Introduction
Hypoxia is a common characteristic of all rapidly
growing solid tumors (1). Tumor
hypoxia is caused by a number of factors, including inadequate
blood supply due to abnormal tumor microvasculature, increased
diffusion distances from the blood vessels to the tumor tissues and
a reduced capacity of the blood to carry oxygen due to anemia
(2). Intracellular hypoxic
responses are highly regulated by hypoxia-inducible factors (HIFs),
which are transcription factors with critical roles in the
development and progression of cancer (3–5).
HIFs belong to a family of basic helix-loop-helix-containing
proteins (6). The prototypic
member of this family is HIF-1, which is a heterodimer consisting
of an oxygen-regulated α subunit and a constitutively expressed β
subunit (7–9). While HIF-1β is constitutively
expressed in cells, HIF-1α protein expression is dependent on
intracellular oxygen concentration. Under normoxia, HIF-1α protein
is continuously expressed, but rapidly degraded, as it is
hydroxylated by prolyl hydroxylases (PHDs) at proline residues
within the oxygen-dependent degradation domain, which in turn
mediates its interaction with the von Hippel-Lindau (pVHL) tumor
suppressor protein, eventually leading to HIF-1α ubiquitination and
degradation through a VHL-dependent ubiquitin-proteasome pathway
(10–12). However, under hypoxic conditions,
the O2-dependent PHDs are inhibited, thus the
interaction between HIF-1α and pVHL is prevented. Consequently,
HIF-1α ubiquitination/degradation is inhibited, resulting in an
increase in HIF-1α protein expression (6,13).
The stabilized HIF-1α subunit then translocates to the nucleus
where it heterodimerizes with the HIF-1β subunit (14) and subsequently regulates the
expression of numerous important genes involved in the regulation
of various biological processes (15). HIF-1α overexpression is commonly
found in numerous types of human cancer and is often associated
with tumor progression and poor prognosis (16,17).
One of the mechanisms by which HIF-1 promotes cancer progression is
through the induction of epithelial-mesenchymal transition (EMT), a
process in which epithelial cells lose cell-cell adhesion and cell
polarity, and acquire properties of mesenchymal cells (18–21).
Through the process of EMT, carcinoma cells undergo migration and
invasion, leading to cancer progression and metastasis (22). Thus, hypoxia-induced EMT may be a
promising target for anticancer chemotherapy.
Due to the drug resistance and adverse side-effects
associated with the majority of currently used cancer
chemotherapies, natural products have gained great interest as they
have comparatively few side-effects and have been used clinically
to treat a variety of diseases, including cancer (23,24).
Traditional Chinese medicines (TCMs) are complex combinations of
various natural products, each of which contain numerous chemical
compounds. Thus, TCMs are considered to be multi-component and
multi-target agents that exert their therapeutic activities in a
holistic way. Pien Tze Huang (PZH) is a well-established TCM that
was first prescribed >450 years ago in the Ming Dynasty
(25). PZH has been used in China
and Southeast Asia for centuries as a remedy for various types of
human cancer. We recently demonstrated that PZH suppresses multiple
colorectal cancer-associated signaling pathways, leading to the
promotion of cancer cell apoptosis and the inhibition of cell
proliferation and tumor angiogenesis (26–31).
In the present study, to further elucidate the mechanism underlying
the antitumor activity of PZH, the effect of PZH on EMT under
hypoxia was investigated in a human colon carcinoma cell line.
Materials and methods
Materials and reagents
Roswell Park Memorial Institute (RPMI)-1640 medium,
fetal bovine serum (FBS), penicillin-streptomycin and
TRIzol® reagent, were purchased from Invitrogen Life
Technologies (Carlsbad, CA, USA). HIF-1α, twist basic
helix-loop-helix transcription factor (TWIST1), E-cadherin,
N-cadherin and β-actin antibodies, as well as horseradish
peroxidase (HRP)-conjugated secondary antibodies were purchased
from Cell Signaling Technology Inc. (Danvers, MA, USA).
Transwell® chambers were obtained from Corning Life
Sciences (Tewksbury, MA, USA). Matrigel™ was purchased from BD
Biosciences (San Jose, CA, USA). SuperScript® II reverse
transcriptase was obtained from Promega Corporation (Madison, WI,
USA). The Anoxomat™ Mark II hypoxic cell-culturing system was
purchased from Mart Microbiology B.V. (Drachten, The Netherlands).
Unless stated otherwise, all other chemicals were obtained from
Sigma-Aldrich (St. Louis, MO, USA).
Preparation of PZH
PZH was obtained from and authenticated by the sole
manufacturer Zhangzhou Pien Tze Huang Pharmaceutical Co., Ltd.,
(Zhangzhou, China; Chinese FDA approval no. Z35020242). PZH stock
solution was prepared prior to use by dissolving the PZH powder in
phosphate-buffered saline to a concentration of 20 mg/ml. The
working solutions of PZH were prepared by diluting the stock
solution in the culture medium.
Cell Culture
HCT-8 human colon carcinoma cells were obtained from
Nanjing KeyGen Biotech. Co. Ltd. (Nanjing, China). Cells were
cultured in RPMI-1640 containing 10% (v/v) FBS, 100 U/ml penicillin
and 100 μg/ml streptomycin in a 37°C humidified incubator with 5%
CO2. To induce cell hypoxia, cells were cultured in a
multi-gas Anoxomat Mark II incubator, with 5% CO2 and
0.1% O2 balanced with N2.
Observation of morphological changes
HCT-8 cells were seeded onto six-well plates at a
density of 5×105 cells/well in 2 ml medium. Cells were
cultured under normoxia or hypoxia (0.1% O2), with or
without treatment with various concentrations of PZH for 24 h. Cell
morphology was observed using a Leica phase-contrast microscope
(Leica Microsystems Ltd., Wetzlar, Germany). Images were captured
at a magnification of ×400.
Cell migration and invasion assays
Migration assays were performed using Transwell cell
culture chambers with 8-μm pore filters (Corning Life Sciences).
Following treatment with various concentrations of PZH under
normoxia or hypoxia for 6 h, HCT-8 cells were trypsinized and
resuspended in serum-free RPMI-1640. A total of 5×104
cells in 200 μl serum-free RPMI-1640 were plated in the upper
chambers. RPMI-1640 media containing 10% (v/v) FBS was used in the
lower chambers as a chemoattractant. Cells were allowed to migrate
for 12 h under normoxia, following which the non-migrating cells
were removed from the upper surface of the Transwell membrane in
each Transwell using a cotton swab. Membranes were then stained
with crystal violet. For quantification, the average number of
migrating cells per field was assessed by counting three random
fields under a Leica phase-contrast microscope (Leica, Microsystems
Ltd.) at a magnification of ×200. For the cell invasion assays, the
procedure was the same as that used for the migration assay;
however, the upper chambers were coated with 100 μl/well 0.2 mg/ml
Matrigel Matrix (BD Biosciences) and cell invasion was allowed to
progress for 24 h in normoxia.
Reverse transcription (RT)-PCR
analysis
Total RNA was isolated using TRIzol reagent.
Oligo(dT)-primed RNA (1 μg) was reverse-transcribed using
SuperScript II reverse transcriptase to generate cDNA according to
the manufacturer’s instructions. cDNA was used to determine the
quantity of HIF-1α, TWIST1, E-cadherin and N-cadherin mRNA using
RT-PCR analysis with Taq DNA polymerase (Fermentas, Burlington, ON,
Canada). GAPDH was used as an internal control.
Western blot analysis
HCT-8 cells were seeded into 25 cm2
flasks at a density of 1.5×106 cells/flask in 5 ml
medium. Cells were cultured under normoxia or hypoxia (0.1%
O2), with or without treatment with various
concentrations of PZH for 24 h. Cells were then were lysed with
mammalian cell lysis buffer containing protease and phosphatase
inhibitor cocktails. Total protein concentrations were determined
using a BCA protein assay. Equal quantities of total protein were
resolved using 12% SDS-PAGE and electroblotted onto polyvinylidene
fluoride membranes. Membranes were blocked using 5% skimmed milk
and probed overnight at 4°C with primary antibodies against
N-cadherin, E-cadherin, HIF-1α, TWIST1 and β-actin diluted 1:1,000.
Membranes were then probed with the appropriate HRP-conjugated
secondary antibodies and the immunoreactive bands were visualized
using an enhanced chemiluminescence method (Bio-Rad, Hercules, CA,
USA).
Statistical Analysis
All data are presented as the mean ± standard
deviation of three independent experiments and were analyzed using
SPSS version 18.0 for Windows (SPSS, Inc., Chicago, IL, USA).
Statistical data analyses were performed using the Student’s t-test
and analysis of variance. P<0.05 was considered to indicate a
statistically significant difference.
Results and Discussion
PZH inhibits hypoxia-induced EMT in HCT-8
human colon carcinoma cells
Hypoxia is a common microenvironment for
pathophysiological progresses, including tumor progression and
metastasis (1,2). Metastasis is a complex process that
involves the spread of malignant tumor cells from the primary tumor
site to a secondary organ. This distant organ colonization is
primarily initiated through EMT. Epithelial and mesenchymal cells
are different in phenotype and function. Epithelial cells have an
apical-basal polarity, express high levels of epithelial markers,
including E-cadherin, and form epithelial adherent junctions. By
contrast, mesenchymal cells lack cell polarity, overexpress
mesenchymal markers, including N-cadherin and vimentin, and exhibit
a spindle-like morphology (18–22).
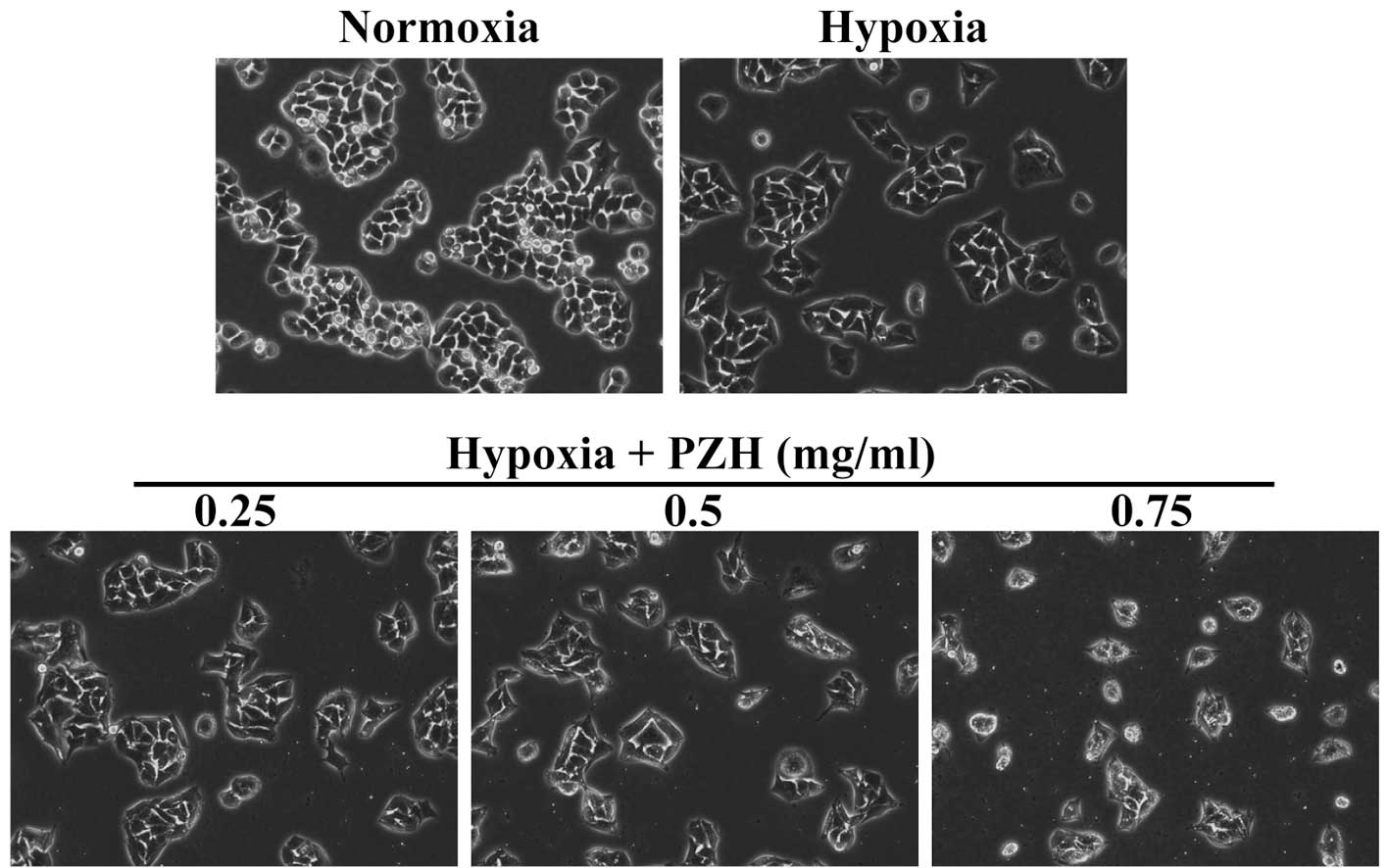
In the present study, in order to enable the effect of PZH on
cancer EMT to be assessed, the morphological changes in HCT-8 cells
under hypoxia were investigated. As shown in Fig. 1, under hypoxia HCT-8 cells exhibit
greater isolation than under normoxia and a more spindle-shaped
fibroblastoid-like morphology, which are typical characteristics
associated with EMT. However, these hypoxia-induced EMT-associated
morphological changes were observed to be inhibited by PZH
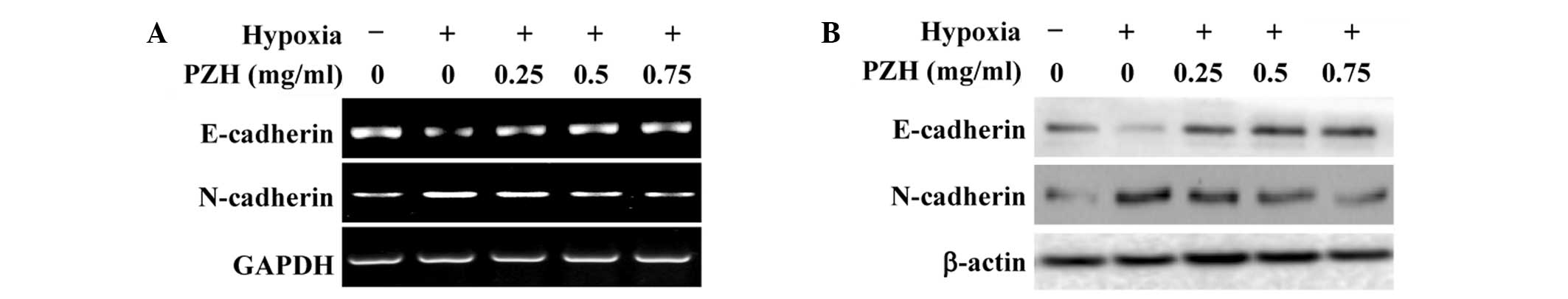
treatment. To further verify these results, the expression of
several critical genes that are involved in the regulation of EMT
was investigated. As shown in Fig.
2, hypoxia was found to significantly reduce the expression of
epithelial cell-specific E-cadherin, and increase that of the
mesenchymal marker N-cadherin. However, the hypoxia-induced
alterations in the expression of EMT-regulatory genes were
attenuated by PZH treatment in the HCT-8 cells.
PZH inhibits the hypoxia-enhanced
migration and invasion of HCT-8 cells
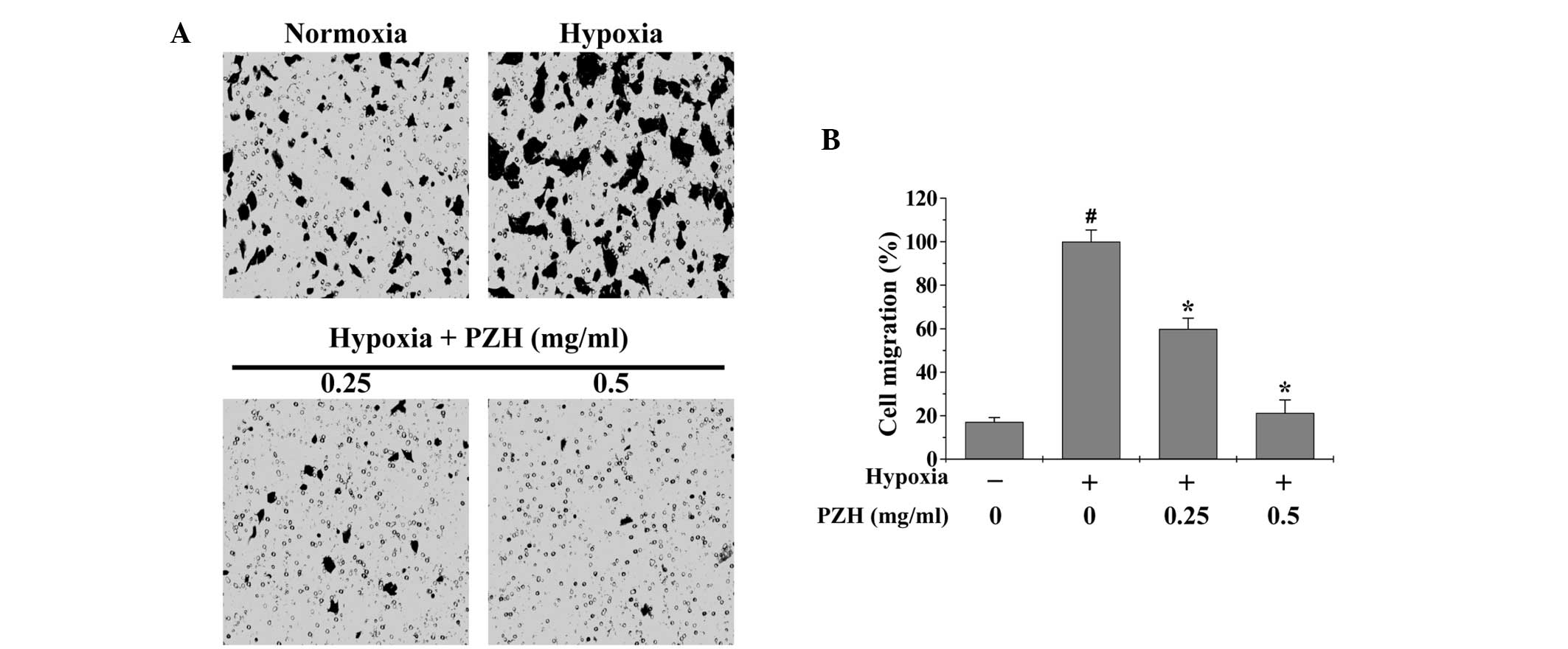
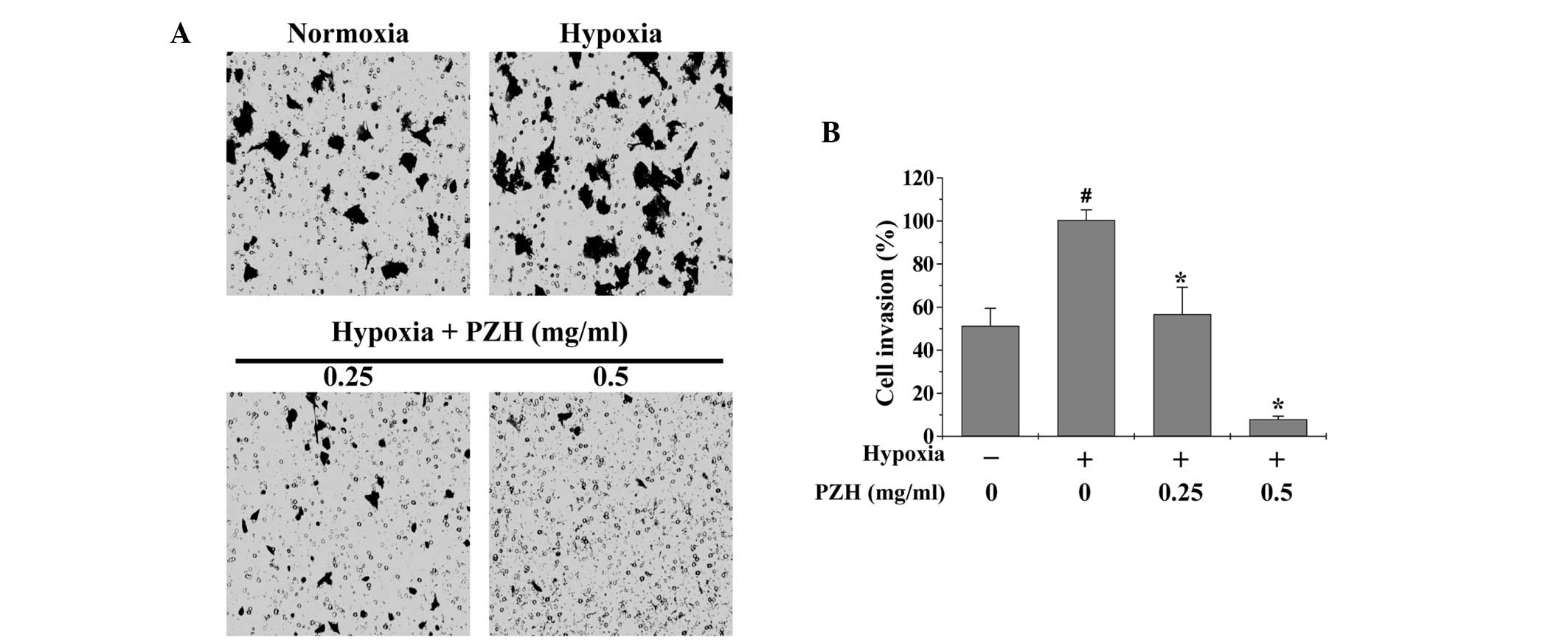
EMT promotes cancer cell metastasis; therefore,
Transwell assays were performed in order to analyze the effect of
PZH on the migration and invasion of HCT-8 cells under hypoxia. As
shown in Figs. 3 and 4, hypoxia was observed to increase HCT-8
cell migration and invasion by 5.8- and 1.9-fold, respectively,
compared with that of the cells cultured under normoxia (both
P<0.05). However, treatment with 0.25–0.5 mg/ml PZH was observed
to significantly decrease the cell migration and invasion rates by
40.1–78.7% and 43.3–92.1% (P<0.05), respectively, suggesting
that PZH concentration-dependently inhibits the hypoxia-induced
metastasis of colon cancer cells.
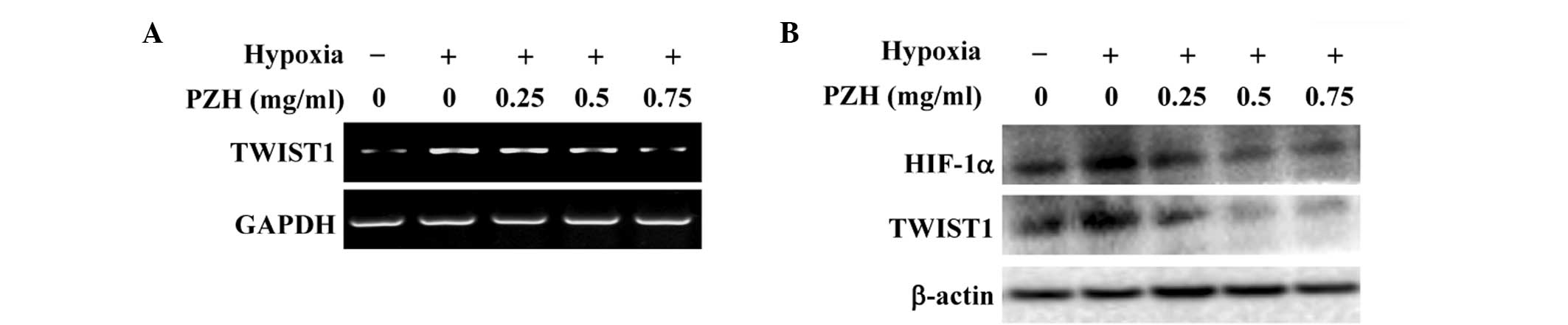
PZH inhibits hypoxia-induced activation
of the HIF-1α pathway in HCT-8 cells
The intracellular response to hypoxia is primarily
controlled by HIF-1, which consists of an oxygen-regulated α
subunit and a constitutively expressed β subunit (3–5). It
has been shown that the hypoxia-induced stabilization of HIF-1α is
strongly associated with EMT (18–22).
The transcription factor TWIST is one of the essential factors
mediating EMT and cancer metastasis and it is highly regulated by
HIF-1. Activation of TWIST represses the expression of epithelial
markers, but upregulates the expression of mesenchymal markers
(9,10). To further investigate the mechanism
underlying the inhibitory activity of PZH against EMT, the effect
of PZH on the activation of the HIF-1 pathway was investigated. As
shown in Fig. 5, hypoxia was
observed to significantly increase the mRNA and protein expression
levels of HIF-1α and TWIST1; these increases were inhibited by PZH
treatment in a concentration-dependent manner.
In conclusion, to the best of our knowledge, the
present study has provided the first evidence that PZH is capable
of inhibiting hypoxia-induced EMT in cancer cells through
suppressing the activation of the HIF-1 pathway. This may be one of
the molecular mechanisms underlying the antitumor activity of
PZH.
Acknowledgements
This study was sponsored by the National Natural
Science Foundations of China (nos. 81202790 and 81373819), and the
China Postdoctoral Science Foundation (no. 2013T60636).
References
|
1
|
Vaupel P, Höckel M and Mayer A: Detection
and characterization of tumor hypoxia using pO2
histography. Antioxid Redox Signal. 9:1221–1235. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Höckel M and Vaupel P: Tumor hypoxia:
definitions and current clinical, biologic, and molecular aspects.
J Natl Cancer Inst. 93:266–276. 2001.PubMed/NCBI
|
|
3
|
Guillemin K and Krasnow MA: The hypoxic
response: huffing and HIFing. Cell. 89:9–12. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar
|
|
5
|
Poon E, Harris AL and Ashcroft M:
Targeting the hypoxia-inducible factor (HIF) pathway in cancer.
Expert Rev Mol Med. 11:e262009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl
Acad Sci USA. 92:5510–5514. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schofield CJ and Ratcliffe PJ: Oxygen
sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 5:343–354.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Semenza GL: Regulation of mammalian
O2 homeostasis by hypoxia-inducible factor 1. Annu Rev
Cell Dev Biol. 15:551–578. 1999.
|
|
9
|
Jiang BH, Rue E, Wang GL, Roe R and
Semenza GL: Dimerization, DNA binding, and transactivation
properties of hypoxia-inducible factor 1. J Biol Chem.
271:17771–17778. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar
|
|
11
|
Ivan M, Kondo K, Yang H, Kim W, Valiando
J, Ohh M, Salic A, Asara JM, Lane WS and Kaelin WG Jr: HIFalpha
targeted for VHL-mediated destruction by proline hydroxylation:
implications for O2 sensing. Science. 292:464–468. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maxwell PH, Wiesener MS, Chang GW,
Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER and
Ratcliffe PJ: The tumour suppressor protein VHL targets
hypoxia-inducible factors for oxygen-dependent proteolysis. Nature.
399:271–275. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Semenza GL: HIF-1, O(2), and the 3 PHDs:
how animal cells signal hypoxia to the nucleus. Cell. 107:1–3.
2001.PubMed/NCBI
|
|
14
|
Kallio PJ, Okamoto K, O’Brien S, Carrero
P, Makino Y, Tanaka H and Poellinger L: Signal transduction in
hypoxic cells: inducible nuclear translocation and recruitment of
the CBP/p300 coactivator by the hypoxia-inducible factor-1alpha.
EMBO J. 17:6573–6586. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bos R, Zhong H, Hanrahan CF, Mommers EC,
Semenza GL, Pinedo HM, Abeloff MD, Simons JW, van Diest PJ and van
der Wall E: Levels of hypoxia-inducible factor-1 alpha during
breast carcinogenesis. J Natl Cancer Inst. 93:309–314. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhong H, De Marzo AM, Laughner E, Lim M,
Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL and Simons
JW: Overexpression of hypoxia-inducible factor 1alpha in common
human cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
17
|
Cheng ZX, Sun B, Wang SJ, Gao Y, Zhang YM,
Zhou HX, Jia G, Wang YW, Kong R, Pan SH, Xue DB, Jiang HC and Bai
XW: Nuclear Factor-κB-dependent epithelial to mesenchymal
transition induced by HIF-1α activation in pancreatic cancer cells
under hypoxic conditions. PLoS One. 6:e237522011.
|
|
18
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Turley EA, Veiseh M, Radisky DC and
Bissell MJ: Mechanisms of disease: epithelial-mesenchymal
transition - does cellular plasticity fuel neoplastic progression?
Nat Clin Pract Oncol. 5:280–290. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bates RC and Mercurio A: The
epithelial-mesenchymal transition (EMT) and colorectal cancer
progression. Cancer Biol Ther. 4:365–370. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gordaliza M: Natural products as leads to
anticancer drugs. Clin Transl Oncol. 9:767–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ji HF, Li XJ and Zhang HY: Natural
products and drug discovery. Can thousands of years of ancient
medical knowledge lead us to new and powerful drug combinations in
the fight against cancer and dementia? EMBO Rep. 10:194–200.
2009.PubMed/NCBI
|
|
25
|
Chinese Pharmacopoeia Commission.
Pharmacopoeia of the People’s Republic of China. 1. Chinese Medical
Science and Technology Press; Beijing: pp. 573–575. 2010
|
|
26
|
Lin JM, Wei LH, Chen YQ, Liu XX, Hong ZF,
Sferra TJ and Peng J: Pien Tze Huang induced apoptosis in human
colon cancer HT-29 cells is associated with regulation of the Bcl-2
family and activation of caspase 3. Chin J Integr Med. 17:685–690.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhuang Q, Hong F, Shen A, Zheng L, Zeng J,
Lin W, Chen Y, Sferra T, Hong Z and Peng J: Pien Tze Huang inhibits
tumor cell proliferation and promotes apoptosis via suppressing the
STAT3 pathway in a colorectal cancer mouse model. Int J Oncol.
40:1569–1574. 2012.PubMed/NCBI
|
|
28
|
Shen AL, Hong F, Liu LY, Lin JM, Zhuang
QC, Hong ZF and Peng J: Effects of Pien Tze Huang on angiogenesis
in vivo and in vitro. Chin J Integr Med. 18:431–436. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shen A, Hong F, Liu L, Lin J, Wei L, Cai
Q, Hong Z and Peng J: Pien Tze Huang inhibits the proliferation of
human colon carcinoma cells by arresting G1/S cell cycle
progression. Oncol Lett. 4:767–770. 2012.PubMed/NCBI
|
|
30
|
Shen A, Chen Y, Hong F, Lin J, Wei L, Hong
Z, Sferra TJ and Peng J: Pien Tze Huang suppresses IL-6-inducible
STAT3 activation in human colon carcinoma cells through induction
of SOCS3. Oncol Rep. 28:2125–2130. 2012.PubMed/NCBI
|
|
31
|
Shen A, Lin J, Chen Y, Lin W, Liu L, Hong
ZF, Sferra TJ and Peng J: Pien Tze Huang inhibits tumor
angiogenesis in a mouse model of colorectal cancer via suppression
of multiple cellular pathways. Oncol Rep. 30:1701–1706.
2013.PubMed/NCBI
|