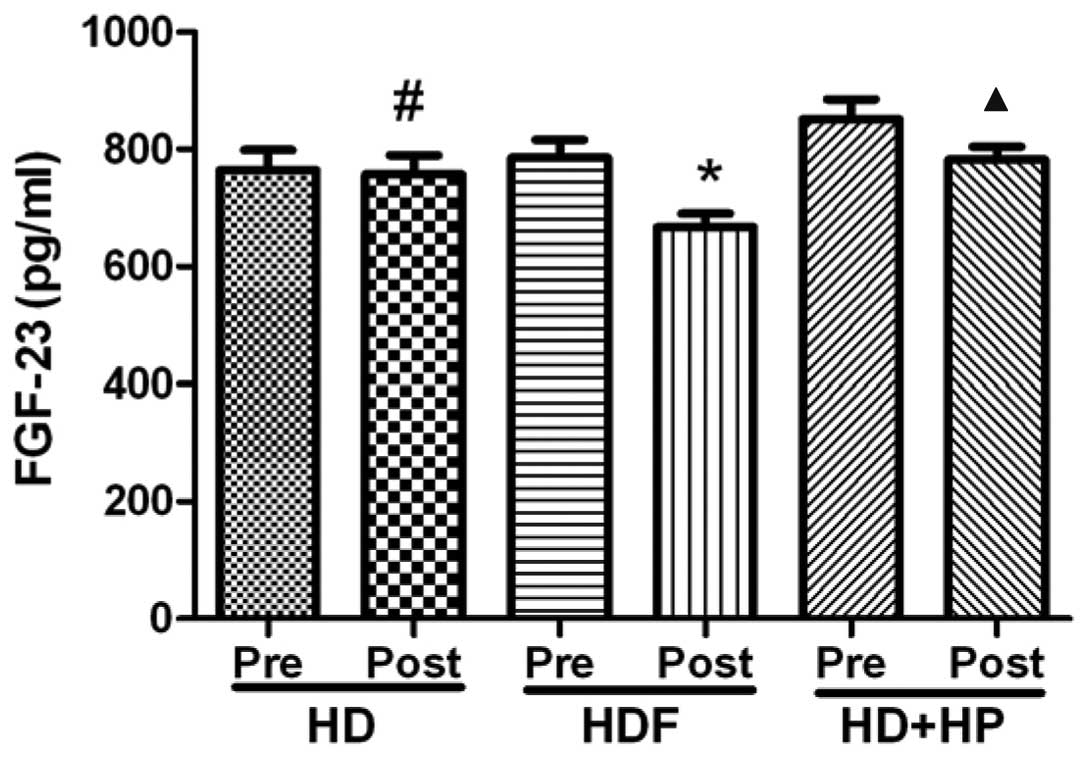
The serum FGF-23 levels of the HD group decreased
from 764.3±109.8 pg/ml pre-hemodialysis to 756.9±103.6 pg/ml
post-hemodialysis, the difference was not identified to be
significant (Fig. 4; P>0.05).
The HDF and HD+HP groups demonstrated significant decreases in
serum FGF-23 levels from 785.5±125.5 to 667.2±94.1 pg/ml
(P<0.05) and from 850.9±108.6 to 782.2±71.9 pg/ml, respectively
(Fig. 4; P<0.05).
Comparison of blood purification
methods
A significant difference was observed in the FGF-23
reduction rate between any two of the three types of blood
purification method (Fig. 5;
P<0.05).
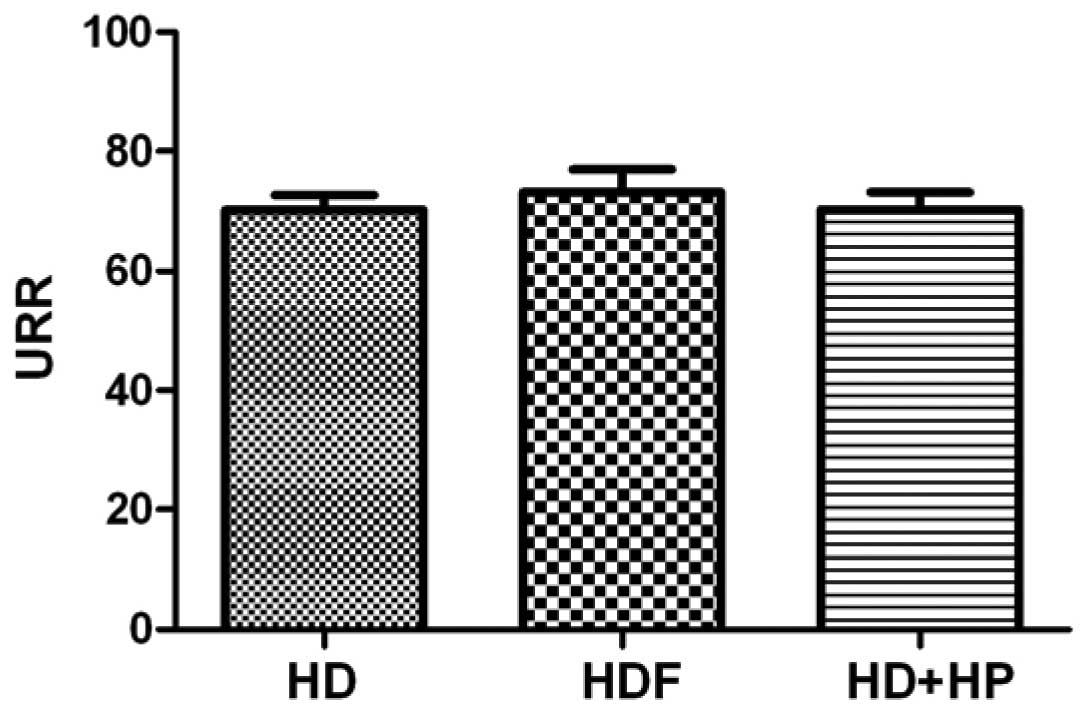
A comparison of the URR of the three blood
purification methods (HD, HDF and HD+HP) revealed values of:
70.27±2.55, 73.31±3.813 and 70.38±2.908, respectively; there were
no statistically significant differences identified between any two
of the three groups (Fig. 6;
P>0.05).
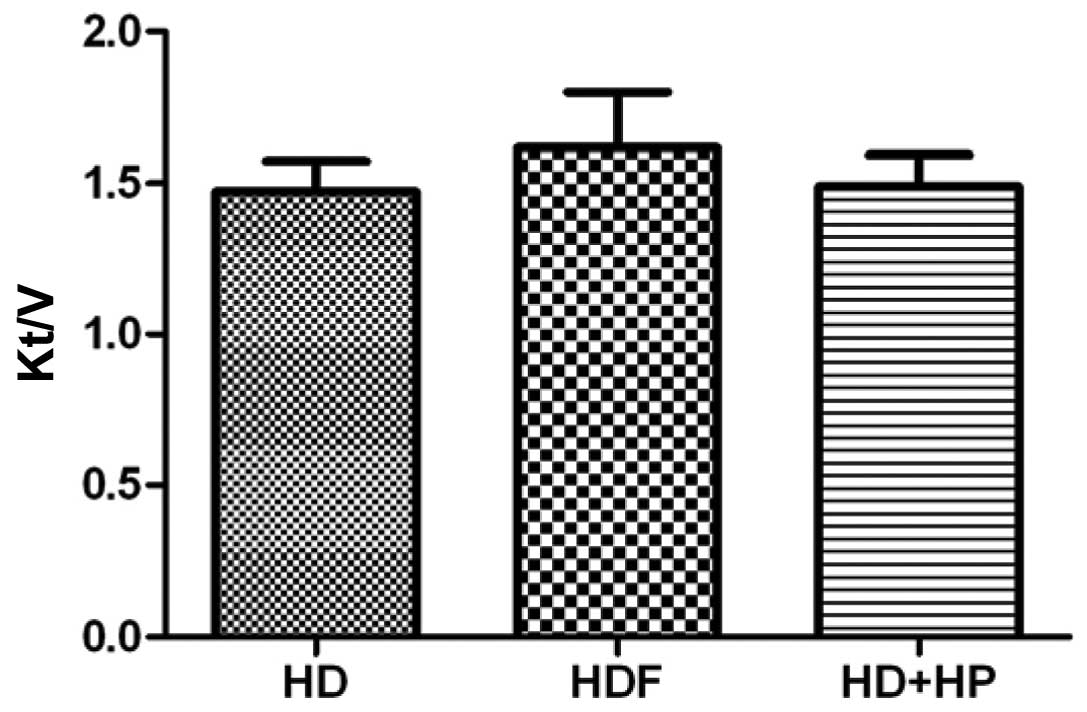
A comparison of the three blood purification methods
(HD, HDF and HD+HP) regarding the Kt/V resulted in values of
1.473±0.099, 1.61±0.18 and 1.49±0.103, respectively, with no
statistically significant differences observed between any two of
the three groups (Fig. 7;
P>0.05).
Discussion
In the present study, three different blood
purification methods were performed, HD, HDF and HD+HP, in patients
that were exhibiting hyperphosphatemia and undergoing MHD.
The findings of the present study demonstrated a
positive correlation between FGF-23 and Pi via a variable line
correlation analysis of FGF-23 with pre-dialysis (Fig. 1). However, the underlying mechanism
of this clinical manifestation remains unclear and may be
associated with various factors. Perwad et al (17) conducted an in vitro study,
which indicated that FGF-23 influenced 1a-hydroxylase expression
via activation of the extracellular signal-regulated kinases-1/2
signaling pathway, which regulates the Pi levels. When kidney
function declines, serum levels of FGF-23 increase, whereas the Pi
values are not considered to be abnormal until the eGFR decreases
to <30 ml/min/1.73 m2 (14). Thus, it has been hypothesized that
there may be a negative feedback loop existing between FGF-23
levels and Pi disorders (9).
Consistent with these findings, an injection of the FGF23
C-terminal tail peptide in healthy rats was shown to inhibit renal
phosphate excretion and induce hyperphosphatemia (18). The results of the present study
were consistent with previous studies (9,14,17,18)
and demonstrated that high levels of FGF-23 were closely associated
with hyperphosphatemia in MHD patients. Therefore, cleaning the
FGF-23 of the MHD patients, and then reducing hyperphosphatemia due
to elevated serum FGF-23 level, and ultimately resulting in
improvement of the long-term prognosis of patients, are the
important issues of blood purification.
In the present study, the three blood purification
methods were effective in the clearance of Pi (Fig. 2; P<0.05); however, there were no
statistically significant differences identified between the groups
regarding the Pi reduction rate (Fig.
3; P>0.05), which demonstrated that the efficacy of the
three Pi clearance methods was similar. However, the use of blood
purification for Pi removal is not considered to be sufficient as
14% of Pi is present in the intracellular fluid and l% is present
in the extracellular fluid; thus, Pi release from the cells into
the blood occurs more slowly compared with Pi removal during
dialysis. Therefore, the treatment of hyperphosphatemia clinically
controls Pi levels, however, is also required to control the serum
Ca, PTH and FGF-23 levels, as dietary Pi restriction and Pi removal
via blood purification alone are insufficient.
In the present study, the efficacy of clearing serum
FGF-23 using three blood purification methods in MHD patients was
compared. It was identified that HD was not able to clear FGF-23
but HDF and HD+HP were able to clear FGF-23 effectively, however,
HDF exhibited the optimum efficacy (Figs. 4 and 5).
FGF-23 is a medium-sized molecule (19); HD was able to clear the small
molecule toxins, such as urea, nitrogen and creatinine via
dispersion, however, the molecular weight cut-offs of the HD
membranes were <5 kDa, therefore removal of important intact
FGF-23 (molecular weight, 32,000) was not possible. This was
consistent with a previous study by Urena Torres et al
(20).
HDF is a type of blood purification technique, which
combines diffusion and convection. Convection is the primary method
of clearing macromolecules and the convective clearance rate is
predominantly dependent on the transmembrane pressure and the
ultrafiltration coefficient of the dialyzer. The maximum
transmembrane pressure of the dialyzer that was used in the present
study was 600 mmHg with an ultrafiltration coefficient of 50 ml/h
mmHg. Taken together, these factors validate the predominant role
that convection has in the removal of medium molecular weight
molecules, such as FGF-23. However, in the present study, the
clearing effect of FGF-23 in the HDF group was lower than the
values reported by Patrier et al (21). This may be due to the HDF treatment
effect being dose-dependent and the filter ultrafiltration
coefficient, membrane area, blood flow and displacement liquid,
which were used in the present study, being lower than those that
were reported in previous studies.
HP involves extraction of the patient’s blood and
the harmful metabolites are subsequently removed through a neutral
macroporous resin apparatus, in vitro. The HA130-type blood
perfusion resin that was used in the present study is a novel
neutral synthetic resin with an average pore diameter of 13–15 nm
and a specific surface area of ≤1000–1500 m2/g. Due to
physical adsorption and the interaction of the hydrophobic group,
the resin strongly and non-specifically adsorbs medium-sized
molecules, such as FGF-23. The adsorbent is not able to effectively
remove water, urea, nitrogen or other small molecules, therefore,
the adsorption treatment in MHD patients often uses a combination
of HD+HP blood purification methods to completely remove the
metabolic waste products.
In the present study, the clearance of FGF-23 in the
HD+HP group was observed to be less effective than that of the HDF
group. A possible mechanism may be related to the furin proteolytic
enzyme cleavage of 179 arginine/180 serine within FGF-23, which may
have been separated into N-terminal (18 kDa) and C-terminal (12
kDa) fragments. The distribution of varying molecular weights and
different forms of FGF-23 in the blood circulation is unknown.
Therefore, convection by HDF is considered to be more effective
than adsorption by HP for the clearance of FGF-23 (18).
Comparisons between the three blood purification
methods regarding URR and the Kt/V indicated no statistically
significant differences. Therefore, it was hypothesized that the
difference between the three blood purification methods regarding
serum FGF-23 clearance was not due to the adequacy of dialysis
(Figs. 6 and 7).
In conclusion, the expression of serum FGF-23 was
positively correlated with the levels of Pi in MHD patients
exhibiting hyperphosphatemia. In addition, FGF-23 was identified to
be an important regulator of the clearance of Pi from the blood.
However, a limitation of the present study was that the long-term
clinical significance of the different blood purification methods
was not observed, therefore, future studies are required for
further investigation.
Acknowledgements
This study was supported by Changzhou Health
Administration Instructional Technology Projects (grant no.
wz201008) and The Hospital-Level Care Special Pre-Research Fund of
The First People’s Hospital of Changzhou (Changzhou, China; grant
no. yy2012016).
References
|
1
|
National Kidney Foundation. K/DOQI
clinical practice guidelines for chronic kidney disease:
evaluation, classification, and stratification. Am J Kidney Dis.
39(2 Suppl 1): S1–S266. 2002.PubMed/NCBI
|
|
2
|
Vassalotti JA, Stevens LA and Levey AS:
Testing for chronic kidney disease: a position statement from the
National Kidney Foundation. Am J Kidney Dis. 50:169–180. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stevens LA and Levey AS: Current status
and future perspectives for CKD testing. Am J Kidney Dis. 53(3
Suppl 3): S17–S26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Levey AS and Coresh J: Chronic kidney
disease. Lancet. 379:165–180. 2012. View Article : Google Scholar
|
|
5
|
Hsu CY, Ordoñez JD, Chertow GM, Fan D,
McCulloch CE and Go AS: The risk of acute renal failure in patients
with chronic kidney disease. Kidney Int. 74:101–107. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
James MT, Hemmelgarn BR, Wiebe N, et al;
Alberta Kidney Disease Network. Glomerular filtration rate,
proteinuria, and the incidence and consequences of acute kidney
injury: a cohort study. Lancet. 376:2096–2103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
James MT, Quan H, Tonelli M, et al;
Alberta Kidney Disease Network. CKD and risk of hospitalization and
death with pneumonia. Am J Kidney Dis. 54:24–32. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hailpern SM, Melamed ML, Cohen HW and
Hostetter TH: Moderate chronic kidney disease and cognitive
function in adults 20 to 59 years of age: Third National Health and
Nutrition Examination Survey (NHANES III). J Am Soc Nephrol.
18:2205–2213. 2007.
|
|
9
|
Miyamoto K, Ito M, Tatsumi S, Kuwahata M
and Segawa H: New aspect of renal phosphate reabsorption: the type
IIc sodium-dependent phosphate transporter. Am J Nephrol.
27:503–515. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu P, Xuan Q, Hu B, Lu L, Wang J and Qin
YH: Fibroblast growth factor-23 helps explain the biphasic
cardiovascular effects of vitamin D in chronic kidney disease. Int
J Biol Sci. 8:663–671. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Slatopolsky E and Moe S: 50 years of
research and discovery in chronic kidney disease and mineral &
bone disorder: the central role of phosphate. Kidney Int Suppl.
S1–S2. 2011.PubMed/NCBI
|
|
12
|
Shigematsu T, Nakashima Y, Ohya M, et al:
The management of hyperphosphatemia by lanthanum carbonate in
chronic kidney disease patients. Int J Nephrol Renovasc Dis.
5:81–89. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gutierrez O, Isakova T, Rhee E, et al:
Fibroblast growth factor-23 mitigates hyperphosphatemia but
accentuates calcitriol deficiency in chronic kidney disease. J Am
Soc Nephrol. 16:2205–2215. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bia M, Adey DB, Bloom RD, Chan L, Kulkarni
S and Tomlanovich S: KDOQI US commentary on the 2009 KDIGO clinical
practice guideline for the care of kidney transplant recipients. Am
J Kidney Dis. 56:189–218. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Noordzij M, Korevaar JC, Boeschoten EW, et
al; Netherlands Cooperative Study on the Adequacy of Dialysis
(NECOSAD) Study Group. The Kidney Disease Outcomes Quality
Initiative (K/DOQI) Guideline for Bone Metabolism and Disease in
CKD: association with mortality in dialysis patients. Am J Kidney
Dis. 46:925–932. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Daugirdas JT: Second generation
logarithmic estimates of single-pool variable volume Kt/V: an
analysis of error. J Am Soc Nephrol. 4:1205–1213. 1993.PubMed/NCBI
|
|
17
|
Perwad F, Zhang MY, Tenenhouse HS and
Portale AA: Fibroblast growth factor 23 impairs phosphorus and
vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin
D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal
Physiol. 293:F1577–F1583. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goetz R, Nakada Y, Hu MC, et al: Isolated
C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting
FGF23-FGFR-Klotho complex formation. Proc Natl Acad Sci USA.
107:407–412. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen HC, Lim LM, Chang JM and Misra M:
Save life and improve quality: Report from the 5th Congress of
International Society for Hemodialysis. Hemodial Int. Jul
30–2013.(Epub ahead of print).
|
|
20
|
Urena Torres P, Friedlander G, de
Vernejoul MC, Silve C and Prié D: Bone mass does not correlate with
the serum fibroblast growth factor 23 in hemodialysis patients.
Kidney Int. 73:102–107. 2008.
|
|
21
|
Patrier L, Dupuy AM, Granger Vallée A, et
al: FGF-23 removal is improved by on-line high-efficiency
hemodiafiltration compared to conventional high flux hemodialysis.
J Nephrol. 26:342–349. 2013. View Article : Google Scholar : PubMed/NCBI
|