Introduction
Endothelial dysfunction is closely associated with
the occurrence and development of atherosclerotic disease and
numerous studies have confirmed that coronary artery disease (CAD)
is often accompanied by endothelial dysfunction (1,2). A
number of studies have demonstrated that hyperhomocysteinemia, one
of the risk factors of CAD, promotes the occurrence and development
of CAD by damaging vascular endothelial function (3–5).
Experimental studies (6–8) and epidemiological data (9) have demonstrated that combined folic
acid (400 μg–5 mg daily) and vitamin B therapy may be involved in
the regulation of vascular endothelial structure and function.
However, there is no definitive conclusion with regard to this
effect lowering the concentration of plasma homocysteine. Whether
long-term high-dose folic acid (5 mg daily) alone may effectively
improve vascular endothelial function and lower the concentration
of plasma homocysteine in patients with CAD remains controversial
(10–12). Therefore, a meta-analysis of
randomized controlled trials (RCTs) with regard to folic acid
treatment for CAD was performed to verify whether folic acid is
capable of improving endothelial function and reducing the
concentration of plasma homocysteine in patients with CAD.
Materials and methods
Search strategy for RCTs
In order to compare the efficacy of folic acid
supplementation or placebos for CAD, an extensive literature search
on PubMed was performed in order to locate relevant RCTs that were
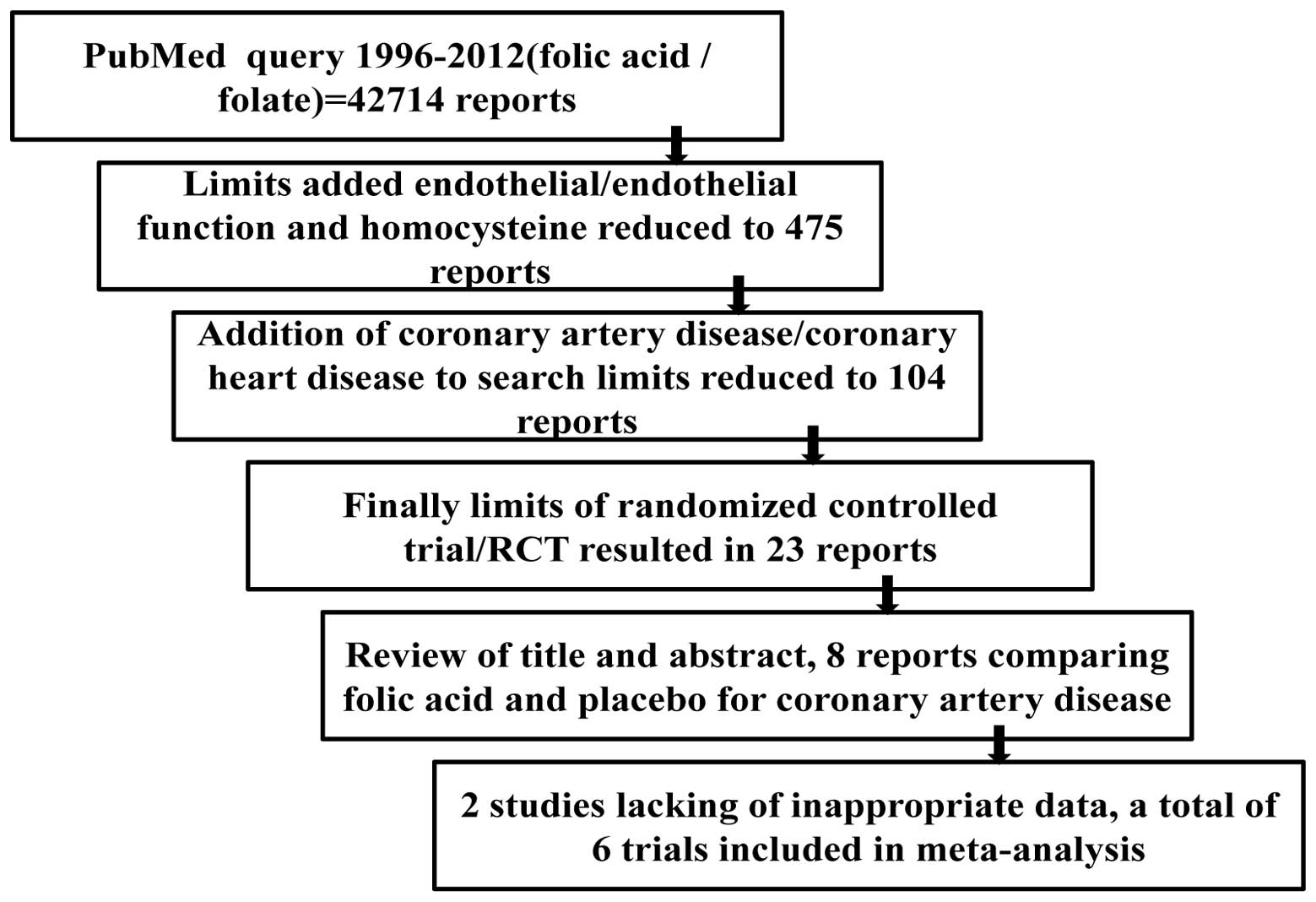
published between January 1966 and September 2012. A flow chart of
the search methodology used in this meta-analysis is provided in
Fig. 1. A total of 42,714 reports
were identified using a PubMed query of ‘folic acid’ or ‘folate’.
Limits of ‘endothelial/endothelial function’ and ‘homocysteine’
reduced the number of reports to 475. Further refinement of the
search criteria with the addition of ‘coronary artery
disease/coronary heart disease’ reduced the number of reports to
104. Finally, limits of ‘randomized controlled trial/RCT’ resulted
in a total of 23 reports (6,13–31).
The titles and abstracts of the reports were reviewed for terms,
including folic acid or folate, endothelial function, CAD or
coronary heart disease. Following careful review, eight randomized
studies were identified that discussed folic acid and placebo
treatment of CAD. However, two studies (10,28)
were excluded for their imprecise information with regard to
flow-mediated dilation (FMD) and end diastolic diameter (EDD) in
the folic acid and placebo-treated groups. As a result, a total of
six trials (11,12,17,25,27,31)
were used for this meta-analysis. The studies were reviewed by two
independent authors in order to assess their quality. Any
discrepancies in their judgments were resolved by joint discussion
or discussion with a third reviewer, referencing the original
report. Variable trials that were assessed included an accurate
description of methods including study design, inclusion criteria,
exclusion criteria, the statistical tests used, the baselines
between the patients undergoing folic acid or placebo treatment and
the outcome of the measures reported along with the results of the
follow-up.
Inclusion/exclusion criteria
Studies were eligible for inclusion if they met the
following criteria: i) the study was a RCT; ii) the study was
conducted using human subjects with CAD; iii) active treatment
consisted of folic acid supplementation (without additional vitamin
B supplementation); iv) folic acid was administered orally with a
dose of 5 mg/day; v) the duration of active treatment was ≥4 weeks
and ≤16 weeks; vi) plasma homocysteine concentration was provided;
vii) the study reported the mean FMD and/or EDD for the treatment
and placebo groups. Studies that reported either FMD or EDD changes
alone, assuming all other criteria were met, were included in this
meta-analysis.
Although 23 potentially relevant studies were
identified and screened, 17 trials did not meet the inclusion
criteria for this meta-analysis. Major reasons for the exclusion of
a study were i) the patients were also treated with vitamin B; ii)
the subject populations did not have CAD; iii) the dosage of folic
acid was not 5 mg/day; iv) the trials were not randomized; v) there
was an absence of data by which to calculate the changes in FMD or
EDD.
Data abstraction and statistical
analysis
Information with regard to study design, sample
size, duration, clinical characteristics and the medication of the
participant, as well as biochemical parameters and treatment
results regarding endothelial function, were independently
abstracted from the six clinical trials and subsequently entered as
standard forms into a Microsoft Excel (Microsoft Corporation,
Redmond, Washington, WA, USA) spreadsheet to calculate the overall
efficacy following folic acid supplementation compared with that
following the administration of a placebo. The risk of bias was
assessed as recommended in the Cochrane Handbook by RevMan 5.0 (The
Cochrane Collaboration) and the standards of assessment were as
follows: i) adequate sequence generation; ii) allocation
concealment; iii) incomplete outcome data were addressed; iv) free
of selective reporting; v) free of other bias. On the basis of this
assessment methodology, the two reviewers provided each eligible
study with an overall rating of low, high or an unclear risk of
bias. Once the outcomes had been evaluated, a table summarizing the
observations was created using the Grading of Recommendations
Assessment, Development and Evaluation (GRADE) system.
The data were analyzed according to the
intention-to-treat principle. RevMan 5.0 was used for the
meta-analysis. The mean difference (MD) with 95% confidence
interval (CI) were calculated as a measure of the correlation
between folic acid supplementation and endothelial function/plasma
homocysteine concentration. A two-sided P<0.05 was considered to
indicate a statistically significant difference.
P values from χ2 statistical analysis and
I2 were used for the heterogeneity test. Heterogeneity
was considered to be significant when P<0.05. If P>0.1 or
I2<50%, ‘there may be no heterogeneity among included
studies and summarize data across the trials by selecting a
fixed-effects model with the software RevMan 5.0’ and pooled data
across the trials by selecting a fixed-effects model based on
inverse variance methods. Otherwise, the results were considered to
have ‘considerable heterogeneity’ and were compared using a
random-effects model. Publication bias was assessed by funnel plots
with the standard error of the intervention effect on the vertical
axis and MD measuring the effect of intervention on the horizontal
axis.
Results
Characteristics of included RCTs
Participant and study design characteristics for the
six RCTs included in the meta-analysis are shown in Table I. Among these clinical studies,
four trials had a parallel double-blind design and two had a
crossover double-blind design. Of the 377 patients included, 191
patients underwent folic acid supplementation and 186 patients
underwent placebo treatment. The majority of trials included aged
male participants. Trial duration varied between eight weeks and
four months. The medication administered in the variable trials is
presented in Table II. There were
no differences in the baseline clinical or biochemical parameters
(Tables I and II). The majority of patients were
treated with antiplatelet therapy, lipid-lowering therapy,
β-blockers, angiotensin-converting-enzyme inhibitors, nitrates and
other drug therapies. Biochemical parameters, including total
cholesterol, triglycerides, low-density lipoprotein (LDL) and
high-denstity lipoprotein cholesterol, plasma folic acid, vitamin
B12, creatinine and glucose, are shown in Table III. Furthermore, baseline
endothelial function data of the folic acid and placebo groups are
presented in Table IV.
 | Table IClinical characteristics of studies
included in the meta-analysis. |
Table I
Clinical characteristics of studies
included in the meta-analysis.
| Yilmaz et al
2007 (17) | Moat et al
2006 (11) | Doshi et al
2004 (12) | Doshi et al
2002 (25) | Doshi et al
2001 (27) | Title et al
2000 (31) |
|---|
|
|
|
|
|
|
|
|---|
|
Characteristics | Placebo | Folic acid | Placebo | Folic acid | Placebo | Folic acid | Placebo | Folic acid | Placebo | Folic acid | Placebo | Folic acid |
|---|
| Study design | PD | PD | CD | PD | CD | PD |
| Cases (n) | 20 | 20 | 29 | 25 | 50 | 50 | 17 | 16 | 50 | 50 | 25 | 25 |
| Age (years) | 65.5±7.6 | 52.2±11.9 | 61±7 | 60±7 | NA | 57±8 | 56±7 | 55±7 | NA | 57±8 | 60.6±8.6 | 57.2±9.8 |
| Gender, M/F
(n) | 18/2 | 13/7 | 25/4 | 21/4 | NA | 44/6 | 16/1 | 14/2 | NA | 44/6 | 21/4 | 19/6 |
| Follow-up
(weeks) | 8.4±1.1 | 8.4±1.1 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 16 | 16 |
| BMI
(kg/m2) | 28.3±4 | 27.2±3.8 | 29.6±4.1 | 29.9±4.4 | NA | 28.5±4.4 | NA | NA | NA | 28.5±4.4 | NA | NA |
| Diabetes mellitus
(n) | 8 | 4 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Hypertension
(n) | 17 | 13 | 9 | 11 | NA | NA | 7 | 5 | NA | 20 | 11 | 11 |
| Hyperlipidemia
(n) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Myocardial
infarction (n) | NA | NA | 16 | 12 | NA | 33 | 10 | 9 | NA | 33 | 14 | 11 |
| Cerebrovascular
event (n) | NA | NA | 2 | 1 | NA | NA | 1 | 0 | NA | 4 | NA | NA |
| Smoking (n) | 2 | 6 | 21 | 21 | NA | NA | 13 | 10 | NA | 36 | 6 | 6 |
| Family history of
CAD (n) | 7 | 10 | 18 | 12 | NA | NA | 11 | 9 | NA | 26 | NA | NA |
 | Table IIClinical medication of studies
included in the meta-analysis. |
Table II
Clinical medication of studies
included in the meta-analysis.
| Yilmaz et al
2007 (17) | Moat et al
2006 (11) | Doshi et al
2004 (12) | Doshi et al
2002 (25) | Doshi et al
2001 (27) | Title et al
2000 (31) |
|---|
|
|
|
|
|
|
|
|---|
| Medication | Placebo | Folic acid | Placebo | Folic acid | Placebo | Folic acid | Placebo | Folic acid | Placebo | Folic acid | Placebo | Folic acid |
|---|
| Aspirin | 16 | 20 | NA | NA | NA | 46 | NA | NA | NA | 46 | 22 | 20 |
| Clopidogrel | NA | NA | NA | NA | NA | 2 | NA | NA | NA | 2 | NA | NA |
| Nitrates | 13 | 10 | 6 | 1 | NA | 4 | 2 | 1 | NA | 4 | NA | NA |
| β-blockers | 10 | 14 | 17 | 16 | NA | 33 | 10 | 6 | NA | 33 | 18 | 21 |
| Statins | 14 | 11 | NA | NA | NA | 43 | 14 | 14 | NA | 44 | NA | NA |
| ACE inhibitors | 8 | 14 | 8 | 9 | NA | 7 | 1 | 1 | NA | 7 | 5 | 9 |
| ATII receptor
antagonist | NA | NA | 0 | 1 | NA | 3 | 0 | 1 | NA | 3 | NA | NA |
| Diuretics | 6 | 5 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Calcium channel
blocks | 0 | 1 | 4 | 3 | NA | 12 | 2 | 3 | NA | 12 | 11 | 5 |
| Insulin | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Oral hypoglycemic
agent | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
 | Table IIIBiochemical parameters of the studies
included in the meta-analysis. |
Table III
Biochemical parameters of the studies
included in the meta-analysis.
| Yilmaz et al
2007 (17) | Moat et al
2006 (11) | Doshi et al
2004 (12) | Doshi et al
2002 (25) | Doshi et al
2001 (27) | Title et al
2000 (31) |
|---|
|
|
|
|
|
|
|
|---|
| Parameters | Placebo | Folic acid | Placebo | Folic acid | Placebo | Folic acid | Placebo | Folic acid | Placebo | Folic acid | Placebo | Folic acid |
|---|
| Homocysteine
(μmol/l) |
| Baseline | 18.4±3.2 | 21.7±8.7 | 12.1±3.9 | 12.9±3.9 | 10.5±2.5 | 11.1±2.8 | 10.8±2.2 | 10.6±2.6 | 10.5±2.5 | 11.1±2.8 | 12.1 | 12.3 |
| Follow-up | 20.7±7.1 | 12.5±2.5 | 12.6±4.7 | 9.9±2.6 | 10.8±2.4 | 9.3±2.4 | 10.8±2.1 | 8.3±1.3 | 10.8±2.4 | 9.3±2.4 | 11.8 | 10.9 |
| Folic acid | 12.7±4.1 ng/ml | 12.1±5.1 ng/ml | 22.7±10.7
nmol/l | 20.2±8.6
nmol/l | 9.3±2.9 μg/l | 8.9±3.5 μg/l | 26.09±6.8
nmol/l | 22.37±8.7
nmol/l | 9.3±2.9 μg/l | 8.9±3.5 μg/l | 14.7 nmol/l | 13.8 nmol/l |
| Vitamin
B12 | 311.9±96.8
pg/ml | 296.0±48.7
pg/ml | 318±110 pmol/l | 366±127 pmol/l | NA | NA | 312±105 pmol/l | 306±73 pmol/l | 430±125.75
ng/l | 435±123 ng/l | 218 nmol/l | 227 nmol/l |
| Total
cholesterol | 175.7±51.0
mg/dl | 205.2±37.5
mg/dl | 4.4±0.6 mmol/l | 4.6±0.7 mmol/l | NA | NA | 4.47±0.57
mmol/l | 4.36±0.87
mmol/l | 4.6±0.7 mmol/l | 4.8±0.7 mmol/l | 5.2 mmol/l | 5.3 mmol/l |
| Triglycerides | 108.6±61.1
mg/dl | 122.1±39.7
mg/dl | 1.77±0.63
mmol/l | 1.45±0.69
mmol/l | NA | NA | 1.45±0.7
mmol/l | 1.43±0.45
mmol/l | 1.7±0.9 mmol/l | 1.7±0.9 mmol/l | 2.5 mmol/l | 2.1 mmol/l |
| LDL | 107.6±43.4
mg/dl | 128.2±27.2
mg/dl | 2.5±0.6 mmol/l | 2.7±0.6 mmol/l | NA | NA | 2.65±0.48
mmol/l | 2.7±0.77
mmol/l | 2.8±0.6 mmol/l | 2.8±0.6 mmol/l | 3.3 mmol/l | 3.5 mmol/l |
| HDL | 48.3±9.4 mg/dl | 43.6±11.8
mg/dl | 1.1±0.2 mmol/l | 1.3±0.2 mmol/l | NA | NA | 1.16±0.36
mmol/l | 1.08±0.2
mmol/l | 1.1±0.3 mmol/l | 1.2±0.4 mmol/l | 0.9 mmol/l | 0.9 mmol/l |
| VLDL (mg/dl) | 22.6±11.8 | 23.5±7.8 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Creatinine
(μmol/l) | NA | NA | 88.1±12.2 | 89.5±14.0 | NA | NA | 95.1±12.1 | 98.7±18.1 | 98.7±13.6 | 98.9±13.7 | 100±19 | 95±20 |
| HbA1C (%) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Glucose
(mmol/l) | NA | NA | 5.5±0.7 | 5.4±0.5 | NA | NA | 5.49±0.75 | 5.6±0.77 | 5.3±0.6 | 5.3±0.7 | NA | NA |
 | Table IVEndothelial function parameters of
the studies included in the meta-analysis. |
Table IV
Endothelial function parameters of
the studies included in the meta-analysis.
| Yilmaz et al
2007 (17) | Moat et al
2006 (11) | Doshi et al
2004 (12) | Doshi et al
2002 (25) | Doshi et al
2001 (27) | Title et al
2000 (31) |
|---|
|
|
|
|
|
|
|
|---|
| Parameters | Placebo | Folic acid | Placebo | Folic acid | Placebo | Folic acid | Placebo | Folic acid | Placebo | Folic acid | Placebo | Folic acid |
|---|
| FMD (μm) |
| Baseline | NA | NA | 20.3±31.0 | 24.4±26.3 | 46±33 | 52±34 | 48±24 | 52.5±29 | 46±33 | 52±34 | 2.7±3.3% | 3.2±3.6% |
| Follow-up | NA | NA | 33.5±21.6 | 99.6±35.7 | 47±35 | 110±43 | 52±19 | 111±28 | 47±35 | 110±43 | 2.9±3.7% | 5.2±3.9% |
| EDD |
| Baseline | 5.8±1.9 % | 5.3±2.2 % | 4.01±0.52 mm | 3.87±0.62 mm | NA | NA | 4.20±0.5 mm | 4.29±0.7 mm | 4.36±0.73 mm | 4.39±0.70 mm | 4.27±0.91 mm | 4.44±0.98 mm |
| Follow-up | 6.1±2.7 % | 12.0±6.3 % | 4.03±0.6 mm | 3.85±0.64 mm | NA | NA | 4.18±0.5 mm | 4.27±0.7 mm | 4.38±0.72 mm | 4.39±0.70 mm | 4.26±0.91 mm | 4.36±0.87 mm |
| GTN diameter change
(μm) |
| Baseline | NA | NA | 451±55 | 444±69 | NA | NA | 396±63 | 415±52 | 340±72 | 340±76 | NA | NA |
| Follow-up | NA | NA | 451±59 | 441±67 | NA | NA | 402±60 | 431±37 | 340±72 | 340±77 | NA | NA |
| Baseline blood flow
(ml/min) |
| Baseline | NA | NA | 70.4±64.0 | 70.4±53.1 | NA | NA | 37±18 | 35±15 | 40±20 | 40±19 | NA | NA |
| Follow-up | NA | NA | 74.6±52.5 | 74.0±44.3 | NA | NA | 33±12 | 34±13 | 40±19 | 40±18 | NA | NA |
| Peak hyperemic flow
(ml/min) |
| Baseline | NA | NA | 220±135 | 220±137 | NA | NA | 189±54 | 180±67 | 196±68 | 202±67 | NA | NA |
| Follow-up | NA | NA | 258±160 | 240±115 | NA | NA | 189±55 | 186±52 | 196±71 | 198±66 | NA | NA |
| Heart rate
(beats/min) |
| Baseline | NA | NA | 61±10 | 61±10 | NA | NA | 61±11 | 58±8 | 59±10 | 59±10 | 57±10 | 60±10 |
| Follow-up | NA | NA | 60±10 | 62±9 | NA | NA | 62±12 | 59±8 | 60±10 | 59±10 | 59±7 | 60±11 |
| Systolic BP
(mmHg) |
| Baseline | 135.0±21.9 | 132.7±21.7 | 128±15 | 131±17 | NA | NA | 131±15 | 133±18 | 132±16 | 133±17 | 132±17 | 132±20 |
| Follow-up | NA | NA | 127±16 | 129±16 | NA | NA | 130±13 | 133±19 | 133±14 | 133±14 | 133±17 | 130±18 |
| Diastolic BP
(mmHg) |
| Baseline | 82.7±11.6 | 82.2±11.7 | 75±7 | 77±8 | NA | NA | 75±10 | 71±6 | 73±9 | 74±9 | 79±10 | 81±10 |
| Follow-up | NA | NA | 76±6 | 77±8 | NA | NA | 72±9 | 70±9 | 71±8 | 73±9 | 79±12 | 79±9 |
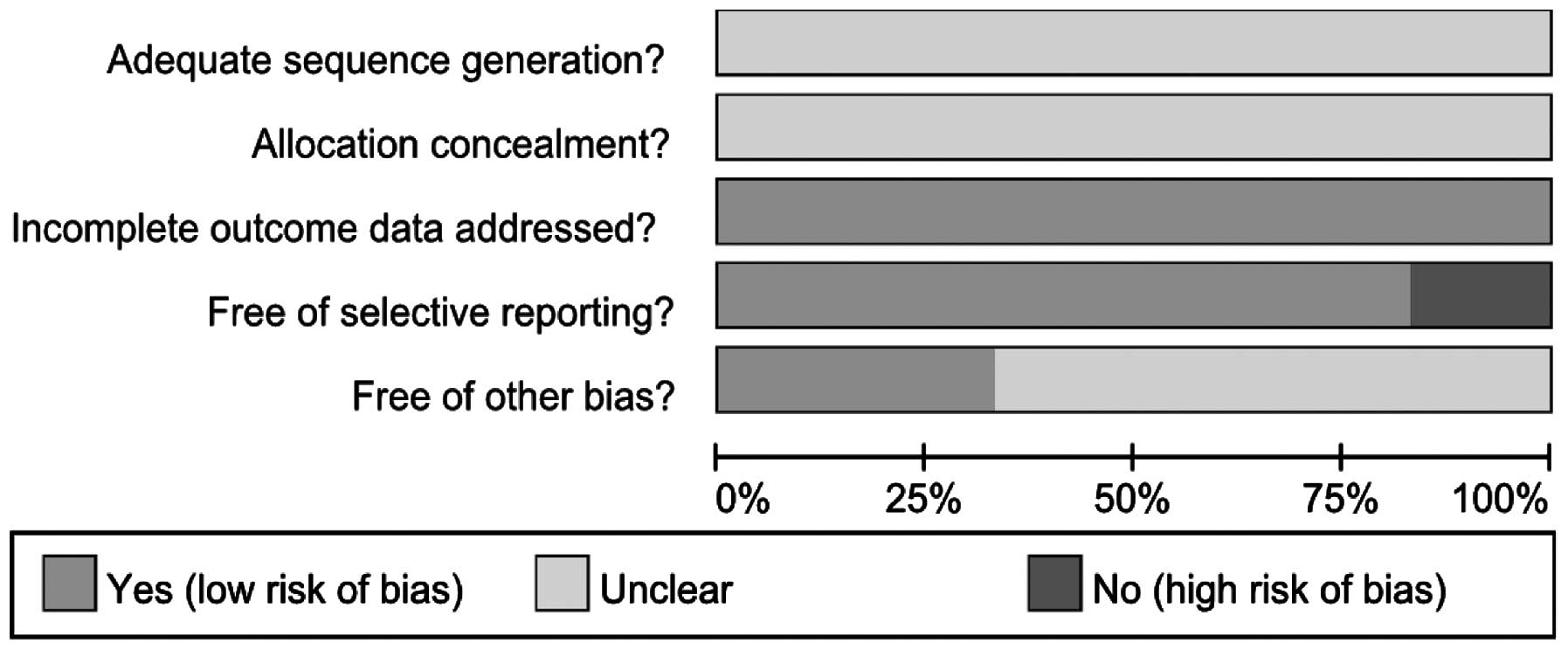
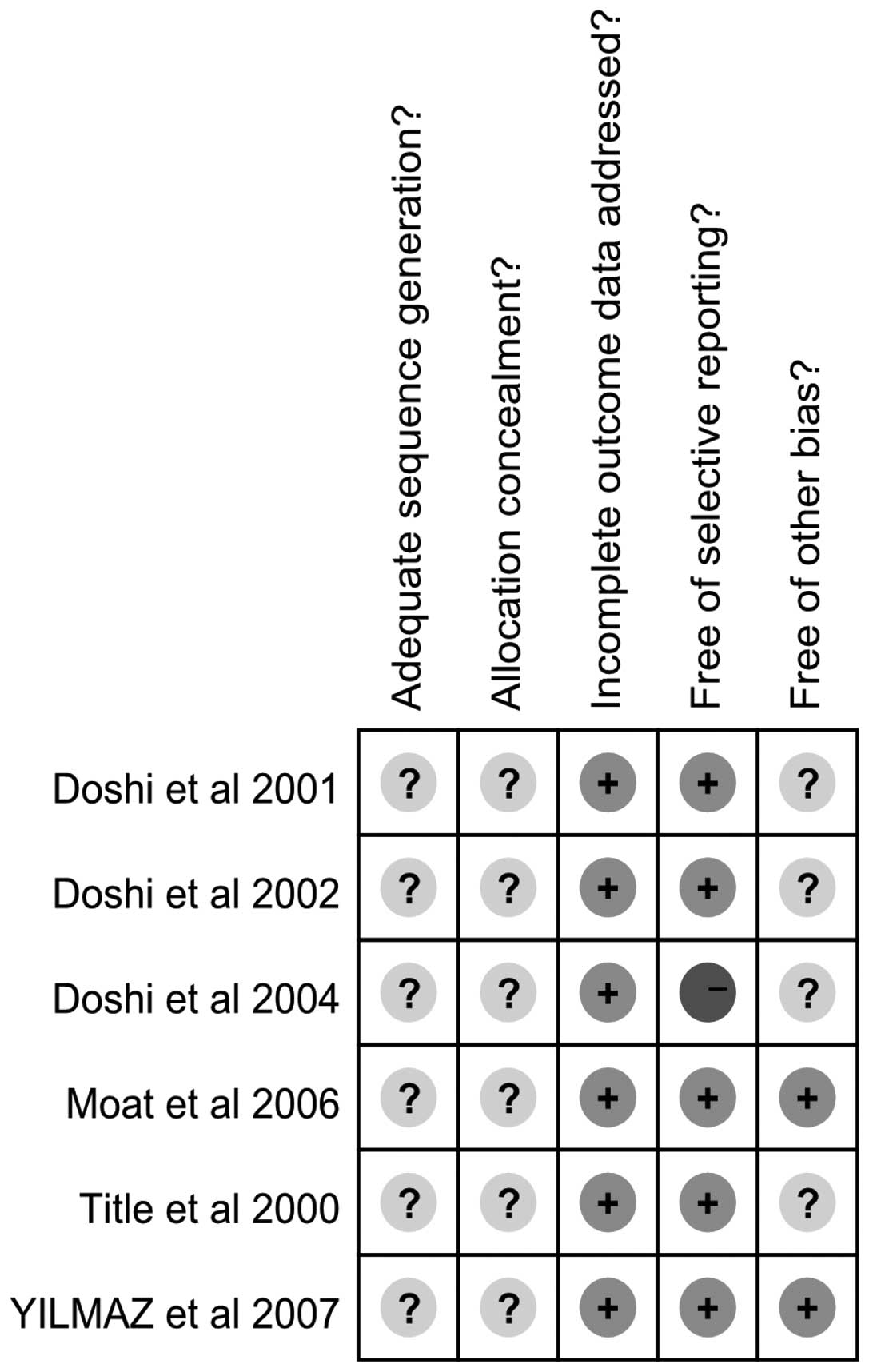
Assessment of the bias risk and
recommended classification of included studies
The bias risk for the included trials was assessed
according to the assessment methodology recommended by The Cochrane
Collaboration (Figs. 2 and
3). Adequate sequence generation
and allocation concealment were not described clearly in all six
studies. The six trials reported complete outcome data, but one
study did not clearly describe the selective reporting (12). Due to incomplete information in
four studies (12,25,27,31),
there may be other biases. The efficacy of folic acid
supplementation on FMD and the concentration of plasma homocysteine
was the main outcome in this meta-analysis. The recommended
classification of FMD was deemed to be of low quality, but the
grade of evidence for the concentration of plasma homocysteine was
deemed to be of moderate quality. Therefore, due to the quality of
evidence according to the GRADE system, the use of folic acid is
recommended to reduce the concentration of plasma homocysteine.
Efficacy of folic acid on endothelial
function
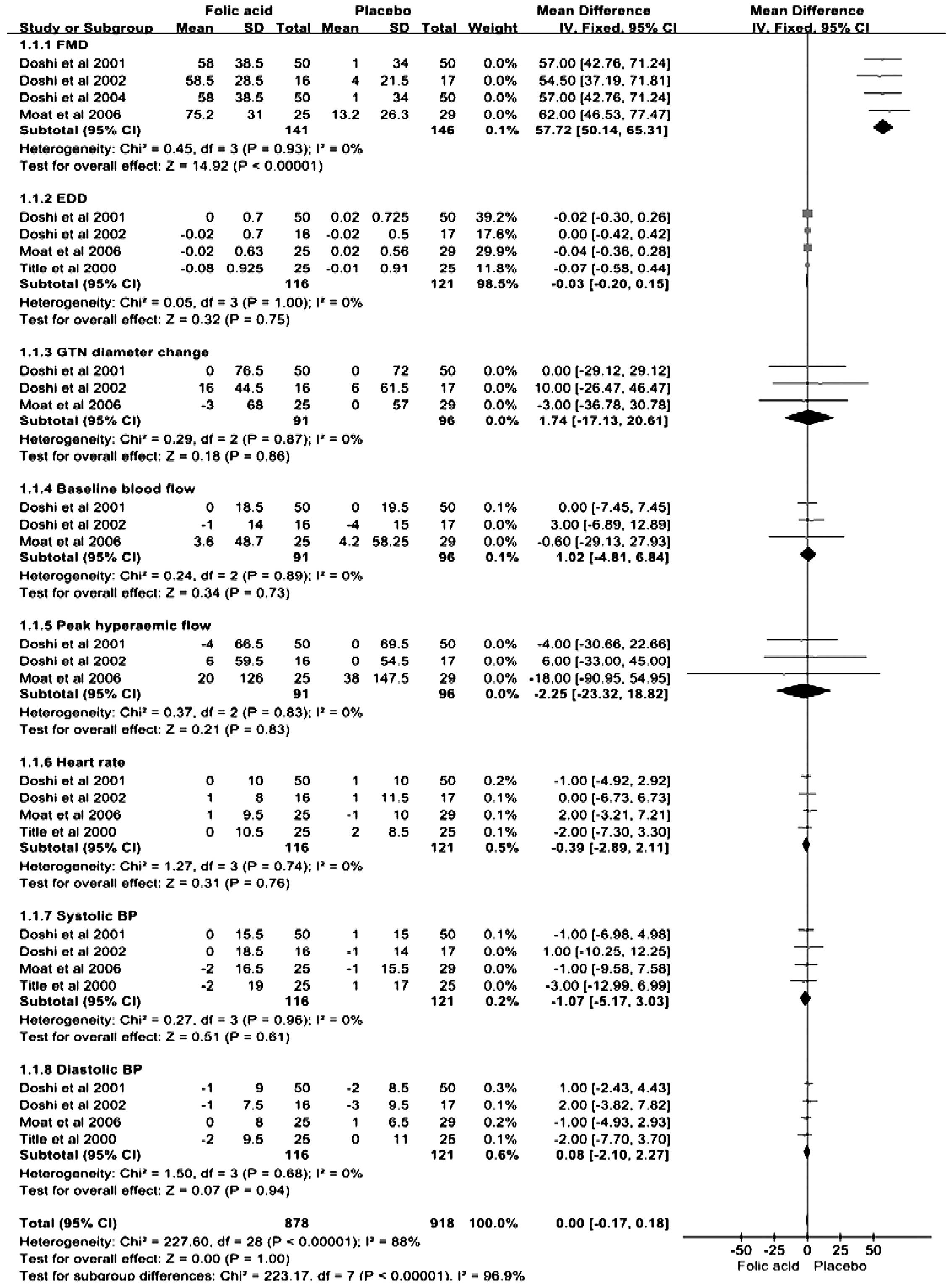
The individual trial results for the effects of
folic acid and placebo therapy on FMD, EDD, glyceryl-trinitrate
(GTN) diameter change, heart rate, baseline and peak hyperemic
flow, systolic and diastolic blood pressure (BP) and the pooled
estimate of the effect are shown in Fig. 4. Of the six trials included, four
studies measured the efficacy of folic acid on FMD; the pooled
estimate from these studies exhibited a marked increase in FMD in
the folic acid-treated group when compared with the placebo group
(MD, 57.72 μm; 95% CI, 50.14–65.31; P<0.05; I2, 0%).
Using a random-effects versus a fixed-effects model did not
markedly alter the pooled estimate. However, the pooled estimate
presented no significant difference in the response to EDD (MD,
−0.03; 95% CI, −0.20–0.15; P=0.75; I2, 0%), GTN diameter
change (MD, 1.74; 95% CI, −17.13–20.61; P=0.86; I2, 0%),
heart rate (MD, −0.39; 95% CI, −2.89–2.11; P=0.76; I2,
0%), baseline hyperemic flow (MD, 1.02; 95% CI, −4.81–6.84; P=0.73;
I2, 0%), peak hyperemic flow (MD, −2.25; 95% CI,
−23.32–18.82; P=0.83; I2, 0%), systolic BP (MD, −1.07;
95% CI, −5.71–3.03; P=0.61; I2, 0%) and diastolic BP
(MD, 0.08, 95% CI, −2.10–2.27; P=0.94; I2, 0%) between
the folic acid and placebo treatment groups when using a
fixed-effects model. A funnel plot of effect size versus study
precision was asymmetrical with a relative dearth of moderately
precise negative studies, indicating the presence of a positive
publication bias (Fig. 5).
 | Figure 4MD with 95% CI estimates for FMD,
EDD, GTN diameter change, heart rate, baseline and peak hyperemic
flow, systolic and diastolic BP (folic acid vs. placebo), by
summarizing different results of included trials in this study. MD,
mean difference; CI, confidence interval; FMD, flow-mediated
dilation; EDD, end diastolic diameter; GTN, glyceryl-trinitrate;
BP, blood pressure. |
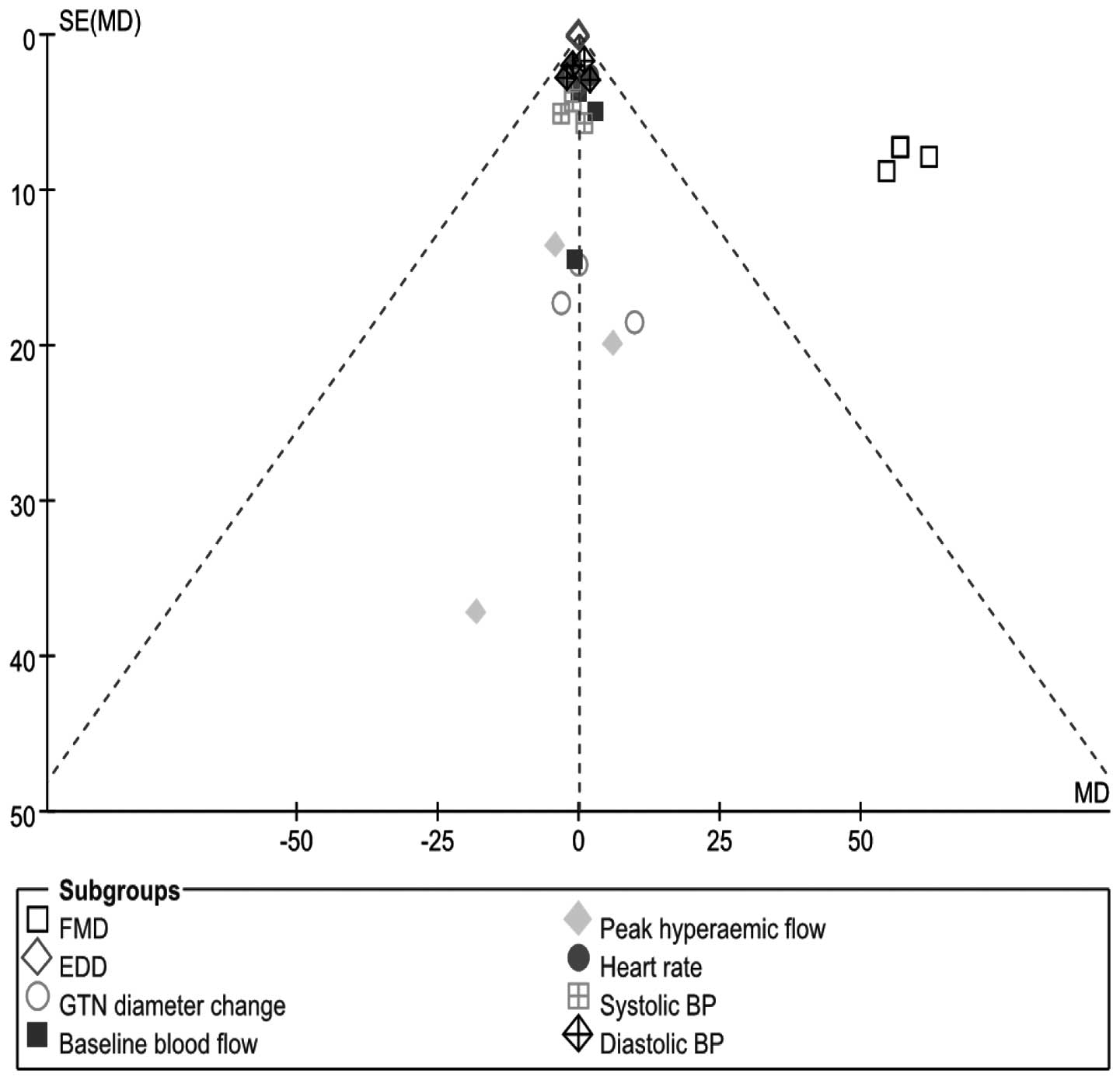 | Figure 5Funnel plot of FMD, EDD, GTN diameter
change, heart rate, baseline and peak hyperemic flow, systolic and
diastolic BP (folic acid vs. placebo), by summarizing different
results of included trials in this study. FMD, flow-mediated
dilation; EDD, end diastolic diameter; GTN, glyceryl-trinitrate;
BP, blood pressure. |
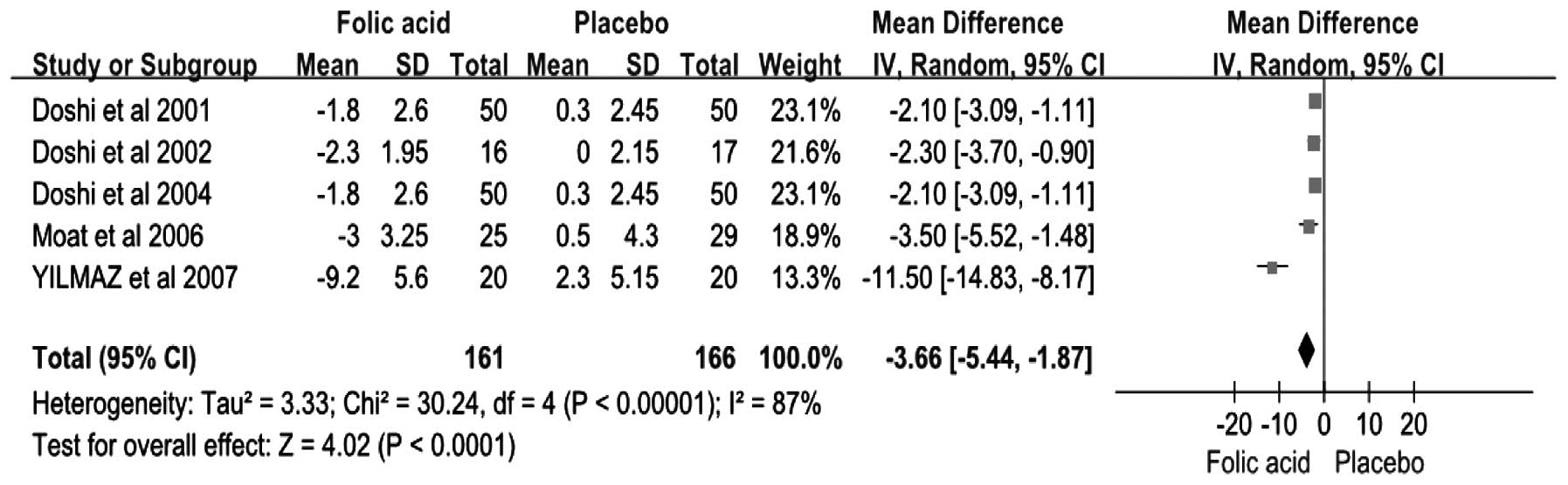
Effect of folic acid on the concentration
of plasma homocysteine
In total, five studies reported a change in the
concentration of plasma homocysteine (Fig. 6). The results from the
random-effects model pooling the MD demonstrated that folic acid
supplementation correlated with a significant reduction in the
concentration of plasma homocysteine (MD, −3.66 μmol/l; 95% CI,
−5.44–−1.87; P<0.05; I2, 87%). Using a fixed-effects
versus random-effects model did not substantially alter the pooled
estimate.
Discussion
The results of the present meta-analysis
demonstrated that an increase in FMD and decrease in plasma
homocysteine concentration in CAD patients were associated with
folic acid supplementation. However, there was no significant
change in EDD, GTN diameter, heart rate, baseline and peak
hyperemic flow and systolic and diastolic BP between the folic acid
supplementation and placebo-treated groups. Measuring the FMD of a
brachial artery using color Doppler ultrasound may accurately
reflect the state of coronary endothelial function and serve as a
non-invasive method to evaluate endothelial function, thus, is of
great value clinically (32,33).
Notably, the results of the current RCT meta-analysis are in
agreement with data from prospective cohort studies, indicating the
efficacy of high-dose folic acid supplementation in improving
endothelial function and lowering the concentration of plasma
homocysteine in subjects with CAD.
It has been recognized that hyperhomocysteinemia is
a risk factor for CAD. Compared with individuals without CAD, the
risk of CAD increases 2-fold in patients with hyperhomocysteinemia
(34). The function of folic acid
is limited to decreasing the levels of plasma cysteine initially,
but subsequent studies have demonstrated that folic acid (400
μg/day) can markedly reduce plasma homocysteine levels, while a
larger dose of folic acid improves endothelial function in patients
with CAD and reduces the incidence of cardiovascular events
(11,13). Doshi et al (25) observed that an improvement in FMD
occurred prior to a significant drop in plasma homocysteine
concentration with folic acid treatment, indicating that the
enhancement was not explained by a reduction in homocysteine
levels. Following the administration of folic acid, FMD improved
markedly at 2 h and peaked 4 h after the first dose. However, there
was no significant decrease in the total or free plasma
homocysteine levels in the 4 h following the initial dose of folic
acid. Verhaar et al (35)
demonstrated that 5-methyltetrahydrofolate, a major circulating
folate, is capable of improving endothelial function in patients
with familial hypercholesterolemia who are free of vascular disease
and are not receiving lipid-lowering treatment. The phenomenon,
which may be mediated by an increase in nitric oxide (NO)
bioavailability and the generation of superoxide ions, was also
confirmed by in vitro laboratory experiments (11,35).
Schwammenthal et al (36),
by performing a meta-analysis of a large number of folic acid
clinical trials, predicted that folic acid supplementation at dose
of 200 μg/day was capable of reducing plasma homocysteine levels by
an average of 4 μmol/l. In addition, the authors hypothesized that
it may be possible to reduce the number of patients succumbing to
cardiovascular disease by 13,500–50,000 each year in the USA. A
recent meta-analysis of 12 RCTs using 16,958 subjects found that
folic acid supplementation had no efficacy on reducing the risk of
CAD (37). However, it should be
noted that half of the trials included in this meta-analysis used
folic acid dosages that were <5,000 μg/day. An additional
meta-analysis (38) observed that
the changes in BP and FMD, along with the concomitant changes in
the risk of coronary heart disease, may only be observed when folic
acid doses are in the order of 5,000 μg/day or greater.
The mechanism by which folic acid improves
endothelial function remains unclear, however, previous studies
have shown that the phenomenon is likely to be associated with the
following mechanisms. Firstly, reduced plasma homocysteine levels.
Homocysteine is capable of promoting the generation of hydrogen
peroxide and oxygen-derived free radicals by the autoxidation of
the sulfhydryl on homocysteine, causing the vascular endothelium to
be damaged. This results in abnormal changes to the vascular
endothelial cell cytoskeleton, accelerating the oxidation of LDL,
increasing the formation of foam cells, thickening the walls of
blood vessels and even leading to occlusion of blood vessels.
Furthermore, homocysteine may also induce apoptosis in endothelial
cells and affect the expression of adhesion factors and cytokines,
reducing NO-dependent vasorelaxation (39). However, as mentioned previously,
high-dose folic acid supplementation improves endothelial function
and reduces plasma homocysteine levels, but does not correlate
positively (25). A second
potential mechanism is that vascular endothelial cells are weakened
by oxidative stress. The biological activity of NO directly affects
endothelial function and NO biological activity is determined by
the activity of nitric oxide synthase (NOS) and NO inactivation.
Various pathophysiological factors are capable of causing the
decoupling of eNOS, the result of which produces NO which is
converted into generating oxygen-derived free radicals. In recent
years, studies have found that the NOS cofactor,
tetrahydrobiopterin, is an important regulator of NOS function,
which maintains the enzymatic coupling of L-arginine oxidation in
order to produce NO (40). NO
inactivation is mostly determined by a variety of reactive
oxygen-derived free radicals. Thirdly, an additional mechanism may
be that folic acid, as a specific type of one-carbon substitution,
may be important in repairing genetic damage and maintaining
genetic stability (41). Finally,
NO production may be directly improved by enhancing the enzymatic
activity of eNOS, however, the scavenging potency is 20-fold lower
than that of vitamin C (42,43).
Imbalance in the secretion and release of vasoactive
substances due to vascular endothelial cell injury leads to
spasming of the coronary artery, rupturing of the coronary artery
plaque, platelet aggregation and thrombus formation. In addition,
it reduces the antithrombotic ability of endothelial cells and
increases blood coagulation, which causes thrombosis, ultimately
promoting the occurrence and development of CAD. Therefore,
improving endothelial function has great clinical value for the
prevention and treatment of CAD. The present meta-analysis predicts
that the long-term use of high-dose folic acid may reduce the
concentration of plasma homocysteine and increase FMD, improving
endothelial function. Subsequently, the prevention and treatment of
CAD may be achieved via clinical trials of folic acid intervention
with CAD patients.
It is possible that high-doses of folic acid (5 mg
daily) administered orally improves endothelial function and lowers
the concentration of plasma homocysteine in CAD patients. Folic
acid supplementation is inexpensive, potentially effective and
temporarily devoid of adverse effects. Therefore, folic acid has an
exceptionally favorable benefit/risk ratio for improving
endothelial function in CAD patients.
There are several limitations to consider when
interpreting the results of the present study. Firstly, only two
trials included in the meta-analysis truly divided the patients
into folic acid supplementation and placebo groups. In the
additional fours studies, the patients were randomized to folic
acid 5 mg or folic acid 400 μg/N-acetylcysteine/folic acid combined
with vitamin B or a placebo. An additional limitation was the small
number of cases. No report documented data on the side effects of
high dose folic acid and the studies also had markedly different
durations and evaluation indices for endothelial function. Thirdly,
the current study is prone to the well-known limitation of
meta-analyses, namely variation in study design and publication
bias. Furthermore, the meta-analytical approach with observational
data is even more fraught with limitations. Thus, additional
double-blind, randomized, placebo-controlled, multicenter studies
with high quality and longer follow-up periods are required to
confirm the conclusions of the present study. It will be useful to
observe whether the efficacy of folic acid supplementation,
particularly on arterial function, is similar among patients with
angina, myocardial infarction and non symptom coronary heart
disease.
In conclusion, the present meta-analysis of RCTs
demonstrates that folic acid supplementation of 5 mg/day for >4
weeks significantly improves FMD and lowers plasma homocysteine
concentration in patients with CAD. Thus, this study has underlined
the importance of high-dose folic acid supplementation for the
improvement of endothelial function. Folic acid supplementation
should be recommended for CAD patients. However, more RCTs are
required in order to confirm this meta-analysis.
Acknowledgements
The authors thank all the members of the Department
of Cardiology and Cardiovascular Research Institute of Renmin
Hospital of Wuhan University for their assistance and supportive
advice. The study was supported by a grant from the National
Natural Science Foundation of China (no. 81170307).
References
|
1
|
Quyyumi AA: Prognostic value of
endothelial function. Am J Cardiol. 91:19H–24H. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weber T, Auer J, O’Rourke MF, et al:
Arterial stiffness, wave reflections, and the risk of coronary
artery disease. Circulation. 109:184–189. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta SK, Kotwal J, Kotwal A, Dhall A and
Garg S: Role of homocysteine & MTHFR C677T gene polymorphism as
risk factors for coronary artery disease in young Indians. Indian J
Med Res. 135:506–512. 2012.
|
|
4
|
Vinukonda G, Shaik Mohammad N, Md Nurul
Jain J, Prasad Chintakindi K and Rama Devi Akella R: Genetic and
environmental influences on total plasma homocysteine and coronary
artery disease (CAD) risk among South Indians. Clin Chim Acta.
405:127–131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin PT, Huang MC, Lee BJ, Cheng CH, Tsai
TP and Huang YC: High plasma homocysteine is associated with the
risk of coronary artery disease independent of
methylenetetrahydrofolate reductase 677C-->T genotypes. Asia Pac
J Clin Nutr. 17:330–338. 2008.
|
|
6
|
Bleie Ø, Strand E, Ueland PM, Vollset SE,
Refsum H, Igland J, Nordrehaug JE and Nygård OK: Coronary blood
flow in patients with stable coronary artery disease treated long
term with folic acid and vitamin B12. Coron Artery Dis. 22:270–278.
2011.PubMed/NCBI
|
|
7
|
MacKenzie KE, Wiltshire EJ, Gent R, Hirte
C, Piotto L and Couper JJ: Folate and vitamin B6 rapidly normalize
endothelial dysfunction in children with type 1 diabetes mellitus.
Pediatrics. 118:242–253. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Till U, Röhl P, Jentsch A, et al: Decrease
of carotid intima-media thickness in patients at risk to cerebral
ischemia after supplementation with folic acid, vitamins B6 and
B12. Atherosclerosis. 181:131–135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Bree A, van Mierlo LA and Draijer R:
Folic acid improves vascular reactivity in humans: a meta-analysis
of randomized controlled trials. Am J Clin Nutr. 86:610–617.
2007.PubMed/NCBI
|
|
10
|
Shirodaria C, Antoniades C, Lee J, et al:
Global improvement of vascular function and redox state with
low-dose folic acid: implications for folate therapy in patients
with coronary artery disease. Circulation. 115:2262–2270. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moat SJ, Madhavan A, Taylor SY, et al:
High- but not low-dose folic acid improves endothelial function in
coronary artery disease. Eur J Clin Invest. 36:850–859. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Doshi SN, Moat SJ, Lewis MJ, McDowell IF,
Giddings JC and Goodfellow J: Short-term high-dose folic acid does
not alter markers of endothelial cell damage in patients with
coronary heart disease. Int J Cardiol. 94:203–207. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo H, Chi J, Xing Y and Wang P: Influence
of folic acid on plasma homocysteine levels & arterial
endothelial function in patients with unstable angina. Indian J Med
Res. 129:279–284. 2009.
|
|
14
|
Tam WY, Chook P, Qiao M, et al: The
efficacy and tolerability of adjunctive alternative herbal medicine
(Salvia miltiorrhiza and Pueraria lobata) on vascular
function and structure in coronary patients. J Altern Complement
Med. 15:415–421. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Myles PS, Leslie K, Peyton P, et al:
Nitrous oxide and perioperative cardiac morbidity (ENIGMA-II)
trial: rationale and design. Am Heart J. 157:488–494. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sultan N, Khan MA and Malik S: Effect of
folic acid supplementation on homocysteine level in postmenopausal
women. J Ayub Med Coll Abbottabad. 19:78–81. 2007.PubMed/NCBI
|
|
17
|
Yilmaz H, Sahin S, Sayar N, et al: Effects
of folic acid and N-acetylcysteine on plasma homocysteine levels
and endothelial function in patients with coronary artery disease.
Acta Cardiol. 62:579–585. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Austen SK, Fassett RG, Geraghty DP and
Coombes JS: Folate supplementation fails to affect vascular
function and carotid artery intima media thickness in cyclosporin
A-treated renal transplant recipients. Clin Nephrol. 66:373–379.
2006. View
Article : Google Scholar
|
|
19
|
Fernández-Miranda C, Yebra M, Aranda JL,
Gómez P, Martínez J, Núñez V and Gómez de la Cámara A: Effect of
folic acid treatment on carotid intima-media thickness of patients
with coronary disease. Int J Cardiol. 118:345–349. 2007.PubMed/NCBI
|
|
20
|
Chia S, Wilson R, Ludlam CA, Webb DJ,
Flapan AD and Newby DE: Endothelial dysfunction in patients with
recent myocardial infarction and hyperhomocysteinaemia: effects of
vitamin supplementation. Clin Sci (Lond). 108:65–72. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Doshi S, McDowell I, Moat S, Lewis M and
Goodfellow J: Folate improves endothelial function in patients with
coronary heart disease. Clin Chem Lab Med. 41:1505–1512. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Woodman RJ, Celermajer DE, Thompson PL and
Hung J: Folic acid does not improve endothelial function in healthy
hyperhomocysteinaemic subjects. Clin Sci (Lond). 106:353–358. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liem A, Reynierse-Buitenwerf GH,
Zwinderman AH, Jukema JW and van Veldhuisen DJ: Secondary
prevention with folic acid: effects on clinical outcomes. J Am Coll
Cardiol. 41:2105–2113. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Willems FF, Aengevaeren WR, Boers GH, Blom
HJ and Verheugt FW: Coronary endothelial function in
hyperhomocysteinemia: improvement after treatment with folic acid
and cobalamin in patients with coronary artery disease. J Am Coll
Cardiol. 40:766–772. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Doshi SN, McDowell IF, Moat SJ, Payne N,
Durrant HJ, Lewis MJ and Goodfellow J: Folic acid improves
endothelial function in coronary artery disease via mechanisms
largely independent of homocysteine lowering. Circulation.
105:22–26. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miner SE, Cole DE, Evrovski J, Forrest Q,
Hutchison S, Holmes K and Ross HJ: Pyridoxine improves endothelial
function in cardiac transplant recipients. J Heart Lung Transplant.
20:964–969. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Doshi SN, McDowell IF, Moat SJ, Lang D,
Newcombe RG, Kredan MB, Lewis MJ and Goodfellow J: Folate improves
endothelial function in coronary artery disease: an effect mediated
by reduction of intracellular superoxide? Arterioscler Thromb Vasc
Biol. 21:1196–1202. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thambyrajah J, Landray MJ, Jones HJ,
McGlynn FJ, Wheeler DC and Townend JN: A randomized double-blind
placebo-controlled trial of the effect of homocysteine-lowering
therapy with folic acid on endothelial function in patients with
coronary artery disease. J Am Coll Cardiol. 37:1858–1863. 2001.
View Article : Google Scholar
|
|
29
|
Spence JD, Howard VJ, Chambless LE,
Malinow MR, Pettigrew LC, Stampfer M and Toole JF: Vitamin
Intervention for Stroke Prevention (VISP) trial: rationale and
design. Neuroepidemiology. 20:16–25. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chambers JC, Ueland PM, Obeid OA, Wrigley
J, Refsum H and Kooner JS: Improved vascular endothelial function
after oral B vitamins: An effect mediated through reduced
concentrations of free plasma homocysteine. Circulation.
102:2479–2483. 2000. View Article : Google Scholar
|
|
31
|
Title LM, Cummings PM, Giddens K, Genest
JJ Jr and Nassar BA: Effect of folic acid and antioxidant vitamins
on endothelial dysfunction in patients with coronary artery
disease. J Am Coll Cardiol. 36:758–765. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Moens AL, Goovaerts I, Claeys MJ and
Vrints CJ: Flow-mediated vasodilation: a diagnostic instrument, or
an experimental tool? Chest. 127:2254–2263. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yoshida T, Kawano H, Miyamoto S, Motoyama
T, Fukushima H, Hirai N and Ogawa H: Prognostic value of
flow-mediated dilation of the brachial artery in patients with
cardiovascular disease. Intern Med. 45:575–579. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fairfield KM and Fletcher RH: Vitamins for
chronic disease prevention in adults: scientific review. JAMA.
287:3116–3126. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Verhaar MC, Wever RM, Kastelein JJ, van
Dam T, Koomans HA and Rabelink TJ: 5-methyltetrahydrofolate, the
active form of folic acid, restores endothelial function in
familial hypercholesterolemia. Circulation. 97:237–241. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schwammenthal Y and Tanne D: Homocysteine,
B-vitamin supplementation, and stroke prevention: from
observational to interventional trials. Lancet Neurol. 3:493–495.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bazzano LA, Reynolds K, Holder KN and He
J: Effect of folic acid supplementation on risk of cardiovascular
diseases: a meta-analysis of randomized controlled trials. JAMA.
296:2720–2726. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
McRae MP: High-dose folic acid
supplementation effects on endothelial function and blood pressure
in hypertensive patients: a meta-analysis of randomized controlled
clinical trials. J Chiropr Med. 8:15–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dionisio N, Jardín I, Salido GM and Rosado
JA: Homocysteine, intracellular signaling and thrombotic disorders.
Curr Med Chem. 17:3109–3119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Crabtree MJ and Channon KM: Synthesis and
recycling of tetrahydrobiopterin in endothelial function and
vascular disease. Nitric Oxide. 25:81–88. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Smulders YM and Stehouwer CD: Folate
metabolism and cardiovascular disease. Semin Vasc Med. 5:87–97.
2005. View Article : Google Scholar
|
|
42
|
Stroes ES, van Faassen EE, Yo M, Martasek
P, Boer P, Govers R and Rabelink TJ: Folic acid reverts dysfunction
of endothelial nitric oxide synthase. Circ Res. 86:1129–1134. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hyndman ME, Verma S, Rosenfeld RJ,
Anderson TJ and Parsons HG: Interaction of 5-methyltetrahydrofolate
and tetrahydrobiopterin on endothelial function. Am J Physiol Heart
Circ Physiol. 282:H2167–H2172. 2002.PubMed/NCBI
|