Introduction
Cilostazol, a selective inhibitor of
phosphodiesterase III, is able to inhibit PDE-3 activity with the
increase in intracellular cAMP concentration which regulates cell
function, inhibits platelet aggregation, prevents thrombosis,
relaxes vascular smooth muscle, inhibits cell proliferation and
regulates blood lipid level. Apoptosis-inducing factor (AIF) is
associated with nerve cell death. The excessive activation of poly
ADP-ribose polymerase (PARP) sends the nuclear signal to the
mitochondrion, triggering AIF release from the mitochondrion to the
cell nucleus with chromatin condensation and DNA fragments.
Cilostazol has protective effects on cerebral ischemia-reperfusion
injury and the protective effects are associated with PARP
inhibition (1).
The main apoptosis-mediated signaling pathways
include the caspase-8-mediated death receptor pathway, the
caspase-9-mediated mitochondrial pathway and the
caspase-12-mediated endoplasmic reticulum stress pathway. However,
non-caspase-mediated apoptotic pathways also exist, including the
poly ADP-ribose polymerase (PARP)/apoptosis-inducing factor
(AIF)-mediated apoptotic pathway (2–4). The
PARP/AIF-mediated apoptotic pathway is an important pathway that is
present in numerous eukaryotic organisms. In this study, the
effects of cilostazol on the PARP/AIF-mediated apoptotic pathway in
a rat model of cerebral ischemia-reperfusion injury were
investigated, providing an experimental basis for the prevention
and treatment of cerebrovascular disease.
Materials and methods
Ethical approval
All study methods were approved by the Ethics
Committee of the First Affiliated Hospital, Liaoning Medical
University (Jinzhou, China).
Animal and reagents
Sprague Dawley rats (n=135), weighing between 280
and 320 g, were purchased from the Experimental Animal Center,
Liaoning Medical University. Cilostazol was purchased from Otsuka
Pharmaceutical Company (Suzhou, China). Rabbit anti-mouse AIF
polyclonal antibody was purchased from Bioss Company (Beijing,
China). Antibodies against Histone H1 was purchased from Santa Cruz
(Dallas, TX, USA) and antibodies against PARP was purchased from
Alexis (New York, NY, USA). Mouse anti-apoptotic reagent was
purchased from Alexis Biochemicals (Ann Arbor, MI, USA). The
immunohistochemical staining kit was purchased from Wuhan Boster
Biological Engineering Co., Ltd (Wuhan, China). The AIF and β-actin
primers were synthesized by Shanghai Generay Biotechnology Co., Ltd
(Shanghai, China), and the total RNA extraction kit was purchased
from Shanghai Generay Biotechnology Co., Ltd.
Grouping and model preparation
The rats were randomly divided into three groups:
Sham-surgery, ischemia-reperfusion and cilostazol (n=45/group). Rat
models of right middle cerebral artery occlusion were prepared
using the thread occlusion method (5). The blood flow was restored 2 h after
ischemia. Rats that exhibited Horner’s sign, adduction and flexion
of the left forelimb, and that had a tendency to fall to the left
or crawl in a counterclockwise direction. For the sham-surgery
group, the thread was inserted into the right middle cerebral
artery and then immediately removed. For the cilostazol group, 30
mg/kg intragastric cilostazol was administered 6 and 2 h before
brain ischemia, respectively. Samples were collected at different
time-points (6, 24 and 72 h) after reperfusion, and each group was
further divided into three subgroups (n=15/subgroup).
Brain samples
At the different time-points after reperfusion (6,
24 and 72 h), five rats were taken from each subgroup. The brain
was removed from each rat following decapitation and divided into
four equal parts (A, B, C and D) from the frontal region to the
occipital region. Part C was dehydrated with alcohol, embedded in
paraffin and sectioned at a thickness of 5 μm for
immunohistochemical staining. A further 10 rats from each subgroup
were sacrificed at different time-points (6, 24 and 72 h) after
reperfusion, and the hippocampus of each rat was placed in liquid
nitrogen. The hippocampi of five of the rats were used for western
blot analysis, whilst the hippocampi of the remaining five rats
were used for reverse transcription-polymerase chain reaction
(RT-PCR).
Analysis of nerve cell apoptosis using
the terminal deoxynucleotidyl-transferase-mediated dUTP nick end
labeling (TUNEL) method
Nerve cell apoptosis was detected using a TUNEL kit
provided by Sigma (St. Louis, MO, USA). One CA1 hippocampus slice
was randomly selected from each rat, and five fields were then
randomly selected from the CA1 hippocampus slice (Olympus,
Hatagaya, Japan). The number of TUNEL-positive cells was counted in
the five fields. Since there were five rats in each subgroup, the
average number of TUNEL-positive cells in 25 fields was
calculated.
Western blot analysis
Cell lysis solution was added to the samples and
nuclei were extracted from the cells using the differential
centrifugation method. Nuclear lysates were added prior to
centrifugation in order to extract nucleoprotein. The quantity of
protein extracted was determined using the Bradford assay. Western
blotting was performed according to a previously described method
(6). The electrophoresis pattern
was analyzed using a gel imaging analysis system (GDS-8000 type;
UVP, Upland, CA, USA). The absorbance of AIF and PARP was detected
and histone H1 was used as an internal control. AIF and PARP
protein expression was calculated relative to the control, histone
H1.
Analysis of AIF mRNA expression using
RT-PCR
Hippocampal tissue was stored at −80°C for future
use. Total RNA was extracted using the Trizol method. Total RNA
underwent reverse transcription for the preparation of cDNA
templates. PCR amplification was performed using 2 μl cDNA, and
β-actin was used as an internal control. AIF primer sequences were
as follows: Forward, 5′-CCCCGATGTTGGCTATGA-3′ and reverse,
5′-TCCTGACTGCTCTGTGGC-3′, with an amplified fragment of 115 base
pairs (bp). β-actin primer sequences were as follows: Forward,
5′-CACCAACTGGGACGACAT-3′ and reverse, 5′-ACAGCCTGGATAGCAACG-3′,
with an amplified fragment of 189 bp. The PCR conditions were as
follows: Pre-denaturing at 94°C for 5 min, denaturing at 94°C for
30 sec, reannealing at 54°C for 30 sec and elongation at 72°C for 5
min. PCR products were then analyzed using 30 g/l agarose gel
electrophoresis. Image scanning was performed using Gene Tools
software (Syngene, Frederick, MD, USA). The level of AIF mRNA was
calculated according to the gray-level value.
Statistical analysis
Statistical analysis was performed using SPSS 13.0
software (SPPS, Inc., Chicago, IL, USA). Data are expressed as the
mean ± standard deviation. A Student’s t-test was used for the
comparison between two samples and repeated measures analysis of
variance was used in the comparison of multiple samples. P<0.05
was considered to indicate a statistically significant
difference.
Results
Effect of cilostazol pretreatment on
nerve cell apoptosis
In the sham-surgery group, only a few apoptotic
cells were observed at the different time-points and no statistical
difference was found in the number of apoptotic cells among the
different time-points (P>0.05). In the ischemia-reperfusion
group, the number of apoptotic cells was markedly increased 24 h
after reperfusion and was decreased 72 h after reperfusion compared
with the 6-h time-point. However, compared with the sham-surgery
group, the apoptosis rate in the ischemia-reperfusion group was
significantly increased at all time-points (P<0.05). Compared
with the ischemia-reperfusion group, the apoptosis rate was
significantly decreased in the cilostazol group at all time-points
(P<0.05). For the ischemia-reperfusion and cilostazol groups,
the apoptosis rate was highest after 24 h (P<0.05) (Table I).
 | Table ITUNEL-positive cells in the CA1 region
of the hippocampus at different time-points in each group
(n=5). |
Table I
TUNEL-positive cells in the CA1 region
of the hippocampus at different time-points in each group
(n=5).
| TUNEL-positive cells
(%) | | |
|---|
|
| | |
|---|
| Groups | 6 h | 24 h | 72 h | F-value | P-value |
|---|
| Sham-surgery | 0.949±0.162 | 1.031±0.153 | 1.146±0.494 | 6.624 | 0.076 |
|
Ischemia-reperfusion | 23.845±3.633b | 48.531±7.810b,c | 15.029±3.718b | 3.626 | 0.044 |
| Cilostazol | 12.763±3.470a | 25.678±9.206a,c | 4.341±2.929a | 12.413 | <0.001 |
| F-value | 7.819 | 13.448 | 3.666 | - | - |
| P-value | 0.003 | <0.001 | 0.043 | - | - |
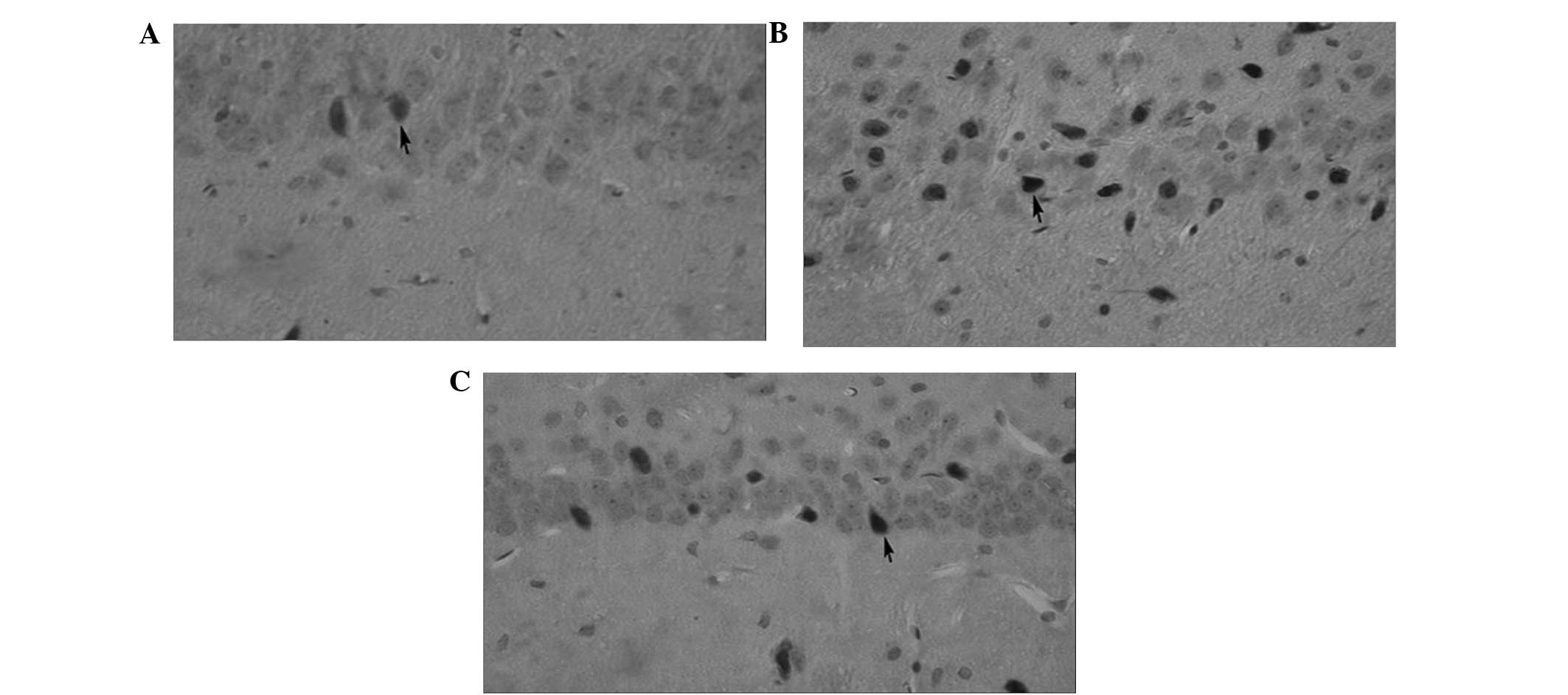
At the 24-h time-point, a few TUNEL-positive cells
were observed in the sham-surgery group. The number of
TUNEL-positive cells observed in the ischemia-reperfusion group was
markedly increased in comparison with the sham-surgery group and
the number of TUNEL-positive cells observed in the cilostazol group
was markedly decreased compared with the ischemia-reperfusion group
(Fig. 1).
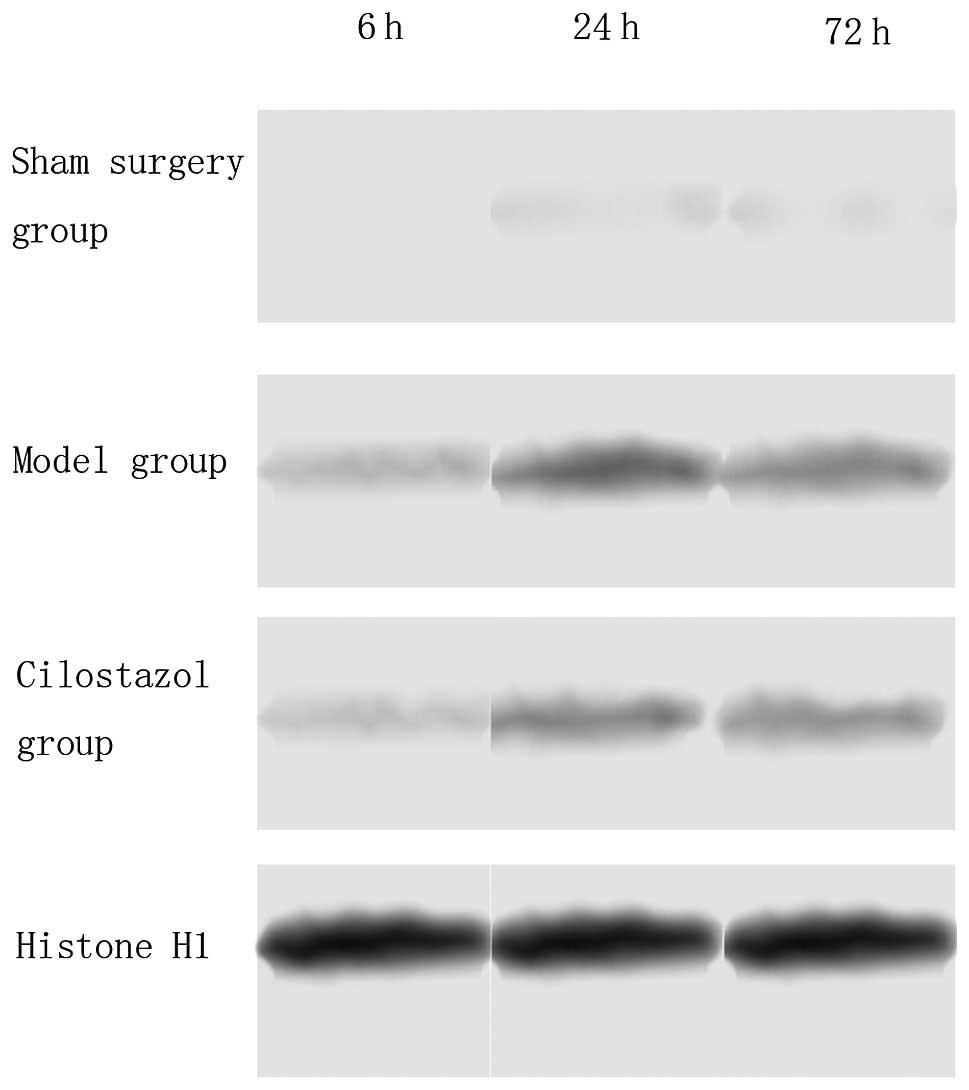
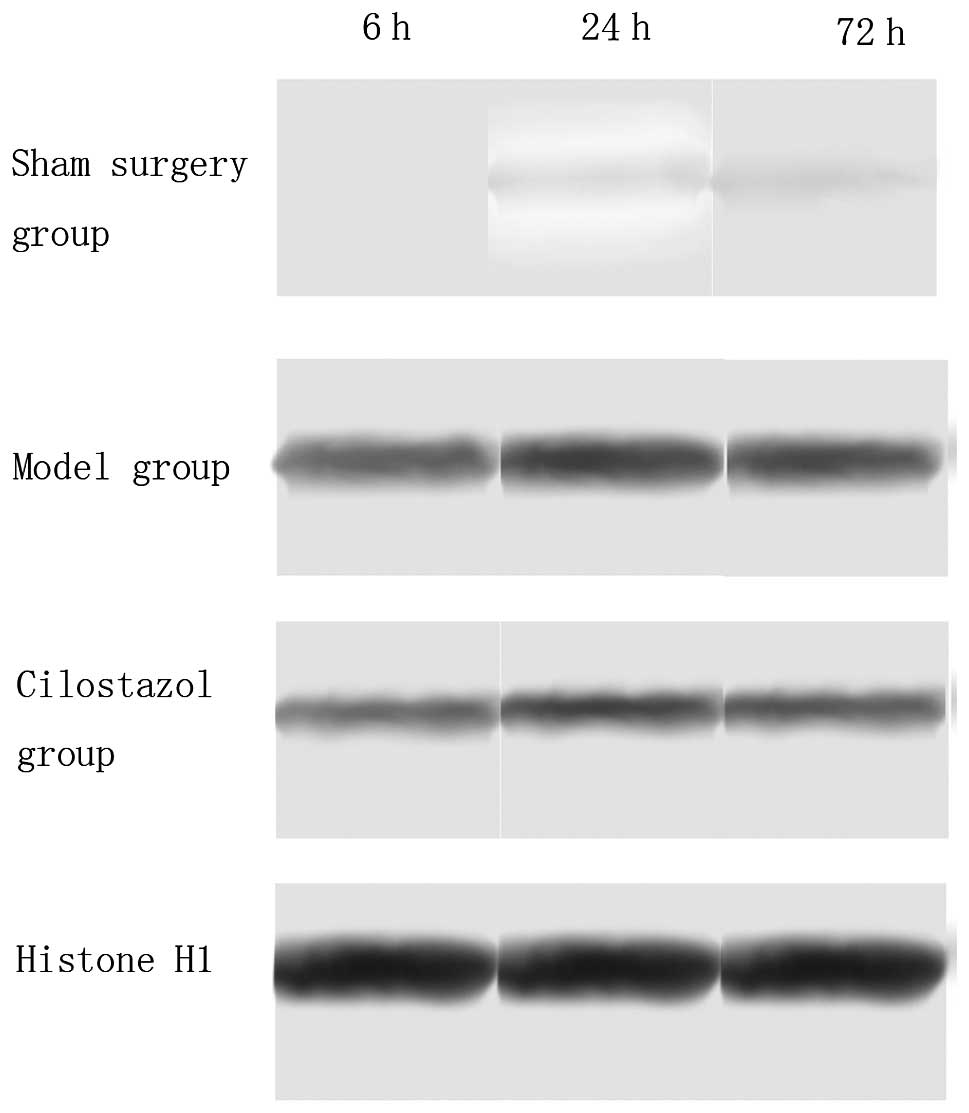
Effects of cilostazol pretreatment on AIF
nuclear translocation and PARP
The results of the western blot analysis indicated
that, in the sham-surgery group, there was no PARP and AIF nuclear
translocation; however, in the ischemia-reperfusion group, PARP and
AIF nuclear translocation occurred 6 h after reperfusion, was
markedly increased 24 h after reperfusion and then markedly
decreased after 72 h. Compared with the ischemia-reperfusion group,
PARP and AIF nuclear translocation was significantly decreased in
the cilostazol group at all time-points (P<0.05). In the
ischemia-reperfusion and cilostazol groups, PARP and AIF nuclear
translocation at 24 h was the highest among the different
time-points (P<0.05) (Tables
II and III, Figs. 2 and 3).
 | Table IIAIF protein expression in the CA1
region of the hippocampus at different time-points in each group
(n=5). |
Table II
AIF protein expression in the CA1
region of the hippocampus at different time-points in each group
(n=5).
| AIF protein
expression (%) | | |
|---|
|
| | |
|---|
| Groups | 6 h | 24 h | 72 h | F-value | P-value |
|---|
|
Ischemia-reperfusion | 0.721±0.005 | 1.240±0.041b | 0.621±0.015 | 14.355 | <0.001 |
| Cilostazol | 0.715±0.008a | 0.869±0.016a,b | 0.411±0.007a | 28.561 | <0.001 |
| T-value | 3.785 | 5.148 | 3.640 | - | - |
| P-value | 0.007 | 0.010 | 0.008 | - | - |
 | Table IIIPARP protein expression in the CA1
region of the hippocampus at different time-points in each group
(n=5). |
Table III
PARP protein expression in the CA1
region of the hippocampus at different time-points in each group
(n=5).
| PARP protein
expression (%) | | |
|---|
|
| | |
|---|
| Groups | 6 h | 24 h | 72 h | F-value | P-value |
|---|
|
Ischemia-reperfusion | 53.001±6.477 | 79.251±7.073b | 33.001±8.521 | 78.451 | <0.001 |
| Cilostazol | 49.243±5.025a | 61.743±15.54a,b | 21.743±4.696a | 47.263 | <0.001 |
| T-value | 10.503 | 2.487 | 3.841 | - | - |
| P-value | <0.001 | 0.042 | 0.006 | - | - |
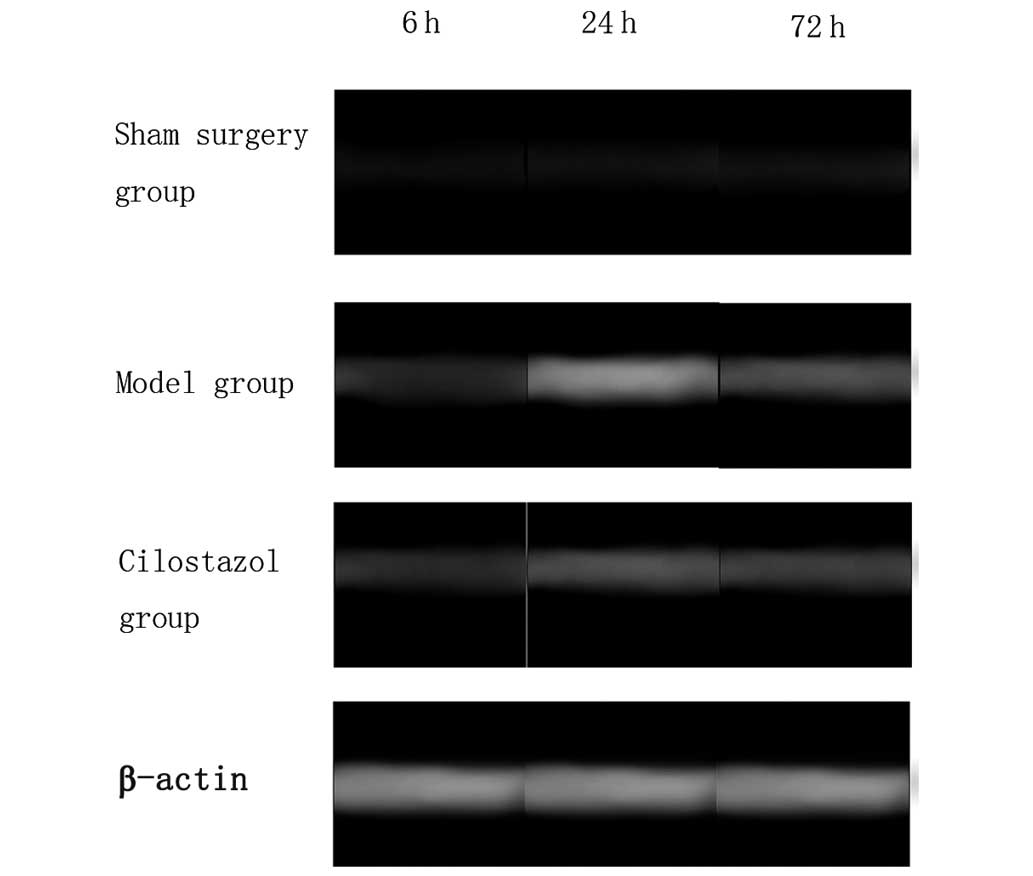
Effect of cilostazol pretreatment on AIF
mRNA expression levels
In the sham-surgery group, there was little
expression of AIF mRNA at all time-points. In the
ischemia-reperfusion group, AIF mRNA expression was observed 6 h
after reperfusion, and was markedly increased 24 h after
reperfusion, prior to being reduced again 72 h after reperfusion.
However, compared with the sham-surgery group, a significant
difference was observed in AIF mRNA expression in the
ischemia-reperfusion group at all time-points (P<0.05). Compared
with the ischemia-reperfusion group, AIF mRNA expression was
significantly decreased in the cilostazol group at all time-points
(P<0.05). In the ischemia-reperfusion and cilostazol groups, AIF
mRNA expression was highest 24 h after reperfusion (P<0.05)
(Table IV and Fig. 4).
 | Table IVmRNA expression of AIF in the CA1
region of the hippocampus at different time-points in each group
(n=5). |
Table IV
mRNA expression of AIF in the CA1
region of the hippocampus at different time-points in each group
(n=5).
| AIF mRNA expression
(%) | | |
|---|
|
| | |
|---|
| Groups | 6 h | 24 h | 72 h | F-value | P-value |
|---|
| Sham-surgery | 0.175±0.031 | 0.220±0.106 | 0.155±0.034 | 16.928 | 0.052 |
|
Ischemia-reperfusion | 0.955±0.084b | 2.056±0.367b,c | 0.966±0.093b | 9.345 | 0.001 |
| Cilostazol | 0.575±0.330a,b | 1.378±0.171a,b,c | 0.596±0.389a,b | 5.809 | 0.010 |
| F-value | 5.342 | 5.470 | 4.369 | - | - |
| P-value | 0.013 | 0.012 | 0.026 | - | - |
Discussion
Aspirin is an effective and economical antiplatelet
drug that is widely used in clinical practice. However, certain
patients have aspirin resistance or are not able to tolerate the
drug. Cilostazol is a novel antiplatelet drug that exerts similar
therapeutic effects, but has a reduced risk of bleeding and
cerebral infarction compared with aspirin (7). Cilostazol is a selective inhibitor of
the cyclic nucleotide PDE-3 and increases cyclic adenosine
monophosphate (cAMP) levels in platelets and vascular endothelial
cells by inhibiting PDE-3 activity and cAMP degradation. This
inhibits platelet aggregation, relaxes the vasculature and
regulates blood lipids, preventing atherosclerosis and vascular
occlusion (8). Cilostazol has
generated significant interest in the treatment of cerebrovascular
disease. It has been proposed that the protective effect of
cilostazol on cerebral ischemia-reperfusion injury is associated
with the inhibition of apoptosis (1).
The present study demonstrated that, in the
ischemia-reperfusion group, the number of apoptotic cells was
increased 24 h after reperfusion compared with the 6-h time-point.
A significant difference was observed in the apoptosis rate in the
ischemia-reperfusion group at all time-points compared with the
sham-surgery group (P<0.05). However, the apoptosis rate was
significantly decreased in the cilostazol group at all time-points
compared with the ischemia-reperfusion group (P<0.05). In the
ischemia-reperfusion and cilostazol groups, the apoptosis rate at
24 h was the highest among the different time-points (P<0.05).
The results demonstrated that cilostazol was able to inhibit
apoptosis caused by cerebral ischemia-reperfusion injury; however,
the mechanism underlying the action of cilostazol was not clear. In
order to investigate this further, the PARP/AIF apoptotic pathway
was studied. PARP, a type of ribozyme, identifies and repairs DNA
single-strand breaks in order to maintain genome integrity, and has
beneficial and harmful effects on cell injury and repair. In mild
ischemia, nerve cells survive as a result of PARP repairing damaged
DNA; however, in severe ischemia, there is significant DNA damage,
which causes the excessive activation of PARP, leading to the
consumption of nicotinamide adenine dinucleotide (NAD), the
formation of NAD+ and the consumption of ATP (9). Energy depletion leads to cell damage,
apoptosis or necrosis (10). In
this study, the effects of cilostazol on PARP expression were
observed 6, 24 and 72 h after cerebral ischemia-reperfusion. The
results indicated that cerebral ischemia-reperfusion injury
activates PARP expression, since PARP expression was markedly
increased 24 h after cerebral ischemia-reperfusion injury, and that
cilostazol is capable of decreasing PARP expression. The results
suggest that the anti-apoptotic effect of cilostazol is associated
with the downregulation of PARP expression in cerebral
ischemia-reperfusion injury.
PARP is capable of inducing changes in mitochondrial
membrane permeability, leading to the release of AIF. The excessive
activation of PARP located in the mitochondria directly causes
mitochondrial injury and the release of pro-apoptotic proteins,
leading to AIF nuclear translocation (1). Apoptotic signals cause the excessive
activation of PARP which results in AIF release from the
mitochondrion to the cell nucleus (11). The excessive activation of PARP
consumes NAD+, leading to energy depletion (12). In the present study, the effect of
cilostazol on AIF protein expression in cerebral
ischemia-reperfusion was observed. The results showed that AIF
expression in the ischemia-reperfusion group was increased 24 h
after reperfusion compared with that at the 6-h time-point, prior
to decreasing again 72 h after reperfusion. This may be due to the
fact that AIF in the mitochondria has a protective role in early
reperfusion; with the prolongation of reperfusion, AIF was released
from the mitochondria and mitochondrial integrity was damaged. This
result suggests that, in order to reduce AIF nuclear translocation,
drug intervention should be adopted within 24 h after reperfusion.
Compared with the ischemia-reperfusion group, AIF expression was
significantly decreased in the cilostazol group, demonstrating that
cilostazol was able to inhibit AIF nuclear translocation. The
results also indicated that AIF mRNA expression was reduced in the
sham-surgery group compared with the ischemia-reperfusion and
cilostazol groups. In the ischemia-reperfusion group, AIF mRNA
expression was observed 6 h after reperfusion, reaching a peak 24 h
after reperfusion and then decreasing again after 72 h. This
suggests that apoptotic signals stimulate AIF transcription. The
dynamic changes in AIF mRNA expression were consistent with those
in nerve cell apoptosis, suggesting that AIF gene transcription is
involved in apoptosis. Compared with the ischemia-reperfusion
group, AIF mRNA expression was significantly decreased in the
cilostazol group at all time-points, suggesting that cilostazol is
capable of inhibiting AIF expression at the transcriptional level,
and then inhibiting apoptosis to exert protective effects on the
brain.
In conclusion, in a rat model of cerebral
ischemia-reperfusion, apoptotic cells, increased expression of
PARP, NAD depletion and AIF nuclear translocation were observed.
Cilostazol was found to inhibit apoptosis, the excessive activity
of PARP, NAD depletion and AIF nuclear translocation, suggesting
that the anti-apoptotic effects of cilostazol may be associated
with the inhibition of excessive PARP activation and AIF mRNA
expression, as well as AIF nuclear translocation.
References
|
1
|
Lee JH, Kim KY, Lee YK, Park SY, Kim CD,
Lee WS, et al: Cilostazol prevents focal cerebral ischemic injury
by enhancing casein Kinase 2 phosphorylation and suppression of
phosphatase and tensin homolog deleted from chromosome 10
phosphorylation in rats. J Pharmacol Exp Ther. 308:896–903.
2004.
|
|
2
|
Stegmüller J, Huynh MA, Yuan Z, Konishi Y
and Bonni A: TGFbeta-Smad2 signaling regulates the Cdhl-APC/SnoN
pathway of axonal morphogenesis. J Neurosci. 28:1961–1969.
2008.PubMed/NCBI
|
|
3
|
Liu W, Wu G, Li W, Lobur D and Wan Y:
Cdhl-anaphase-promoting complex targets Skp2 for destruction in
transforming growth factor beta-induced growth inhibition. Mol Cell
Biol. 27:2967–2979. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stegmüller J, Konishi Y, Huynh MA, Yuan Z,
Dibacco S and Bonni A: Cell-intrinsic regulation of axonal
morphogenesis by the Cdhl-APC target SnoN. Neuron. 50:389–400.
2006.PubMed/NCBI
|
|
5
|
Lin ZZ and Pi RB: Advances on rodent
cerebral ischemia-reperfusion models. Chinese Journal of Nervous
and Mental Diseases. 33:574–576. 2007.(In Chinese).
|
|
6
|
Xiao CY, Chen M, Zsengellér Z, Li H, Kiss
L, Kollai M, et al: Poly (ADP-Ribose) polymerase promotes cardiac
remodeling, contractile failure, and translocation of
apoptosis-inducing factor in a murine experimental model of aortic
banding and heart failure. J Pharmacol Exp Ther. 312:891–898. 2005.
View Article : Google Scholar
|
|
7
|
Heeres JT and Hergenrother PJ:
Poly(ADP-ribose) makes a date with death. Curr Opin Chem Biol.
11:644–653. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schrör K: The pharmacology of cilostazol.
Diabetes Obes Metab. 4(Suppl 2): S14–S19. 2002.
|
|
9
|
Li GY and Osborne NN: Oxidative-induced
apoptosis to an immortalized ganglion cell line is caspase
independent but involves the activation of poly (ADP-ribose)
polymerase and apopotosis-inducing factor. Brain Res. 1188:35–43.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shah GM, Shah RG and Poirier GG: Different
cleavage pattern for poly (ADP-ribose) polymerase during necrosis
and apoptosis in HL-60 cells. Biochem Biophys Res Commun.
229:838–844. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hong SJ, Dawson TM and Dawson VL: Nuclear
and mitochondrial conversations in cell death: PARP-l and AIF
signaling. Trends Pharmacol Sci. 25:259–264. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moroni F: Poly(ADP-ribose) polymerase 1
(PARP-l) and postischemic brain damage. Curr Opin pharmacol.
8:96–103. 2008. View Article : Google Scholar : PubMed/NCBI
|