Introduction
Sepsis-associated mortality rates have not decreased
despite powerful technical support and development of advanced
treatments. Severe sepsis remains the most common cause of
mortality in intensive care units (ICUs) (1). The mortality rate of severe sepsis is
25–30%, whereas the mortality rate of septic shock at the most
severe phase of sepsis is as high as 40–70% (2). Therefore, identifying septic patients
that may have the worst outcomes is crucial. Certain clinical
indices, including multiple organ dysfunction and high disease risk
score, have been shown to be associated with clinical prognosis
(3); however, the application of
these indices is difficult. Therefore, relevant laboratory
variables are required for sepsis prognosis. In recent years, the
renin-angiotensin system (RAS) has received increasing attention in
the field of sepsis, but the clinical research results on RAS are
inconsistent. Previous studies have shown that RAS may be activated
in patients with severe sepsis (4), which may subsequently result in
ischemic reperfusion injury (5) or
energy metabolic abnormality (6).
RAS antagonists may also be applied for the treatment of sepsis
(7). In addition, a previous study
demonstrated that following the occurrence of sepsis, the
expression of the angiotensin receptor (ATR) was downregulated
(8). Exogenous angiotensin II
(AngII) may be used to enhance urine volume and the creatinine
clearance rate (9), as well as
treat specific patients with septic shock who are insensitive to
norepinephrine (10). Therefore,
the aim of the present study was to monitor the dynamic changes of
RAS in patients with severe sepsis. The significance of RAS in the
prognosis of sepsis was evaluated by comparing the RAS levels in
patients with various clinical outcomes.
Subjects and methods
Subjects
Patients with severe sepsis (including septic shock)
were included in this study. The individuals were admitted to the
ICU of Yantai Mountain Hospital (Yantai, China) between January
2011 and December 2011. All the patients satisfied the diagnostic
criteria of the Conference of Washington in 1992 (11). Sepsis was diagnosed by the presence
of systemic inflammatory reaction syndrome and bacterial infection.
Severe sepsis includes complications such as organ dysfunction or
tissue hypoperfusion. Septic shock is a type of hypotension in
which fluid resuscitation is ineffective. Patients suffering from
septic shock have a systolic blood pressure (SBP) of <90 mmHg (1
mmHg = 0.133 kPa) or a mean arterial pressure of <70 mmHg.
Patients may also exhibit an SBP decrease of >40 mmHg or
reduction of 2 standard deviation based on age that is >2 if no
other evident causes of hypoperfusion are observed. All the
patients in this study were observed within 24 h after the onset of
severe sepsis or septic shock. The study was conducted in
accordance with the Declaration of Helsinki and with approval from
the Ethics Committee of Qilu Hospital of Shandong University
(Yantai, China). Written informed consent was obtained from all
participants.
Exclusion criteria
Patients under the following conditions were
excluded from the study: Patients with chronic renal failure that
had received hemodialysis or ultrafiltration; patients with acute
renal failure that had received urgent hemodialysis; patients with
terminal conditions whose life expectancy was <48 h; patients
who were pregnant or lactating; and patients aged <18 years.
Collection of specimens
Venous blood samples were collected from patients,
who satisfied the diagnostic criteria of severe sepsis, on day 1
(D1) and 3 (D3) after diagnosis. For each sample, the levels of
AngII, angiotensin-converting enzyme (ACE), AngII type 1 receptor
(AT1R) antagonist and AngII type 2 receptor (AT2R) antagonist were
measured, as well as the levels of pro-brain natriuretic peptide
(pro-BNP), troponin T (TNT), C-reactive protein (CRP) and lactate.
Acute Physiology and Chronic Health Evaluation II (APACHE II) and
Sepsis-related Organ Failure Assessment (SOFA) scores were
calculated within the first 24 h. Patient medical and drug usage
history, specifically ACE inhibitor (ACEI) or AngII receptor
antagonist (ARB), were recorded. Observation lasted for 28 days.
Follow-up was conducted via telephone calls for patients who had
left the ICU or hospital prior to day 28.
Treatment principles of severe
sepsis
Upon admission, the patients received crystal
solution or colloid solution within the first 6 h for early
recovery, based on the Surviving Sepsis Campaign Guidelines for
Management of Severe Sepsis and Septic Shock in 2008 (2). Imaging examination was conducted
immediately to detect potential infectious lesions. Within 1 h
following definitive diagnosis of severe sepsis or septic shock,
wide-spectrum antibiotics were administered. If blood pressure
remained <65 mmHg following fluid resuscitation, norepinephrine
or dopamine were jointly administered to stabilize circulation. If
this was unsuccessful in controlling the blood pressure to an ideal
level, a daily dose of 200 mg succinyl hydrocortisone was
administered. For patients with acute lung injury/acute respiratory
distress syndrome (ALI/ARDS), ventilation with a small tidal volume
or inhibition of pause pressure was applied and the management of
blood glucose was enhanced.
Diagnostic criteria for acute kidney
injury (AKI)
Diagnostic criteria for AKI according to the AKI
Network (12) were as follows:
Sudden loss of renal function (within 48 h), which manifests as
absolute elevation of serum creatinine levels to ≥0.3 mg/dl (≥26.4
mmol/l), an increase of serum creatinine levels from the baseline
of ≥50% or decreased urine volume to <0.5 ml/kg/h lasting >6
h.
Diagnostic criteria for ALI/ARDS
Diagnostic criteria for ALI/ARDS, as recommended by
the American Thoracic Society and European Society of Intensive
Care Medicine in 1992 (13), were
as follows: i) Acute onset; ii) Diagnosis of ALI if the arterial
blood oxygen partial pressure/content of oxygen inhalation
(PaO2/FiO2) is ≤300 mmHg (without considering
if the positive end expiratory pressure was used or not) and
diagnosis of ARDS if PaO2/FiO2 is ≤200 mmHg;
iii) X-ray chest film showing infiltrates in both lobes of the
lung; and iv) pulmonary artery wedge pressure ≤2.4 kPa (18 mmHg) or
no clinical evidence of high left atrial pressure.
Testing of the specimens
The method used to detect the levels of AngII, ACE,
AT1R and AT2R was as follows: 2-ml blood samples were collected and
centrifuged at 1,760 × g for 10 min to separate the serum and
erythrocytes. The samples were then stored in a refrigerator at
−80°C. Levels of the variables were determined using an
enzyme-linked immunosorbent assay (Shanghai Yuanye Biotechnology
Co. Ltd., Shanghai, China). Pro-BNP and TNT kits were provided by
Roche R&D Center (Shanghai, China) and the levels of pro-BNP
and TNT were determined using electroluminescence in the clinical
laboratory of our hospital. The levels of CRP (Beckman Coulter
Inc., Miami, FL, USA) and lactate (Radiometer Medical ApS,
Brønshøj, Denmark) were also determined in Yantaishan Hospital. The
CRP kits were provided by Beckman Coulter Inc. The levels of CRP
were determined using scattering immunonephelometry by IMMAGE-800
specific protein detection equipment (Beckman Coulter Inc.) in the
clinical laboratory of Yantaishan Hospital. The lactate kits were
provided by Radiometer Medical (ApS, Brønshøj, Denmark). The levels
of lactate were determined by ABL520 (Radiometer Medical ApS,
Brønshøj, Denmark) in the clinical laboratory of Yantaishan
Hospital. The levels of pro-BNP and TNT were determined by PPE
Roche automatic biochemical immunological analyzer (Mannheim,
Germany).
Statistical analysis
Statistical analysis was performed using SPSS
software version 21.0 (IBM, Armonk, NY, USA). Data are expressed as
the mean ± SD. Normal distributions of measuring materials for the
two groups were compared using the univariate t-test. Measuring
materials not within a normal distribution were converted to an
exponential form and revalidated to identify whether they were in
the normal distribution. If not, the rank-sum test was applied.
Counting materials were compared using the χ2 test.
Intergroup comparison was conducted using univariate analyses. In
the univariate analysis, step-wise selection was used for variables
with P<0.1 to build logistic regression models and to calculate
the odds ratio and 95% confidence intervals for the risk factors
and mortality. Receiver operating characteristic (ROC) curves were
constructed with risk factors as the test variables and mortality
as the state variable. The area under the curve (AUC) was
calculated to evaluate the accuracy of the prognosis forecast.
Models with accuracy of >0.7 were considered to be of clinical
value. P<0.05 was considered to indicate a statistically
significant difference. The variables with prognosis significance
were analyzed to determine the critical value, sensitivity and
specificity.
Results
General information
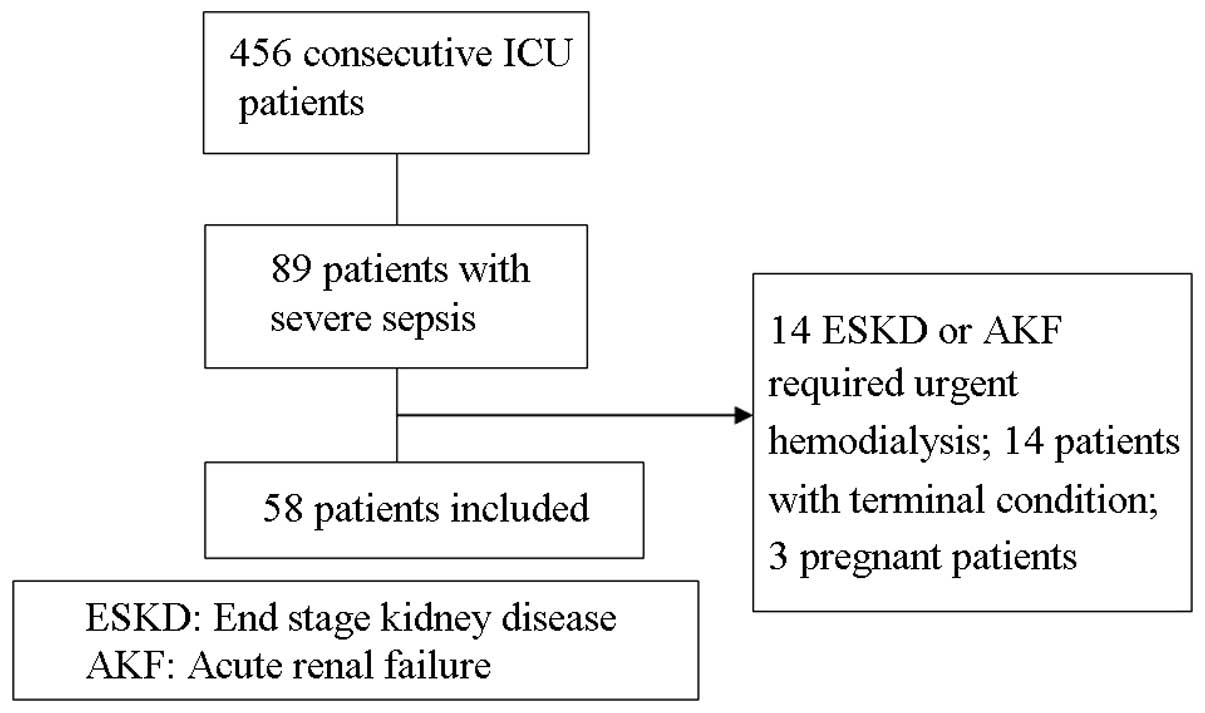
Among the 456 continuous patients admitted to the
ICU, 89 cases were diagnosed with severe sepsis (including septic
shock). These 89 cases included 14 cases of end-stage kidney
disease with long-term hemodialysis or acute renal failure/urgent
hemodialysis or hemofiltration, 14 cases of terminal stage sepsis
(6 cases in which the patient succumbed upon admission and 8 cases
in which the patient succumbed within 48 h) and three pregnant
females. These 31 patients were excluded from the study. Thus, a
total of 58 patients were included in the study as shown in
Fig. 1. The 58 patients had a mean
age of 75 years and 43 patients were male. Thrombosis was the most
common disease, followed by chronic obstructive pulmonary disease
(COPD) and coronary heart disease. Five patients had received ACEI
or ARB prior to admission. On D1 of admission, the mean APACHE II
and SOFA scores were 22.2 and 6.1, respectively. The lung was the
most common infection site. The majority of this group were medical
patients. A total of 50 patients were admitted to the ICU due to
respiratory failure and 34 patients had unstable circulation.
Following admission, 49 patients required mechanical ventilation,
34 patients received pressor agents and 30 patients were
administered cortical hormones. Among the 58 patients, 24 patients
succumbed and 34 patients survived, resulting in a 28-day mortality
rate of 41.3% (Table I).
 | Table IClinical data of patients with severe
sepsis. |
Table I
Clinical data of patients with severe
sepsis.
| Item | Survival group
(n=34) | Mortality group
(n=24) | Total (n=58) | P-value |
|---|
| Male, n (%) | 26 (76.4) | 17 (70.8) | 43 (74.1) | 0.629 |
| Age, years | 69.2±17.5 | 74.6±10.8 | 71.5±15.2 | 0.157 |
| No comorbidity | 4 (11.8) | 0 | 4 (6.9) | 0.082 |
| Comorbidity, n
(%) |
| Cerebral
infarction | 12 (35.3) | 9 (37.5) | 21 (36.2) | 0.863 |
| COPD | 11 (32.4) | 3 (12.5) | 14 (24.1) | 0.082 |
| CHD | 6 (17.6) | 8 (33.3) | 14 (24.1) | 0.169 |
| HTN | 4 (11.8) | 5 (20.8) | 9 (15.5) | 0.347 |
| ACEI/ARB | 3 (8.8) | 2 (8.3) | 5 (8.6) | 0.948 |
| Diabetes
mellitus | 4 (11.8) | 3 (12.5) | 7 (12.1) | 0.933 |
| Pneumoconiosis | 0 | 3 (12.5) | 3 (5.2) | 0.034 |
| APACHE II | 19.8±6.3 | 25.5±6.0 | 22.2±6.7 | 0.001 |
| SOFA | 5.1±2.2 | 7.3±1.7 | 6.1±2.3 | <0.001 |
| Source of infection,
n (%) |
| Pneumonia | 27 (79.4) | 22 (91.7) | 49 (84.5) | 0.204 |
| Urosepsis | 4 (11.8) | 0 | 4 (6.9) | 0.082 |
| Biliary
infection | 1 (2.9) | 2 (8.3) | 3 (5.2) | 0.361 |
| Soft tissue
infection | 2 (5.8) | 0 | 2 (3.4) | 0.227 |
| Multiple foci | 3 (8.8) | 2 (8.3) | 5 (8.6) | 0.948 |
| Treated type, n
(%) |
| Elective
surgery | 1 (2.9) | 0 | 1 (1.7) | 0.397 |
| Emergency
surgery | 5 (14.7) | 2 (8.3) | 7 (12.1) | 0.463 |
| Medical | 28 (82.4) | 22 (91.7) | 50 (86.2) | 0.311 |
| Cause of ICU
admission, n (%) |
| Respiratory
failure | 29 (85.3) | 21 (87.5) | 50 (86.2) | 0.810 |
| Shock | 14 (41.2) | 20 (83.3) | 34 (58.6) | 0.001 |
| Treatment in ICU, n
(%) |
| Mechanical
ventilation | 25 (73.5) | 24 (100) | 49 (84.5) | 0.032 |
| Vasopressor
agents | 14 (41.2) | 20 (83.3) | 34 (58.6) | 0.001 |
| Use of
glucocorticoids | 12 (35.3) | 18 (75) | 30 (51.7) | 0.003 |
| Complication, n
(%) |
| Shock | 14 (41.2) | 20 (83.3) | 34 (58.6) | 0.001 |
| AKI | 11 (32.4) | 17 (70.8) | 28 (48.3) | 0.004 |
| ALI/ARDS | 12 (35.3) | 18 (75) | 30 (51.7) | 0.003 |
Comparison between the survival and
mortality groups
Basic information from the survival and mortality
groups was used for univariate analysis. All the patients in the
mortality group presented with basic diseases, particularly
thrombosis. In the survival group, the most common disease was
chronic obstructive lung disease. Three patients with lung disease
complicated with infection were treated, however all patients
succumbed. APACHE II and SOFA scores were significantly higher in
the mortality group compared with the survival group. The most
common infection site was the lung in the two groups. All patients
with infections in the urinary system or skin soft tissues
survived. In terms of infection in multiple sites, the survival
group had two cases with lung and urinary infections and one case
with lung and biliary infections, whereas the mortality group had
two cases with biliary and lung infections (Table I).
The disease source was not postoperative in either
group and there were no intergroup differences. The major reason
for admission to the ICU was respiratory failure and/or septic
shock. The number of respiratory failures did not differ between
the groups; however, the number of shocks was significantly larger
in the mortality group compared with the survival group. The number
of patients who were administered vasoactive agents and
glucocorticoids was markedly larger in the mortality group compared
with the survival group. With regard to complications, the number
of patients with shock, AKI and ARDS was significantly larger in
the mortality group compared with the survival group.
Univariate analysis
Intergroup comparisons of laboratory variables,
including the levels of AngII on D1 and D3 and ACE on D1, revealed
the variable levels to be significantly higher in the survival
group compared with the mortality group. However, the pro-BNP and
lactic acid levels on D3 were higher in the mortality group
(Table II). Variables that had a
significance value of P<0.1 were included for logistic
regression analysis.
 | Table IILaboratory parameters. |
Table II
Laboratory parameters.
| Item | Survival group
(n=34) | Mortality group
(n=24) | Total (n=58) | P-value |
|---|
| AngII, pg/ml |
| D1 | 91.39±6.04 | 83.29±6.18 | 88.04±7.26 | <0.001 |
| D3 | 72.83±7.53 | 66.23±6.81 | 70.10±7.89 | <0.001 |
| ACE, U/l |
| D1 | 41.45±1.95 | 38.97±1.29 | 40.29±2.02 | <0.001 |
| D3 | 34.30±1.46 | 33.69±1.62 | 34.05±1.54 | 0.160 |
| AT1R, ng/ml |
| D1 | 3.93±0.57 | 3.67±0.47 | 3.82±0.54 | 0.079 |
| D3 | 3.76±0.52 | 3.67±0.37 | 3.72±0.46 | 0.490 |
| AR2R, ng/ml |
| D1 | 4.57±0.72 | 4.20±0.64 | 4.43±0.70 | 0.060 |
| D3 | 4.37±0.72 | 4.26±0.46 | 4.32±0.62 | 0.537 |
| pro-BNP, pg/ml |
| D1 |
4,246.01±9,475.85 |
7,378.27±9,342.36 |
5,691.66±9,454.33 | 0.237 |
| D3 |
2,712.78±4,508.00 |
10,106.44±11,495.76 |
6,215.04±9,227.76 | 0.018 |
| TNT, ng/ml |
| D1 | 0.11±0.23 | 0.11±0.10 | 0.11±0.18 | 0.924 |
| D3 | 0.26±0.49 | 0.20±0.18 | 0.22±0.35 | 0.623 |
| CRP, mg/dl |
| D1 | 95.69±52.28 | 81.84±58.97 | 89.48±55.16 | 0.390 |
| D3 | 71.79±51.39 | 80.74±46.14 | 76.05±48.57 | 0.558 |
| Lac, mmol/l |
| D1 | 1.82±2.67 | 2.52±1.84 | 2.13±2.34 | 0.283 |
| D3 | 1.48±0.69 | 3.90±4.20 | 2.69±3.21 | 0.014 |
Logistic regression analysis
Logistic regression analysis revealed that the
mortality-associated variables included the APACHE II score on D1,
the SOFA score on D1 and high lactic acid levels on D3, as well as
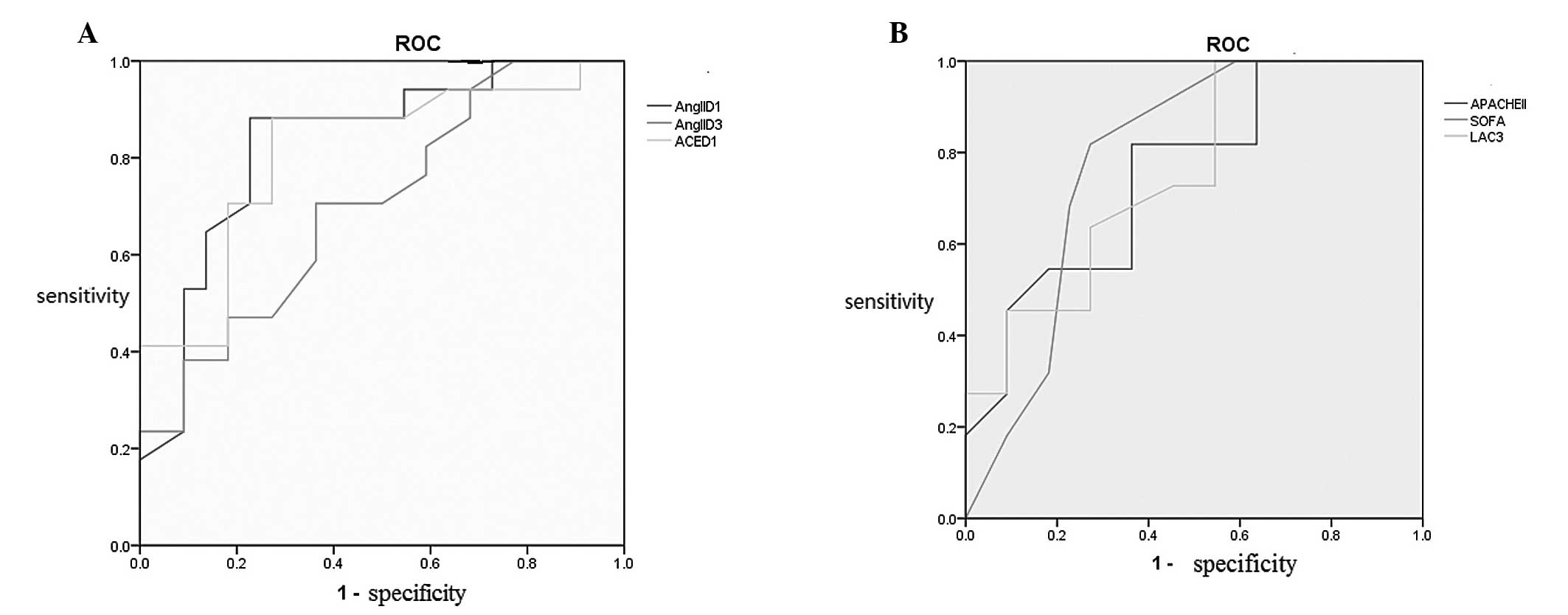
low AngII levels on D1 and D3 and low ACE levels on D1 (Table III). These risk factors,
determined by logistic regression analysis, were used for ROC curve
analysis. APACHE II and SOFA scores on D1 and high lactic acid
levels on D3 were valuable for mortality prediction. In addition,
low AngII levels on D1 and D3, as well as low ACE levels on D1, may
predict poor prognosis (Fig. 2).
Critical values, sensitivity and specificity were calculated for
the variables with an AUROC of >0.7, based on Youden’s index.
The results demonstrated that AngII and ACE levels on D1 had the
highest sensitivity and specificity for the prediction of
mortality, followed by the SOFA score. APACHE II score showed high
sensitivity but low specificity, whereas lactate levels on D3
showed high specificity but low sensitivity (Table IV).
 | Table IIIMultifactor logistic regression
analysis associated with mortality from severe sepsis. |
Table III
Multifactor logistic regression
analysis associated with mortality from severe sepsis.
| Item | B | SE | OR | 95% CI | P-value |
|---|
| AngII (D1) | −0.219 | 0.061 | 0.803 | 0.712–0.905 | 0.001 |
| AngII (D3) | −0.132 | 0.045 | 0.877 | 0.802–0.958 | 0.004 |
| ACE (D1) | −0.804 | 0.236 | 0.448 | 0.282–0.711 | 0.001 |
| Lac (D3) | 1.231 | 0.534 | 3.426 | 1.203–9.757 | 0.021 |
| SOFA (D1) | 0.538 | 0.165 | 1.713 | 1.24–2.367 | 0.001 |
| APACHE II (D1) | 0.153 | 0.054 | 1.166 | 1.050–1.295 | 0.004 |
 | Table IVCritical values, sensitivity and
specificity of the mortality-associated variables. |
Table IV
Critical values, sensitivity and
specificity of the mortality-associated variables.
| Item | Critical
values | Sensitivity
(%) | Specificity
(%) |
|---|
| AngII (D1) | 86.1 | 88.2 | 77.3 |
| ACE (D1) | 39.2 | 88.2 | 72.7 |
| SOFA (D1) | 5.5 | 81.8 | 72.7 |
| AngII (D3) | 67.9 | 70.6 | 63.6 |
| Lac (D3) | 2.3 | 45.5 | 91.1 |
| APACHE II (D1) | 19 | 81.8 | 36.4 |
Discussion
In the present study, 58 cases of severe sepsis
(including septic shock) were analyzed for the detection of RAS
activity-associated, myocardial damage (TNT), pro-BNP, response
tissue perfusion (lactate) and inflammatory (CRP) variables.
Patients were medical patients with a mean age of 75 years. The
major reason for admission into the ICU was due to respiratory
failure or complications caused by septic shock. The lung was the
most common infection site. The majority of patients in the two
groups required mechanical ventilation. With regard to basic
diseases, COPD was common in the survival group, but not in the
mortality group. The major reason for the use of mechanical
ventilation was acute exacerbations of COPD in the survival group
and ARDS in the mortality group. The mean APACHE II score on D1 was
22. The 28-day mortality rate was 41%, which is consistent with the
mortality rate indicated in the Guidelines for Management of Severe
Sepsis and Septic Shock in 2008 (2).
Previous studies have demonstrated that a number of
factors, including age, severity of basic diseases, number of
injured organs/systems, disease severity score, lactic acid levels
and cellular factors, affect the prognosis of severe sepsis and
septic shock (14–18). Among the 58 patients in the present
study, the APACHE II and SOFA scores and disease and organ/system
damage severities were found to be associated with poor prognosis,
which was consistent with previous studies (19,20).
In addition, high lactic acid levels on D3 indicated a high
mortality risk, whereas continuously high lactic acid levels on D3
following early positive treatment and recovery capacity may
indicate a severe condition and high mortality risk.
The present study on RAS variables has demonstrated
that relatively low expression levels of RAS are associated with
poor prognosis. RAS is an important neuroendocrine system. In cases
with insufficient capacity or decrease of blood pressure,
circulating angiotensin I, under the action of ACE, is hydrolyzed
to AngII, which is the primary active peptide in RAS. AngII
functions primarily by combining with ATR to cause systemic
micro-artery contraction and increase peripheral resistance and
blood pressure. AngII may also enhance the release of
norepinephrine from sympathetic nerve endings. Results from
previous studies are controversial. One study demonstrated that
sepsis is likely to result in high expression levels of RAS
(4) and that RAS was involved in
several developmental stages of sepsis. AngII may promote the
synthesis of proinflammatory cell factors and chemokines, aggravate
inflammatory reaction and increase the production of active oxygen
(21). Endotoxin-treated mice
showed high expression levels of RAS, which was associated with
oxidative stress and endodermic dysfunction (22). A number of animal experiments have
shown that RAS antagonists may be used to alleviate inflammatory
reactions in septic animals and protect organ/system functions
(23–25). Therefore, RAS antagonists are
recommended for the treatment of sepsis (7). However, this topic remains
controversial. Escherichia coli endotoxins may inhibit the
activity of renin renal mesangial cells, resulting in low
expression of RAS (26). In sepsis
models, adrenal ATR is expressed in low levels, alleviating the
irritation of AngII to the adrenal gland and thereby resulting in a
decrease in the release of catecholamine and an induction of septic
shock (27). Endotoxins can
deactivate ACE and therefore decrease the levels of AngII (28). From a therapeutic perspective,
Yunge and Petros used exogenous AngII to treat two children under
septic shock who were insensitive to norepinephrine and the
conditions improved (10).
Additional studies have shown that RAS antagonists do not improve
the prognosis of animals under septic shock (29,30).
However, the expression levels of AngII may differ from phase to
phase (31).
The results of the present study showed that the
expression levels of AngII and ACE were low in the mortality group.
This group exhibited complications due to septic shock, thus,
vasoactive agents should be used in combination to maintain blood
pressure. Therefore, patients under septic shock may react slightly
to microcirculatory disturbance. Considering the lack of RAS
excitation, we hypothesize that relatively low levels of AngII
reduce the irritation of the adrenal cortex to release
catecholamine or inhibit the ATR on the surface of adrenal gland,
thereby resulting in relatively less endogenous catecholamine.
Consequently, the body depends on exogenous catecholamines to
maintain blood pressure. Such patients may present downregulated
excitability in other systems, including the sympathetic nervous
system and pituitary-adrenal axis, since the mortality group
require more glucocorticoids for treatment. However, in using this
method, patients are more prone to develop organ damage (high SOFA
score) or have a high risk of mortality (32).
The current results are not entirely consistent with
previous studies due to the following reasons. Firstly, certain
patients with severe sepsis in the present study developed shock,
although others did not. Secondly, differences between the survival
and mortality groups were compared for the first time. However,
previous studies were conducted mostly with animals and
determination of variable levels was mainly performed at certain
time point. Based on the current results, we hypothesize that at
various levels or stages of sepsis, the expression levels of RAS
may differ. Relatively low levels of RAS expression upon onset
demonstrate significance for the poor prognosis of sepsis. However,
the sample size in the present study was small; therefore, future
studies with larger sample sizes are required for further analyses
to support the conclusions.
References
|
1
|
Bone RC: A critical evaluation of new
agents for the treatment of sepsis. JAMA. 266:1686–1691. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hicks P and Cooper DJ; Australian and New
Zealand Intensive Care Society (ANZICS) Board and Clinical Trials
Group Executive Committee. The Surviving Sepsis Campaign:
International guidelines for management of severe sepsis and septic
shock: 2008. Crit Care Resusc. 10:82008.
|
|
3
|
Brun-Buisson C, Doyon F, Carlet J, et al:
Incidence, risk factors, and outcome of severe sepsis and septic
shock in adults. A multicenter prospective study in intensive care
units French ICU Group for Severe Sepsis. JAMA. 274:968–974. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tamion F, Le Cam-Duchez V, Menard JF,
Girault C, Coquerel A and Bonmarchand G: Erythropoietin and renin
as biological markers in critically ill patients. Crit Care.
8:R328–R335. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Higuchi S, Ohtsu H, Suzuki H, Shirai H,
Frank GD and Eguchi S: Angiotensin II signal transduction through
the AT1 receptor: novel insights into mechanisms and
pathophysiology. Clin Sci (Lond). 112:417–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Crouser ED: Mitochondrial dysfunction in
septic shock and multiple organ dysfunction syndrome.
Mitochondrion. 4:729–741. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Salgado DR, Rocco JR, Silva E and Vincent
JL: Modulation of the renin-angiotensin-aldosterone system in
sepsis: a new therapeutic approach? Expert Opin Ther Targets.
14:11–20. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schmidt C, Höcherl K, Kurt B, Moritz S,
Kurtz A and Bucher M: Blockade of multiple but not single cytokines
abrogates downregulation of angiotensin II type-I receptors and
anticipates septic shock. Cytokine. 49:30–38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wan L, Langenberg C, Bellomo R and May CN:
Angiotensin II in experimental hyperdynamic sepsis. Crit Care.
13:R1902009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yunge M and Petros A: Angiotensin for
septic shock unresponsive to noradrenaline. Arch Dis Child.
82:388–389. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
No authors listed. American College of
Chest Physicians/Society of Critical Care Medicine Consensus
Conference: definitions forsepsis and organ failure and guidelines
for the use of innovative therapies in sepsis. Crit Care Med.
20:864–874. 1992. View Article : Google Scholar
|
|
12
|
Mehta RL, Kellum JA, Shah SV, Molitoris
BA, Ronco C, Warnock DG and Levin A; Acute Kidney Injury Network.
Acute Kidney Injury Network: report of an initiative to improve
outcomes in acute kidney injury. Crit Care. 11:R312007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bernard GR, Artigas A, Brigham KL, et al;
The American-European Consensus Conference of ARDS. Definitions,
mechanisms, relevant outcomes, and clinical trial coordination. Am
J Respir Crit Care Med. 149(3 Pt 1): 818–824. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kreger BE, Craven DE and McCabe WR:
Gram-negative bacteremia. IV Re-evaluation of clinical features and
treatment in 612 patients. Am J Med. 68:344–355. 1980.PubMed/NCBI
|
|
15
|
Bone RC, Fischer CJ Jr, Clemmer TP,
Slotman GJ, Metz CA and Balk RA: Sepsis syndrome: a valid clinical
entity. Methylprednisolone Severe Sepsis Study Group. Crit Care
Med. 17:389–393. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sprung CL, Peduzzi PN, Shatney CH, et al:
Impact of encephalopathy on mortality in the sepsis syndrome. Crit
Care Med. 18:801–806. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Calandra T, Baumgartner JD, Grau GE, et
al: Prognostic values of tumor necrosis factor/cachectin,
interleukin-1, interferon-alpha, interferon-gamma in the serum of
patients with septic shock. Swiss-Dutch J5 Immunoglobulin Study
Group. J Infect Dis. 161:982–987. 1990. View Article : Google Scholar
|
|
18
|
Clemmer TP, Fischer CJ Jr, Bone RC,
Slotman GJ, Metz CA and Thomas FO: Hypothermia in the sepsis
syndrome and clinical outcome. The Methylprednisolone Severe Sepsis
Study Group. Crit Care Med. 20:1395–1401. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zabolotskikh IB, Musaeva TS and Denisova
EA: Validity of APACHE II, APACHE III, SAPS 2, SAPS 3 and SOFA
scales in obstetric patients with sepsis. Anesteziol Reanimatol.
Nov–Dec;55–57. 2012.PubMed/NCBI
|
|
20
|
Chen SJ, Chao TF, Chiang MC, Kuo SC, Chen
LY, Yin T, Chen TL and Fung CP: Prediction of patient outcome from
Acinetobacter baumannii bacteremia with Sequential Organ
Failure Assessment (SOFA) and Acute Physiology and Chronic Health
Evaluation (APACHE) II scores. Intern Med. 50:871–877. 2011.
|
|
21
|
Suzuki Y, Ruiz-Ortega M, Lorenzo O,
Ruperez M, Esteban V and Egido J: Inflammation and angiotensin II.
Int J Biochem Cell Biol. 35:881–900. 2003. View Article : Google Scholar
|
|
22
|
Lund DD, Brooks RM, Faraci FM and Heistad
DD: Role of angiotensin II in endothelial dysfunction induced by
lipopolysaccharide in mice. Am J Physiol Heart Circ Physiol.
293:H3726–H3731. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yao S, Feng D, Wu Q, Li K and Wang L:
Losartan attenuates ventilator-induced lung injury. J Surg Res.
145:25–32. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wiel E, Pu Q, Leclerc J, et al: Effects of
the angiotensin-converting enzyme inhibitor perindopril on
endothelial injury and hemostasis in rabbit endotoxic shock.
Intensive Care Med. 30:1652–1659. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hagiwara S, Iwasaka H, Hidaka S, Hasegawa
A, Koga H and Noguchi T: Antagonist of the type-1 ANG II receptor
prevents against LPS-induced septic shock in rats. Intensive Care
Med. 35:1471–1478. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Almeida WS, Maciel TT, Di Marco GS,
Casarini DE, Campos AH and Schor N: Escherichia coli
lipopolysaccharide inhibits renin activity in human mesangial
cells. Kidney Int. 69:974–980. 2006. View Article : Google Scholar
|
|
27
|
Bucher M, Hobbhahn J and Kurtz A: Nitric
oxide-dependent down-regulation of angiotensin II type 2 receptors
during experimental sepsis. Crit Care Med. 29:1750–1755. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dunn CW and Horton JW: Role of angiotensin
II in neonatal sepsis. Circ Shock. 40:144–150. 1993.PubMed/NCBI
|
|
29
|
Graninger M, Marsik C, Dukic T, Wagner OF,
Blann AD and Jilma B: Enalapril does not alter adhesion molecule
levels in human endotoxemia. Shock. 19:448–451. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bexelius TS, Blomberg J, Lu YX, et al:
Losartan to prevent hyperenzymemia after endoscopic retrograde
cholangiopan-creatography: A randomized clinical trial. World J
Gastrointest Endosc. 4:506–512. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dong LW, Chang YZ, Tong LJ, Tang J, Su JY
and Tang CS: Role of regulatory peptide in pathogenesis of shock.
Sci China B. 37:162–169. 1994.PubMed/NCBI
|
|
32
|
Annane D, Sébille V, Troché G, Raphaël JC,
Gajdos P and Bellissant E: A 3-level prognostic classification in
septic shock based on cortisol levels and cortisol response to
corticotropin. JAMA. 283:1038–1045. 2000. View Article : Google Scholar : PubMed/NCBI
|