Introduction
Pituitary prolactinoma is an estrogen-related tumor
(1). Estrogen regulates the
synthesis and secretion of prolactin (PRL) from lactotrophs
(2). Numerous studies have
demonstrated that estrogen induces lactotroph proliferation and
results in prolactinoma formation (3,4). The
effects of estrogen are mediated by estrogen receptors (ERs)
(5), which are expressed in all
pituitary tumor subtypes (6).
Furthermore, ER expression levels are higher in prolactinoma than
in other pituitary tumor types and may be correlated with tumor
size (6,7). Therefore, close correlations exist
among estrogen, ERs and prolactinoma, which provide a viable
therapeutic target in prolactinoma treatment. Certain
antiestrogenic compounds have been shown to exert a suppressive
effect on the cell growth and PRL secretion of normal pituitary
cells and pituitary tumor cells (8).
Resveratrol (RE), a type of phytoestrogen, acts as
an agonist as well as an antagonist for ERs (9). In certain tumors, RE has shown
chemopreventive and chemotherapeutic effects; this may be
attributed to its capacity to inhibit cellular events associated
with the initiation, promotion and progression of tumors (10). These properties indicate the
potential use of RE as an adjuvant in the chemotherapy of
prolactinoma.
GH3 cells, an established estrogen-responsive cell
line from rat pituitary tumor cells, secrete PRL and growth hormone
(GH) (11). These cells are a
useful model for investigating the effects of RE on prolactinoma.
In the present study, the effect of RE on the synthesis of PRL and
cell proliferation in GH3 cells was examined.
Materials and methods
Reagents and chemicals
The following reagents and chemicals were used in
the present study: RE (Sigma, St. Louis, MO, USA); 17β-estradiol
(E2; Sigma); general caspase inhibitor z-VAD-fmk (Promega
Corporation, Madison, WI, USA); and antibodies against rat PRL
(rPRL; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), GH
(Chemicon, Temecula, CA, USA), poly ADP ribose polymerase (PARP;
Invitrogen Life Technologies, Carlsbad, CA, USA) and caspase-3 and
-8 (Santa Cruz Biotechnology, Inc.).
Cell culture
GH3 cells were obtained from the Institute of Basic
Medical Sciences of the Chinese Academy of Medical Sciences
(Beijing, China) and were maintained in Ham’s F-10 medium
(Gibco-BRL, Carlsbad, CA, USA) containing 12.5% horse serum
(Gibco-BRL), 2.5% HyClone™ fetal bovine serum
(ThermoFisher Scientific, Waltham, MA, USA), 2 mmol/l L-glutamine
(Sigma), 0.25 μg/ml Fungizone® (Invitrogen Life
Technologies) and 80 μg/ml gentamicin (Sigma). The cells were
plated at various densities and were incubated for four days in the
maintenance medium. Prior to treatment, the medium was replaced
with the treatment medium, a defined serum-free, phenol red-free
medium, containing Ham’s F-12 medium (Gibco-BRL) with 10 μg/ml
insulin (Sigma), 5 μg/ml transferrin (Sigma) and 0.5 ng/ml
parathyroid hormone (Sigma). After 24 h, cells were treated with or
without E2 and RE at various concentrations for different time
periods.
Cell proliferation assays
Cell proliferation was assessed via an MTT assay
(Sigma) following extensive validation, which included a direct
comparison to tritiated thymidine incorporation and a linear
correlation with the actual cell number. Briefly, 1×105
GH3 cells were plated in 96-well plates. Following treatment, with
or without the addition of E2 and RE at various concentrations for
6 h or three days, 20 μl MTT at a concentration of 5 mg/ml was
added to each well. After 4 h, 200 μl dimethyl sulfoxide was added
and the optical density at 490 nm was observed using a Fluostar
Optima ABS UV/Vis microplate reader (BioTek Instruments, Inc.,
Winooski, VT, USA). Data were presented as a percentage of
vehicle-treated control values.
Apoptosis analysis
GH3 cells were treated with vehicle control or RE,
or RE in the presence or absence of pretreatment with 100 μM
Z-VAD-fmk, a pan-inhibitor of caspase for 1 h. Following treatment,
apoptosis was assessed using the Annexin V-fluorescein
isothiocyanate (FITC)-labeled apoptosis detection kit I (BD
Biosciences Pharmingen, San Diego, CA, USA). Cells were washed
twice with phosphate-buffered saline (PBS), suspended in binding
buffer and stained with Annexin V-FITC and propidium iodide. Cells
undergoing apoptosis were detected by flow cytometry FACS Calibur
(Becton Dickinson, Rutherford, NJ, USA).
Immunofluorescent microscopy
GH3 cells were cultured on glass coverslips in a
24-well plate. Dual-labeling immunofluorescent detection of GH and
PRL was performed using primary antisera obtained from two
different species of animal. The cells were initially incubated in
monkey antiserum against rat GH (1:500) in a humidified chamber for
1 h at room temperature and rinsed with PBS three times, followed
by incubation in rabbit antiserum against rPRL (1:1,000). The
immunoreacted coverslips were washed three times with PBS,
incubated with tetramethylrhodamine isothiocyanate-labeled goat
anti-monkey IgG (Sigma), followed by incubation in FITC-labeled
chicken anti-rabbit IgG (Sigma) in a humidified chamber for 30 min
at room temperature. Immunofluorescence (GH, red; PRL, green) was
observed with a laser scanning confocal fluorescence microscope LSM
710 (Carl Zeiss AG, Oberkochen, Germany). The fields were
systematically scanned across the coverslip to avoid overlap. Each
group consisted of three coverslips and >1,000 cells were
observed per coverslip. The proportions of each cell type were
determined.
Reverse transcription-polymerase chain
reaction (RT-PCR) of PRL
Total RNA was prepared from GH3 cells using TRIzol
LS reagent (Invitrogen Life Technologies) according to the
manufacturer’s instructions. Messenger RNA was reverse transcribed
into single-stranded complementary DNA (cDNA) using 1.0 mg total
RNA, an oligo (dT) primer (Promega Corporation) and Moloney murine
leukemia virus reverse transcriptase (Gibco-BRL). The reaction
mixtures were diluted 20-fold and subjected to PCR amplification of
PRL cDNA, as previously described (12). The PCR primers were designed on the
basis of published sequences of rat PRL (13). Amplification of cDNAs was conducted
using the following primers: Forward: 5′-CCT GAA GAC AAG GAA CAA
GCC-3′ and reverse: 5′-TGG GAA TCC CTG CGC AGG CA-3′. PCR
amplification was conducted using the GeneAmp® PCR
System 2400 (Perkin-Elmer, Norwalk, CT, USA) following denaturation
of the samples for 5 min. Each cycle consisted of: Denaturation at
94°C for 1 min, annealing at 60°C for 1 min and extension at 72°C
for 2 min. Following amplification, there was a final 10 min
extension step at 72°C. qPCR analysis of PRL was conducted over 30
cycles. The PCR products were separated by electrophoresis on a
1.0% agarose gel, visualized with ethidium bromide staining and
quantified via scanning densitometry using NIH Image software,
version 1.61 (National Institutes of Health, Bethesda, MD, USA).
The quantity of PCR products for PRL was normalized to that of the
PCR products of glyceraldehyde-3-phosphate dehydrogenase per
sample.
Western blot analysis
Following treatment, cells were rinsed twice with
ice-cold PBS solution. Lysis buffer (containing 50 mM Tris, pH 7.5;
5 mM ethylene glycol tetraacetic acid, 120 mM NaCl, 20 mM
α-glycerophosphate, 1% NP-40, 15 mM sodium pyrophosphate, 50 mM
sodium fluoride, 10 mM sodium orthovanadate, 0.5 mM
phenylmethanesulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml
leupeptin and 20% glycerol) was added (120–180 μl) and the cells
were incubated for 10–30 min at 4°C. Following cell scraping, cell
lysates were clarified by centrifugation (9,600 × g for 15 min at
4°C) and the proteins in the supernatant were determined via a
bicinchoninic acid protein assay (Pierce Chemical Co., Rockford,
IL, USA). Equal quantities of proteins were used for western blot
analysis. Briefly, 35 μg cell lysate was subjected to
electrophoresis on 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis gel and the separated proteins were transferred
onto polyvinylidene fluoride membranes. The membranes were washed
twice with PBS and Tween-20 (PBST) and incubated with a blocking
buffer (4% non-fat milk) for 1–2 h at room temperature. The
membranes were incubated overnight at 4°C with primary antibodies
(anti-PRL, anti-PARP, anti-caspase-3, anti-caspase-8 and anti-actin
1:5,000), followed by three 10-min washes with PBST. The secondary
antibody was goat anti-rabbit IgG conjugated with horseradish
peroxidase (Amersham Life Sciences, Buckinghamshire, England).
Membranes were incubated with the secondary antibodies at room
temperature for 1 h. Densitometric assays were conducted using a
Kodak Digital Science ID scanner (Eastman Kodak, Rochester, NY,
USA). This experiment was repeated three times.
Statistical analysis
Each experiment was conducted three times,
independently. Data are expressed as the mean ± SD and were
analyzed using SPSS 10.0 software (SPSS Inc., Chicago, IL, USA).
Statistical significance was determined using analysis of variance,
Student’s t-test and a χ2 test. P<0.01 was considered
to indicate a statistically significant difference.
Results
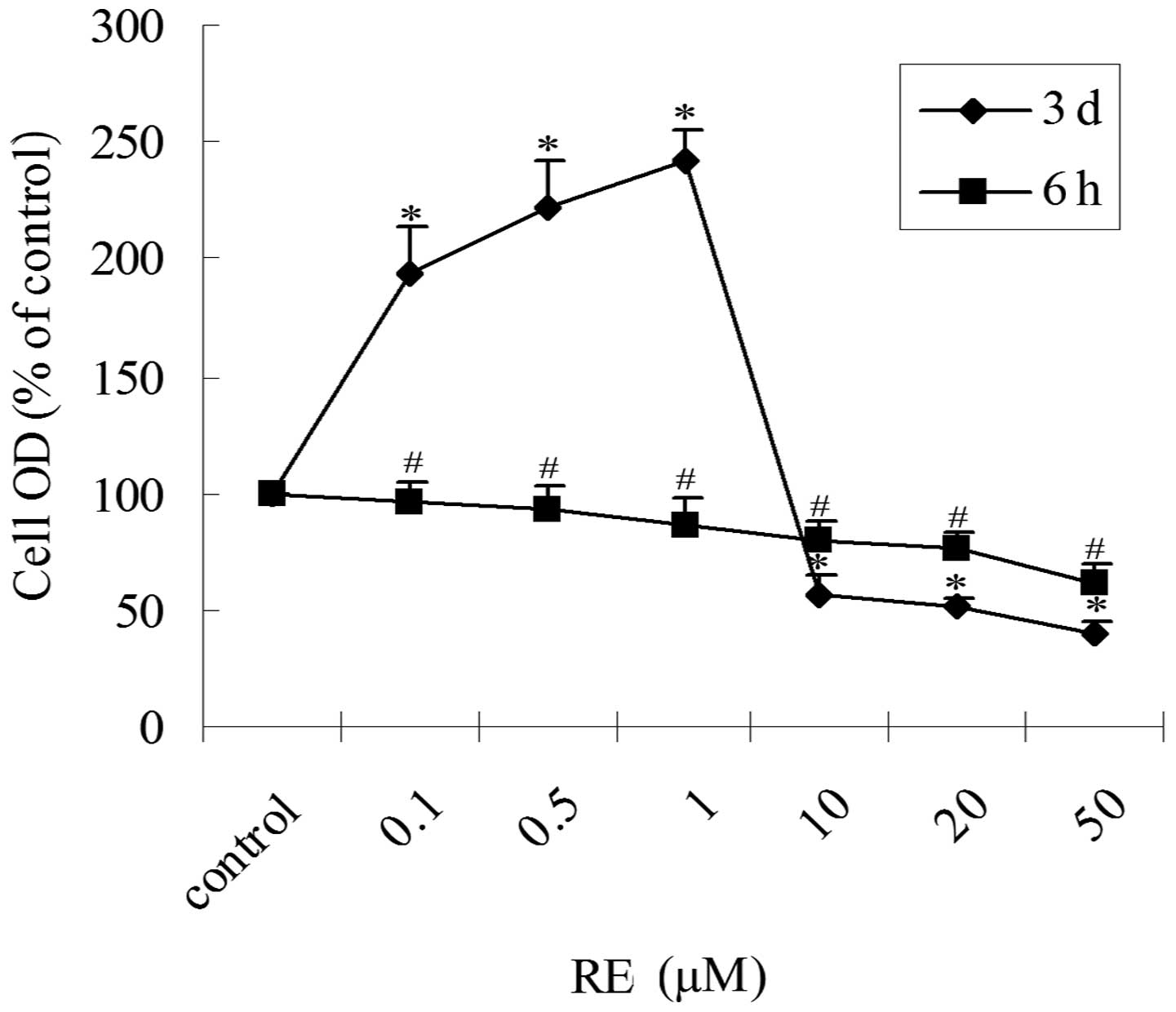
Biphasic effect of RE on the
proliferation of GH3 cells
To determine the proliferation-related effects of
RE, GH3 cells were treated with increasing concentrations of RE in
serum-free, phenol red-free medium for 6 h or three days and cell
proliferation was assessed via an MTT assay. In the cells treated
with RE for 6 h, an inhibitory effect on cell proliferation was
observed in a concentration-dependent manner (Fig. 1). However, treatment of GH3 cells
with RE for three days had a biphasic effect on cell proliferation
(Fig. 1). RE treatment for three
days at low concentrations (0.1–1 μM) stimulated cell proliferation
(2 to 3-fold) and at high concentrations (10–50 μM) inhibited
proliferation (~2-fold) compared with the control. The half maximal
inhibitory concentration (IC50), of RE was between 10
and 20 μM. Thus, depending on the concentration and duration of
treatment, RE exhibited inhibitory and stimulatory effects on the
proliferation of GH3 cells.
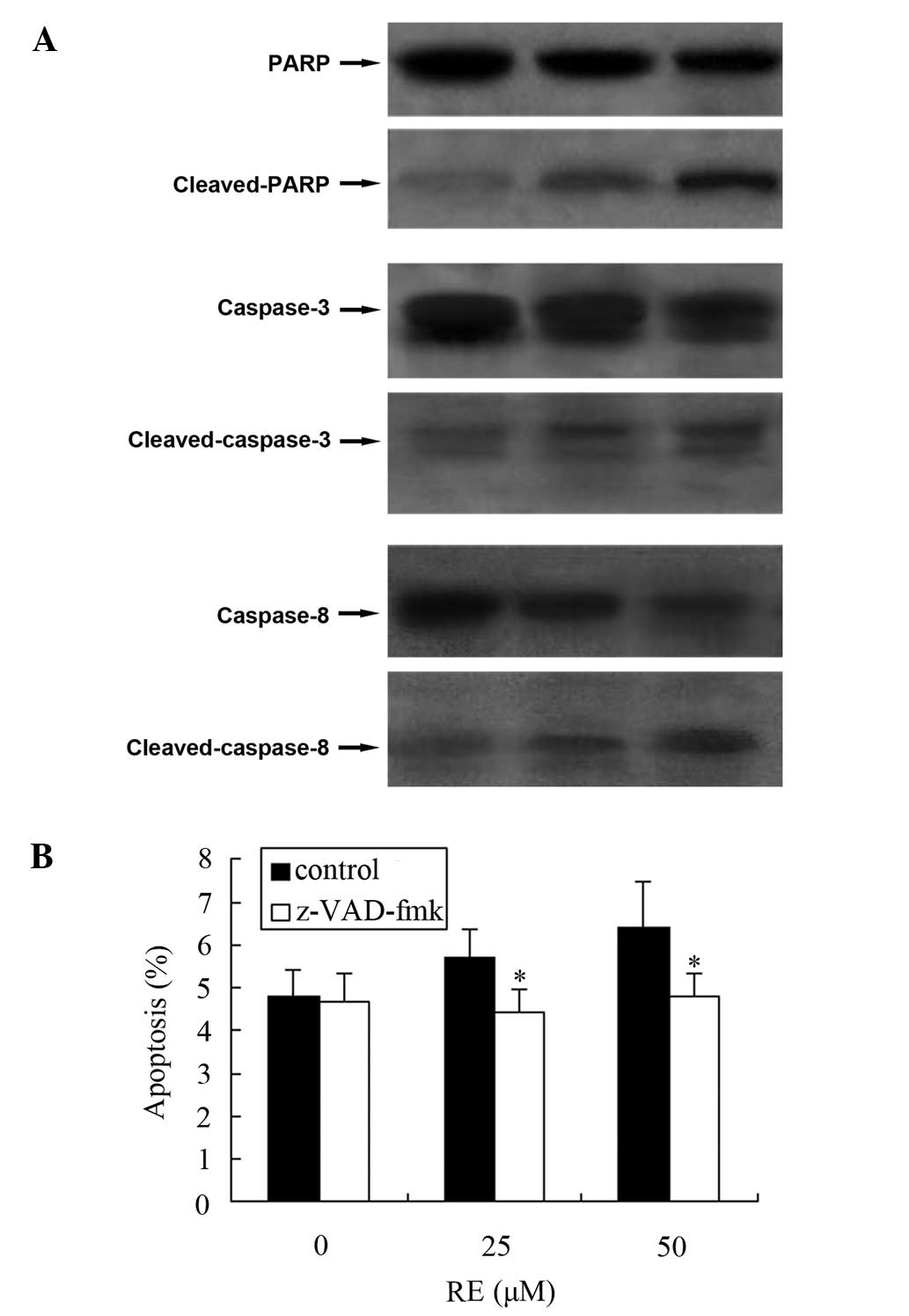
RE-induced cell apoptosis is dependent on
the activation of caspase-3 and -8 in GH3 cells
We have previously demonstrated that RE induces
apoptosis in GH3 cells (14). As
cells undergoing apoptosis execute programmed cell death by the
activation of caspases and the cleavage of PARP, the levels of
cleaved PARP, caspase-3 and -8 were measured. As Fig. 2A demonstrates, cleaved PARP was
detected in GH3 cells, accompanied by cleaved caspase-3 and -8. To
further confirm the involvement of caspase activation in the
RE-induced apoptosis, a broad spectrum caspase inhibitor,
z-VAD-fmk, was employed. After 24 h of RE exposure, the percentage
of apoptotic cells was reduced by z-VAD-fmk (Fig. 2B).
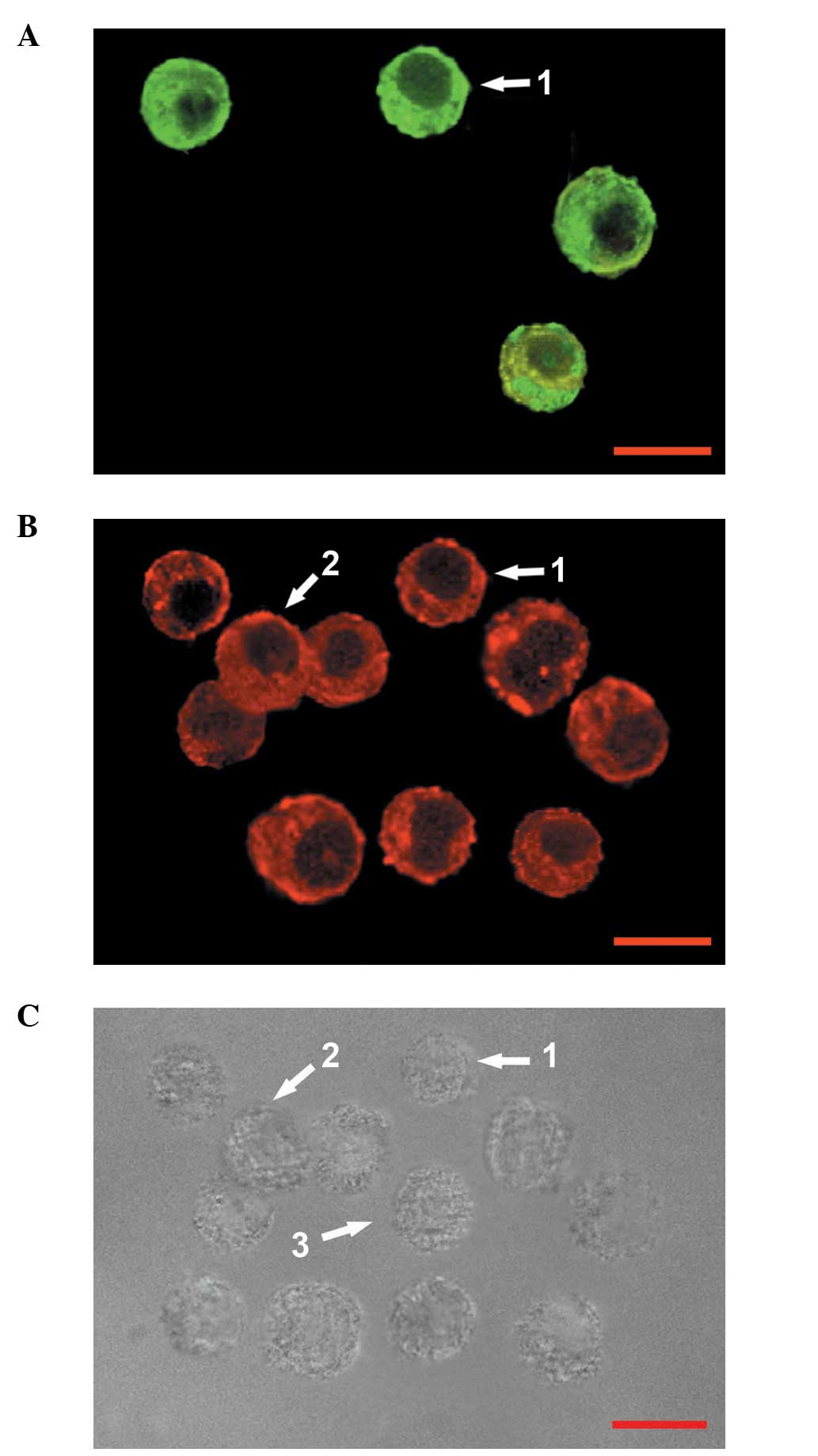
Effect of RE on the proportions of
various cell types in GH3 cells
GH3 cells may be divided into four categories: Cells
immunoreactive for GH alone (GH+ cells); cells
immunoreactive for PRL alone (PRL+ cells); cells
immunoreactive for PRL and GH (PRL+/GH+
cells) and cells in which neither PRL nor GH were detected
(PRL−/GH− cells) (11). In the present study,
double-labeling immunocytochemical analysis of GH3 cells cultured
in serum-free, phenol red-free medium (control) for three days
revealed that the GH3 cells were composed of
PRL+/GH+ cells, GH+ cells and
PRL−/GH− cells (Fig. 3); PRL+ cells were not
detected. At high concentrations (10–50 μM), under the same culture
conditions, RE treatment for three days decreased the proportion of
PRL+/GH+ cells; however, the proportion of
GH+ cells increased. At low concentrations (0.1–1 μM),
RE did not change the relative proportions of the types of GH3
cells (Table I) and
PRL+ cells were not detected. The relative proportion of
PRL−/GH− cells was not affected by RE at the
selected concentrations (0.1–50 μM). Although quantitative analysis
of the immunoreactivity was not conducted at high concentrations of
RE (10–50 μM), PRL immunoreactivity was observed to be decreased
compared with that in the controls.
 | Table IProportion of each cell type in the
GH3 cells, following treatment for three days at different
concentrations of RE. |
Table I
Proportion of each cell type in the
GH3 cells, following treatment for three days at different
concentrations of RE.
| Proportion (%) of
each cell type |
|---|
|
|
|---|
| Treatment |
PRL+/GH+ cells | GH+
cells |
PRL−/GH− cells |
|---|
| Control | 9.2±0.6 | 73.5±2.1 | 17.3±1.4 |
| 0.1 μM RE | 9.1±0.4 | 73.9±1.6 | 17.0±0.6 |
| 0.5 μM RE | 9.3±0.1 | 73.7±0.6 | 17.0±1.0 |
| 1.0 μM RE | 9.2±0.4 | 73.4±2.1 | 17.4±0.7 |
| 10 μM RE | 7.6±0.7a | 75.2±1.6a | 17.2±0.2 |
| 20 μM RE | 7.4±0.1a | 75.3±0.9a | 17.3±0.7 |
| 50 μM RE | 6.2±0.8a | 75.7±1.8a | 17.1±1.0 |
Inhibitory effect of RE on PRL
synthesis
The effect of RE on PRL at the mRNA level was
evaluated using RT-PCR and the content of intracellular PRL was
established by western blot analysis. GH3 cells, cultured in
serum-free, phenol red-free medium (control), synthesized PRL. At
selected concentrations (0.01–10 μM), RE reduced PRL mRNA and
intracellular PRL levels, resulting in decreased PRL production
(Fig. 4).
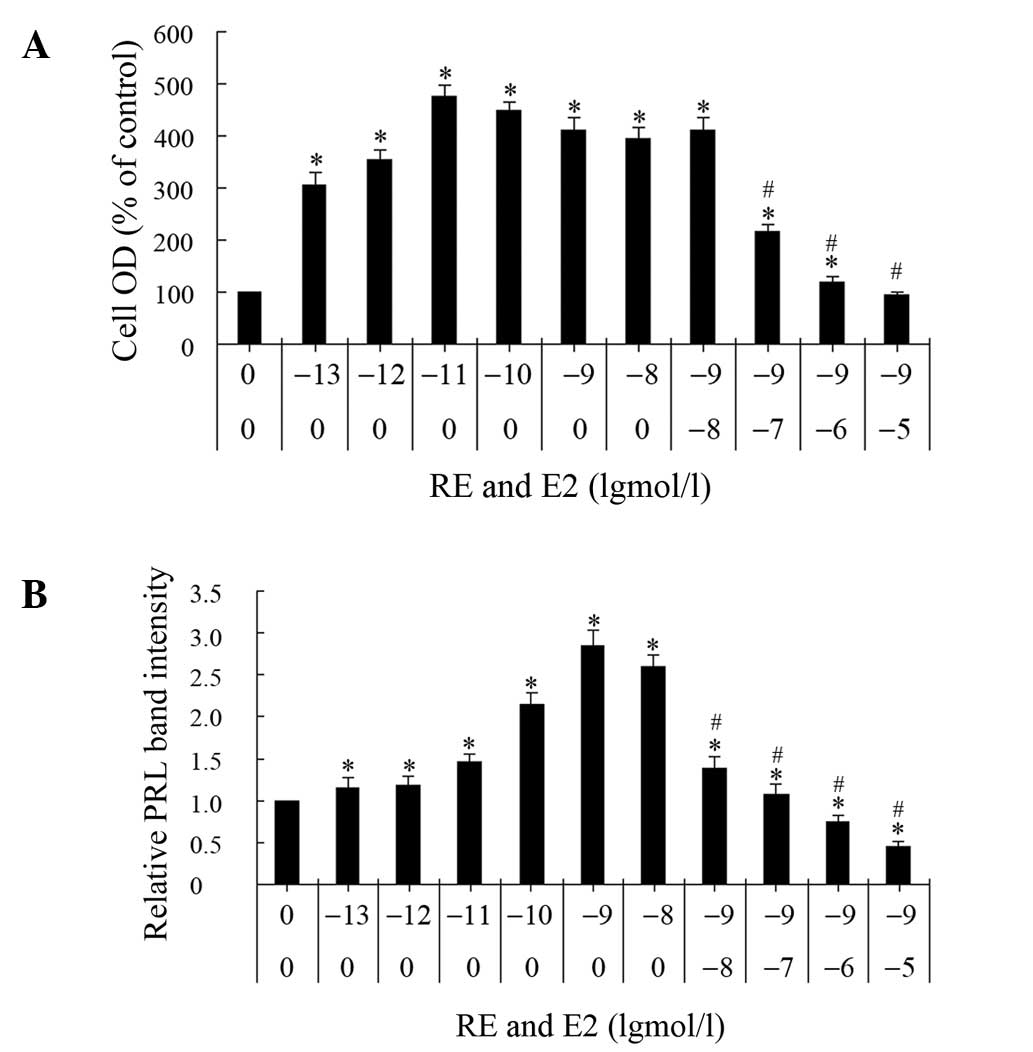
Inhibitory effect of RE on cell
proliferation and PRL synthesis induced by E2
To determine the antiestrogenic effect of RE, GH3
cells were treated with E2 alone, or E2 and RE simultaneously, for
three days and two parameters were analyzed, namely, cell
proliferation and PRL synthesis (Fig.
5). The effect of the E2 concentration on cell proliferation
was measured using an MTT assay. At the selected concentrations
(0.1 pM-10 nM), E2 stimulated proliferation 2- to 4-fold with a
maximum effect at 0.01 nM, whereas a higher concentration decreased
cell growth. The cells maintained full levels of proliferation,
comparable with those induced by E2 alone, when incubated with 1 nM
E2 and 0.01 μM RE. However, 0.1 μM RE decreased the proliferation
induced by 1 nM E2 and 10 μM RE inhibited the 1 nM E2-induced
proliferation to levels comparable with those of the control
group.
The effects of the E2 concentration on PRL synthesis
were analyzed. At concentrations between 0.1 pM and 10 nM, E2
stimulated PRL synthesis. A concentration of 1 nM E2 resulted in a
maximal response and the half maximal induction concentration
(EC50) was ~0.01 nM. When GH3 cells were simultaneously
treated with E2 and RE, with the latter at selected concentrations
between 0.01 and 10 μM, RE inhibited the PRL synthesis that was
induced by 1 nM E2 in a concentration-dependent manner; at a
concentration of 0.01 μM, RE decreased the E2-induced intracellular
PRL content to 50% of the level induced by E2 alone.
Discussion
Estrogen plays a key role in the development and
progression of pituitary prolactinoma (1). Selective estrogen receptor modulators
(SERMs) exhibit antiestrogenic properties (8) and may be adopted in the treatment of
prolactinoma. Numerous studies have identified that RE, a type of
SERM, is able to induce growth inhibition and apoptosis in certain
tumors including GH3 cells (14,15).
Therefore, RE may be a potential therapeutic agent for
prolactinoma. The aim of medical therapy for prolactinoma is to
reduce the volume of the tumor and decrease the level of PRL to
improve the endocrine symptoms.
The present study demonstrated the concentration-
and treatment duration-dependent biphasic effect of RE on the
proliferation of GH3 cells. After three days of treatment, RE
stimulated cell proliferation at low concentrations (0.1–1 μM),
whereas it inhibited cell proliferation at high concentrations
(10–50 μM). The concentration of RE required for 50% inhibition of
the GH3 cell proliferation, as compared with the control, was ~20
μM. When the treatment duration was reduced to 6 h, RE treatment
inhibited proliferation in a concentration-dependent manner.
We previously observed the apoptosis in GH3 cells as
a result of RE exposure (14). Chu
et al (16) demonstrated
that RE induced growth inhibition via cell cycle arrest and
apoptosis in GH3 cells. However, the underlying molecular
mechanisms were not clear. It was hypothesized that RE-induced cell
death is tumor-specific and involves the cluster of differentiation
95 (CD95) or CD95-ligand system as the apoptotic trigger. In the
present study, RE activated the caspase-8 and -3 pathway, which
resulted in the cleavage of PARP. Therefore, RE-induced apoptosis
in GH3 cells was shown to be caspase-dependent.
The results of the immunocytochemical experiments
showed a decreased proportion of PRL+/GH+
cells and an increased proportion of GH+ cells following
treatment with RE. Numerous studies have shown that
PRL+/GH+ cells are capable of bipotential
differentiation into PRL+ cells or GH+ cells
when induced by specific growth factors (11,16,17).
Lee et al (8) demonstrated
that in GH3 cells, the percentage of PRL-immunopositive cells was
increased by E2 and decreased by tamoxifen. Furthermore, E2
combined with epidermal growth factor and insulin, increases the
percentage of PRL+/GH+ cells and stimulates
the development of PRL+ cells (18). In physiological states of estrogen
excess, such as pregnancy or the estrous phase of the estrous
cycle, the percentage of lactotrophs increases in the pituitary.
Prolactinoma growth occurs in ~23% of females harboring
macroprolactinomas during pregnancy; therefore, estrogen is key in
lactotroph proliferation and differentiation (1). In the present study, RE inhibited
proliferation and, therefore, decreased the percentage of
lactotrophs in GH3 cells, thus indicating that RE may affect the
differentiation of GH3 cells.
E2 stimulated proliferation in GH3 cells at a low
concentration (0.1 pM) and GH3 cells exhibited maximum growth with
0.01 nM E2 alone; however, greater concentrations decreased cell
proliferation. When GH3 cells were simultaneously treated with 1 nM
E2 and varying concentrations of RE (0.01–10 μM), 0.01 μM RE
exhibited no effect on E2-induced proliferation. Conversely, at
concentrations between 0.1 and 10 μM, RE inhibited the E2-induced
proliferation. Kansra et al (19) demonstrated that E2-induced
proliferation in GH3 cells may be mediated through ERα, which is
capable of binding to RE with a 7,000-fold lower affinity than E2
(9). This may be the reason that,
compared with the concentration required for stimulating
proliferation by E2, only higher concentrations of RE exhibit the
inhibitory effect on E2-induced proliferation.
RE is able to inhibit PRL gene expression, which may
have contributed to the RE-induced reduction in the proportion of
lactotrophs in GH3 cells. The present study demonstrated that E2
increased PRL production. E2, at a concentration of 1 nM resulted
in a maximal PRL response and the EC50 was ~0.01 nM.
Previous studies have demonstrated that the effects of E2 are
mediated by ERα and ERβ (19,20).
E2 induced the proliferation of GH3 cells and
increased PRL synthesis. GH3 cells showed maximum cell growth at
0.01 nM E2, whereas only half-maximal PRL production occurred at
the same concentration. This indicates that the sensitivity of the
PRL response to E2 was lower than that of the proliferation
response. When these two responses were compared via inhibition of
estrogen activity with increasing quantities of RE, the
proliferation response was not as sensitive to antiestrogen as the
PRL response was. The results indicate that a 0.01 μM concentration
of RE was not able to inhibit 1 nM E2-induced proliferation,
whereas an equal concentration of RE decreased PRL secretion to 50%
of the 1 nM E2-induced, PRL secretion. Four explanations for these
observations are: i) PRL and proliferation responses are mediated
via different ERs; ii) RE and E2 possess different affinities for
ERs; iii) ERs interact with a nuclear factor that is critical for
replication at a greater affinity than its affinity for factors
affecting PRL gene expression; iv) novel ER types exist (21). The inhibitory effect of RE on PRL
synthesis may be useful in the treatment of prolactinoma,
particularly with microprolactinoma where the predominant aim of
therapy is to decrease the high level of PRL and improve endocrine
symptoms.
In conclusion, RE exerts its antitumor effect on GH3
cells in two ways: Through the suppression of cell growth and by
inducing a reduction in PRL expression. Furthermore, its
antiestrogenic effects on cell proliferation and PRL synthesis
indicate that RE may be effective in the chemoprevention and
chemotherapy of pituitary prolactinoma.
Acknowledgements
This study was supported partly by grants from the
Creative Talent Special Foundation of Harbin Science and Technology
Bureau, Heilongjiang Province, China (grant no. 2008RFQXS090) and
the Foundation of Health Department of Heilongjiang Province (grant
no. 2009-199).
References
|
1
|
Heaney AP, Fernando M and Melmed S:
Functional role of estrogen in pituitary tumor pathogenesis. J Clin
Invest. 109:277–283. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Freeman ME, Kanyicska B, Lerant A and Nagy
G: Prolactin: structure, function, and regulation of secretion.
Physiol Rev. 80:1523–1631. 2000.PubMed/NCBI
|
|
3
|
Yin P, Kawashima K and Arita J: Direct
actions of estradiol on the anterior pituitary gland are required
for hypothalamus-dependent lactotrope proliferation and secretory
surges of luteinizing hormone but not of prolactin in female rats.
Neuroendocrinology. 75:392–401. 2002. View Article : Google Scholar
|
|
4
|
Caporali S, Imai M, Altucci L, et al:
Distinct signaling pathways mediate stimulation of cell cycle
progression and prevention of apoptotic cell death by estrogen in
rat pituitary tumor PR1 cells. Mol Biol Cell. 14:5051–5059. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Friend KE, Ang LW and Shupnik MA: Estrogen
regulates the expression of several different estrogen receptor
mRNA isoforms in rat pituitary. Proc Natl Acad Sci USA.
92:4367–4371. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jaffrain-Rea ML, Petrangeli E, Ortolani F,
et al: Cellular receptors for sex steroids in human pituitary
adenomas. J Endocrinol. 151:175–184. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakao H, Koga M, Arao M, et al:
Enzyme-immunoassay for estrogen receptors in human pituitary
adenomas. Acta Endocrinol (Copenh). 120:233–238. 1989.PubMed/NCBI
|
|
8
|
Lee SY, Ahn BT, Baik SH and Lee BL:
Tamoxifen inhibits GH3 cell growth in culture via enhancement of
apoptosis. Neurosurgery. 43:116–123. 1998.PubMed/NCBI
|
|
9
|
Bowers JL, Tyulmenkov VV, Jernigan SC and
Klinge CM: Resveratrol acts as a mixed agonist/antagonist for
estrogen receptors alpha and beta. Endocrinology. 141:3657–3667.
2000.PubMed/NCBI
|
|
10
|
Jang M, Cai L, Udeani GO, et al: Cancer
chemopreventive activity of resveratrol, a natural product derived
from grapes. Science. 275:218–220. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Boockfor FR, Hoeffler JP and Frawley LS:
Cultures of GH3 cells are functionally heterogeneous:
thyrotropin-releasing hormone, estradiol and cortisol cause
reciprocal shifts in the proportions of growth hormone and
prolactin secretors. Endocrinology. 117:418–420. 1985. View Article : Google Scholar
|
|
12
|
Iwasaka T, Umemura S, Kakimoto K, Koizumi
H and Osamura YR: Expression of prolactin mRNA in rat mammary gland
during pregnancy and lactation. J Histochem Cytochem. 48:389–396.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shaw-Bruha CM, Pennington KL and Shull JD:
Identification in the rat prolactin gene of sequences homologous to
the distal promoter of the human prolactin gene. Biochim Biophys
Acta. 1442:304–313. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang C, Hu ZQ, Chu M, et al: Resveratrol
inhibited GH3 cell growth and decreased prolactin level via
estrogen receptors. Clin Neurol Neurosurg. 114:241–248. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Joe AK, Liu H, Suzui M, Vural ME, Xiao D
and Weinstein IB: Resveratrol induces growth inhibition, S-phase
arrest, apoptosis, and changes in biomarker expression in several
human cancer cell lines. Clin Cancer Res. 8:893–903.
2002.PubMed/NCBI
|
|
16
|
Chu M, Hu ZQ, Wei LL, Zhang SZ, Hu EX and
Dong Q: Inhibitory effect of resveratrol in pituitary GH3 cells.
Chin J Neurosurg. 21:105–109. 2005.(In Chinese).
|
|
17
|
Yamashita H, Okadome T, Franzén P, ten
Dijke P, Heldin CH and Miyazono K: A rat pituitary tumor cell line
(GH3) expresses type I and type II receptors and other cell surface
binding protein(s) for transforming growth factor-beta. J Biol
Chem. 270:770–774. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kakeya T, Takeuchi S and Takahashi S:
Epidermal growth factor, insulin, and estrogen stimulate
development of prolactin-secreting cells in cultures of GH3 cells.
Cell Tissue Res. 299:237–243. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kansra S, Yamagata S, Sneade L, Foster L
and Ben-Jonathan N: Differential effects of estrogen receptor
antagonists on pituitary lactotroph proliferation and prolactin
release. Mol Cell Endocrinol. 239:27–36. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schreihofer DA, Stoler MH and Shupnik MA:
Differential expression and regulation of estrogen receptors (ERs)
in rat pituitary and cell lines: estrogen decreases ERalpha protein
and estrogen responsiveness. Endocrinology. 141:2174–2184.
2000.
|
|
21
|
Mitchner NA, Garlick C, Steinmetz RW and
Ben-Jonathan N: Differential regulation and action of estrogen
receptors alpha and beta in GH3 cells. Endocrinology.
140:2651–2658. 1999.PubMed/NCBI
|