Introduction
To improve the rate of successful heart
transplantations, organ preservation should be optimized in cardiac
transplantation surgery. However, the functional depression of
cardiac grafts in postoperative recovery is not exceptional and the
vitality of the transplanted tissue depends considerably on
cardioplegic and storage conditions. At present, heart preservation
is limited to 4–6 h of cold ischemic storage (1). Reperfusion injury occurs when there
has been inadequate myocardial protection during the preceding
ischemic period. Cardiac fatty acid and glucose metabolism are
highly regulated processes that meet the majority of myocardial
energetic requirements. Cardiac ischemia reperfusion (I/R) is
characterized by complex alterations in fatty acid and glucose
oxidation that ultimately have a negative impact on cardiac
efficiency and function. Therefore, targeting metabolic events may
be a promising strategy to reduce I/R injury (2).
Ethyl pyruvate (EP) is a key intermediate in the
metabolism of glucose and is a potent reactive oxygen species (ROS)
scavenger, which may promote the release of high-mobility group
protein B1 (HMGB1). EP has been reported to inhibit myocardial
apoptosis and reduce myocardial I/R injury in a variety of in
vitro and in vivo model systems, including our previous
study (3–5). During cardiac surgery and heart
transplantation, cardioplegic arrest is used to protect the
myocardium against the consequences of ischemia (6). When the heart is protected against
ischemic injury by cardioplegic arrest, it is important to
elucidate which additives have cardioprotective effects against I/R
injury in the cardioplegic solutions. However, there are no data
available on the effects of EP on cardiac function and apoptosis
following prolonged cold ischemic conditions, including those used
for heart transplantation. Therefore, it was hypothesized that EP
may provide protection against reperfusion injury following
prolonged hypothermic storage.
In the present study, isolated rat hearts were
prepared similarly to those used for heart transplantation and were
treated with EP before and/or after 4 h of global cold (4°C)
ischemia. Hemodynamic parameters, adenosine triphosphate (ATP)
levels, malondialdehyde (MDA) content and apoptotic cell
determination were studied as the experimental variables. The aim
of the present study was to determine whether the addition of EP to
storage solutions and perfusion reduced the extent of reperfusion
injury in the isolated rat heart.
Materials and methods
Animals
Adult male Wistar rats (weight, 220±30 g) were
provided by the Experimental Animal Center of Tongji Medical
College (Wuhan, China). All animals were treated in accordance with
the Guide for Care and Use of Laboratory Animals published by the
US National Institutes of Health. The study was approved by the
ethics committee of Hubei Medical College (Shiyan, China). EP was
purchased from Sigma-Aldrich Chemie (St Louis, MO, USA).
Model of isolated and perfused working
rat heart
Rats were anesthetized by intraperitoneal
administration of 1 ml/100 g thiopental sodium and intravenous
injection of 500 IU heparin. The chest was opened by bilateral
sternocostal triangle and the hearts were immediately excised and
placed into a cold bath (4°C) containing Krebs-Henseleit buffer
(KHB; 11 mM glucose, 118 mM NaCl, 1.2 mM MgSO4, 25 mM
NaHCO3, 1.2 mM KH2PO4 and 3 mM
CaCl2). Hearts were fixed through the aortic root and
left atrium on the perfusion cannulas of the Langendorff apparatus
and perfused in Langendorff mode for 15 min (stabilization period)
at a constant pressure of 70 cm H2O. KHB was used as a
perfusion medium and saturated with 95% O2 and 5%
CO2 (pH 7.4) at a stable temperature of 37°C. Hearts
with a heart rate of <270 bpm were excluded from the study. At
the end of the stabilization period, the perfusion mode was
switched to the working heart mode for 15 min (WH-mode). The
pressure in the left atrium was maintained at 10 cm H2O
and fluid was ejected through the aortic root against a stable
pressure of 80 cm H2O in the aortic cannula. After 15
min of perfusion in the WH-mode, the heart was arrested using 20 ml
cardioplegic solution (St. Thomas’ solution; modified at 4°C; 114
mM Na+, 2 mM Ca2+, 20 mM K+, 203
mM Cl− and 16 mM Mg2+) injected via the
aortic cannula deviation under a pressure of 60 cm H2O.
Hearts were disconnected from the circuit, immersed in storage
solution (solution B21; 132.2 Na+, 0.9 Ca2+,
4 K+, 108.4 Cl−4 and 27.6 mM lactate) and
stored in a cold box (4°C) for 4 h. After 4 h of cold ischemia,
reperfusion at 37°C in the Langendorff mode for 15 min was
established to stabilize the basic recovery conditions before the
mode was switched to the WH-mode for 30 min. Throughout each
experiment cardiac parameters, including the heart rate (HR), left
ventricular systolic pressure (LVSP), left ventricular
end-diastolic pressure (LVEDP), left ventricular developed pressure
(LVDP = LVSP-LVEDP) and maximal rise rate of left ventricular
pressure (+dp/dtmax, −dp/dtmax), were
continuously monitored and recorded using a data acquisition system
(PowerLab/8S; ADInstruments, Bella Vista, Australia). Coronary flow
(CF) was measured by timed collection of the coronary effluent
draining from the pulmonary artery cannula and was used as an index
of vascular diastolic function. At the end of reperfusion, the left
ventricle was quickly removed and stored in liquid nitrogen for
additional assays.
Protocols of perfusion
Two experimental groups were evaluated; the control
(n=8) and EP groups (n=8). The hearts of the EP group received 2 mM
EP, as described previously (7).
The two groups underwent the same protocol with the exception that
EP was added to the cardioplegic and storage solutions during
ischemia and added to the KHB solution during reperfusion in the EP
group.
Measurement of myocardial ATP levels
ATP levels were quantified using the commercially
available ENLITEN® ATP Assay System (Promega Corp.,
Madison, WI, USA). At the end of reperfusion, the myocardial tissue
specimens were immediately frozen in liquid nitrogen and
individually pulverized into a fine powder by hand grinding with a
dry ice-chilled steel mortar and pestle (8). Myocardium samples (10 mg) were
homogenized with 1 ml precooled extractant (0.1% trichloroacetic
acid) and centrifuged at 680 × g for 10 min (9). Supernatant (100 μl) was diluted
10-fold with 50 mmol/l Tris-acetate buffer containing 2 mmol/l EDTA
(pH 7.75). Next, 100 μl sample extract or reference standard
solution was placed in a tube luminometer (Turner Designs
Luminometer TD-20/20; Promega Corp.), which was followed by the
auto-injection of 100 μl ATP luciferin/luciferase assay mix for ATP
quantification. Luminescence was measured at a set lag time of 1
sec and integration time of 10 sec.
Measurement of myocardial MDA levels
The MDA assay method, as described by Yagi in 1976
(10), was designed to estimate
the extent of oxidative damage. Heart bioptic samples (500 μl) were
homogenized with 1 ml phosphate-buffered saline (PBS; 15 mM
Na+ and 145 mM K+; pH 7) at 4°C and incubated
with 1.5 ml thiobarbituric acid-reactive substances (TBARS). TBARS
contained thiobarbituric acid (13.5 g; Sigma, St Louis, MO, USA),
trichloracetic acid (TCA; 0.33 g) and hydrochloric acid (HCl; 8.5
ml) in 100 ml distilled water. Successive procedures included: i)
Heating at 100°C for 15 min; ii) cooling and the addition of 1 ml
TCA (70%); and iii) incubation for 20 min. Centrifugation was
performed at 300 × g for 10 min. The MDA concentration was
determined using a spectrophotometer (PerkinElmer LS-5;
PerkinElmer, Inc., Norwalk, CT, USA) at 515 nm excitation and 535
nm emission. Results were expressed as μmol/g protein.
Determination of myocardial apoptotic
cells
Frozen sections from the left ventricle (5-μm thick)
were fixed with 4% paraformaldehyde solution. To detect the extent
of DNA degradation, the terminal deoxynucleotidyl transferase
(TdT)-mediated biotin-dUTP nick-end labeling method was performed
(In situ Cell Death Detection Kit, POD; Boehringer Ingelheim
GmbH, Mannheim, Germany). Slides were incubated with proteinase (20
μg/ml in 10 mM Tris-HCl) for 20 min at room temperature (pH
7.4–8.0). Next, the slides were rinsed with PBS-blocking solution
and incubated with permeabilization solution (0.1% Triton X-100 in
0.1% sodium citrate) for 2 min at 4°C. Following several washes
with PBS, the samples were incubated with TdT and detection buffer
conjugated with horse-radish peroxidase (Converter-POD) in a
humidified chamber at 37°C for 60 min. For visualization, a
diamino-benzidin-chromogen (Boehringer Ingelheim GmbH) was used and
counterstaining with hematoxylin and eosin was performed. All
experiments were performed according to the manufacturer’s
instructions. To analyze the apoptotic cells, a light microscope
was used (magnification, ×200). The apotosis index (AI) was
calculated using the following formula: (Number of apoptotic
cells/total number of cells counted) ×100. Quantitative analysis
was performed by counting the cells in a randomly selected area of
each tissue sample.
Statistical analysis
All data are expressed as the mean ± SD. Analysis of
variance with Tukey’s test was used to perform statistical
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Cardiac function parameters
There were no significant differences in the
functional parameters between the control and EP groups in the
period of pre-ischemia (Table I).
The functional parameters, including LVDP, +LVdp/dtmax,
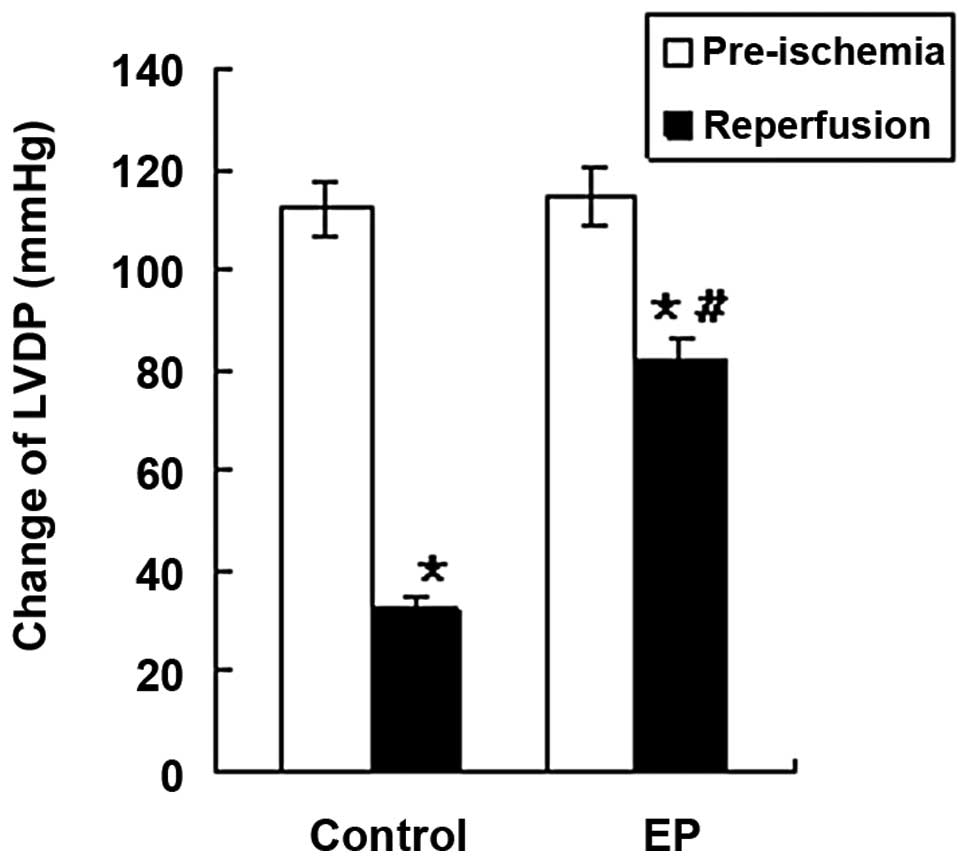
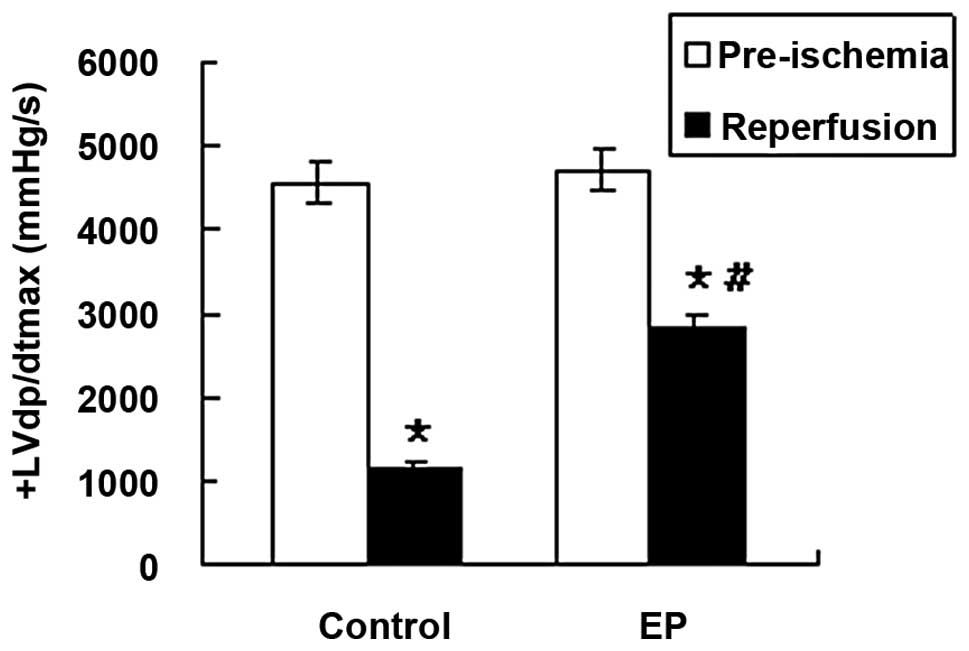
−LVdp/dtmax and CF, decreased significantly in the
control and EP groups during reperfusion (P<0.05; Table I, Figs. 1 and 2), indicating the damaging effect of I/R
on left ventricular function. The rats in the EP group exhibited a
better recovery during reperfusion following ischemia than that of
the control group. The functional parameters in the EP group were
significantly higher compared with those in the control group
during the reperfusion time (P<0.05; Table I, Figs. 1 and 2). No significant differences in HR were
observed in all the rat hearts (Table
I). Thus, the results indicated that EP increased the tolerance
of the hearts to I/R injury.
 | Table IHemodynamic variables. |
Table I
Hemodynamic variables.
| Variables | Control group
(n=8) | EP group (n=8) |
|---|
| Pre-ischemia |
| LVDP (mmHg) | 112.3±14.2 | 114.6±12.1 |
| LVEDP (mmHg) | 12.4±1.8 | 11.8±1.6 |
| +LV
dp/dtmax (mmHg/sec) | 4 562±574 | 4 727±548 |
| −LV
dp/dtmax (mmHg/sec) | −2 548±316 | −2 436±280 |
| HR (beats/min) | 223±26 | 228±24 |
| CF (ml/min) | 12.7±1.8 | 12.5±1.6 |
| Reperfusion |
| LVDP (mmHg) | 32.7±5.1a | 82.4±7.5a,b |
| LVEDP (mmHg) | 65.7±8.3a | 30.3±4.5a,b |
| +LV
dp/dtmax (mmHg/sec) | 1 175±153a | 2845±367a,b |
| −LV
dp/dtmax (mmHg/sec) | −786±104a | −1425±164a,b |
| HR
(beats/min) | 232±56 | 228±53 |
| CF (ml/min) | 3.8±0.5a | 8.2±1.0a,b |
Myocardial ATP and MDA levels
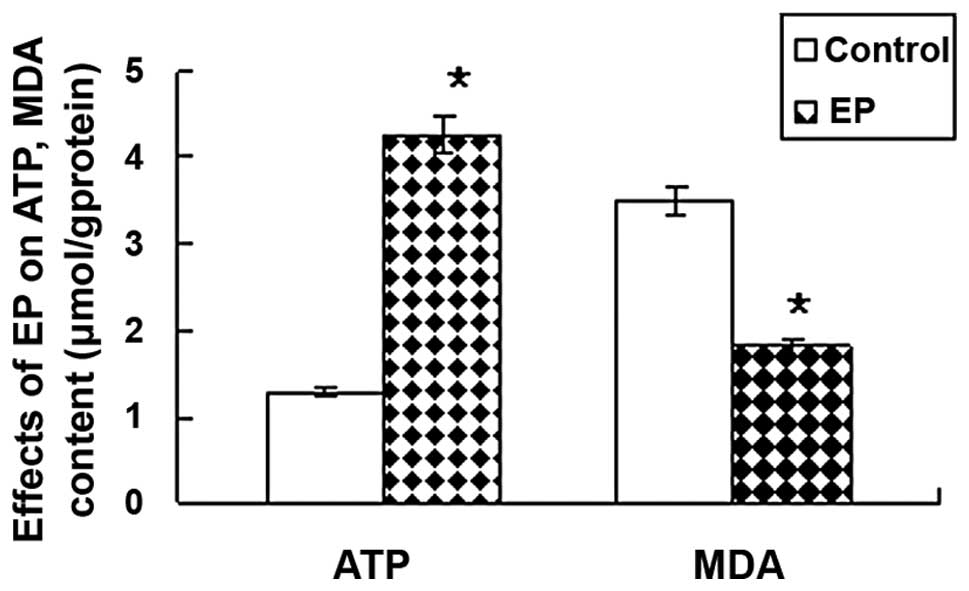
As shown in Table
II, the levels of ATP (4.26±0.43 μmol/g protein) were
significantly higher in the EP group than in the control group
(1.28±0.17 μmol/g protein). The content of MDA was lower in the EP
group (1.8±0.3 μmol/g protein) compared with the control group
(3.5±0.5 μmol/g protein; P<0.05; Fig. 3).
 | Table IIMeasurement of ATP, MDA content and
AI. |
Table II
Measurement of ATP, MDA content and
AI.
| Group | ATP (μmol/g
protein) | MDA (μmol/g
protein) | AI (%) |
|---|
| Control | 1.28±0.17 | 3.5±0.5 | 6.8±1.6 |
| EP | 4.26±0.43a | 1.8±0.3a | 3.1±1.2a |
Apoptotic myocardial cells
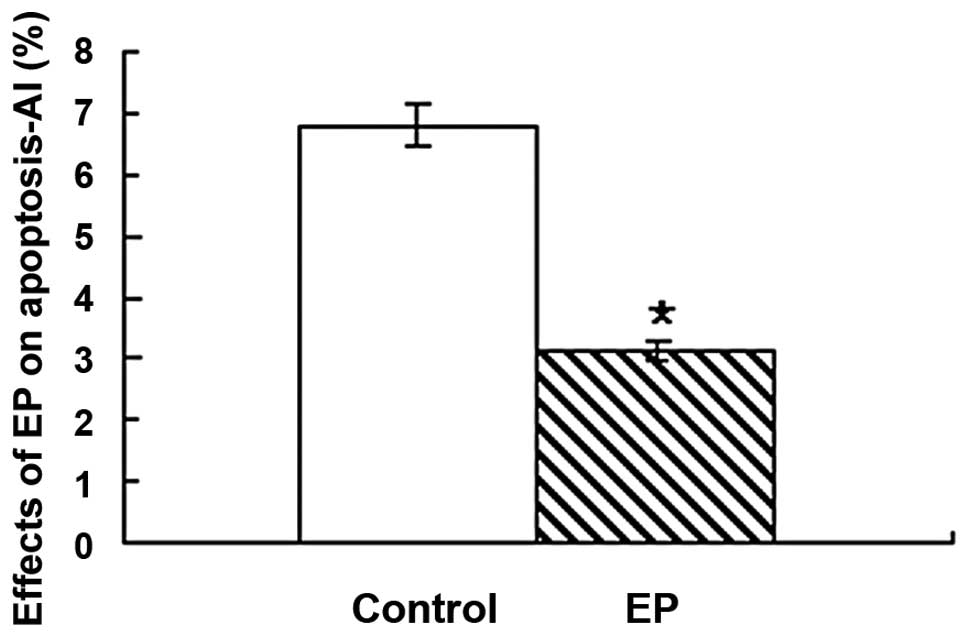
Administration of 2 mM EP significantly reduced the
number of apoptotic cells in the EP group (3.1±1.2%) when compared
with the control group (6.8±1.6%; P<0.05; Table I and Fig. 4).
Discussion
I/R injury of the myocardium is a significant entity
in heart transplantation. Although numerous attempts to study the
molecular interactions and elucidate the onset and time course of
the functional alterations concerning I/R have been made in the
previous two decades, the mechanisms of I/R remain unclear.
Myocardial dysfunction and cellular injury occurs due to metabolic
depletion during ischemia followed by ROS formation during
reperfusion. Significant research efforts have investigated
techniques of protecting the myocardium against I/R injury. The
present study utilized EP as a myocardial protection agent and
administered EP to isolated rat hearts and evaluated a possible
role of EP in promoting cardiac function and preventing apoptosis.
To the best of our knowledge, this study was the first to analyze
the effects of EP in a cardiovascular model of 4 h of cold
cardioplegia and reperfusion to mimic heart preservation in
clinical heart transplantations.
EP parent compound, the glycolytic product pyruvate,
is a natural metabolic fuel and antioxidant in the myocardium and
other tissues, that exerts a variety of cardioprotective actions
when provided at supraphysiological concentrations. Pyruvate
increases the cardiac contractile performance and myocardial energy
state, bolsters endogenous antioxidant systems and has been shown
to attenuate myocardial ischemic injury through metabolic
augmentation and antioxidant mechanisms. However, pyruvate is
limited as a potential therapeutic agent due to extreme aqueous
instability (11–13). EP is an ester derivative of
pyruvate that is used as a food preservative and is highly stable
in calcium containing solutions (14). A previous study demonstrated the
ability of EP to enhance ATP levels, attenuate oxidative stress and
preserve myocardial function in a model of prolonged myocardial I/R
injury (15). EP has subsequently
been studied in trauma, organ protection, critical care literature
with models of organ ischemia, hemorrhagic shock and endotoxemic
sepsis, all demonstrating a cytoprotective effect (16–20).
An additional postulated role of EP is associated with its
anti-inflammatory properties. In vitro, EP appears to
directly inhibit nuclear factor-κB (NF-κB) and p38
mitogen-activated protein kinase pathways of inflammatory cytokine
activation (21). Recently, Jang
et al (3) reported that EP
has the ability to inhibit neutrophil activation, inflammatory
cytokine release and NF-κB translocation, which is associated with
delayed myocardial protective effects following regional I/R injury
in an in vivo rat heart model. In addition, Hu et al
(5) reported that EP reduced
myocardial I/R injury by inhibiting HMGB1 in rats.
In the present study, the addition of EP
significantly prevented post-I/R injury and promoted cardiac
function recovery in isolated rat hearts following 4 h of global
cold I/R. A previous study demonstrated that EP scavenges the
hydroxyl radical (•OH) and the effects are
dose-dependent (22). These
results may explain the better recovery of cardiac function with
administration of 2 mM EP to the perfusion and storage solutions
following cold global ischemia, since •OH is considered
to be the most cytotoxic oxygen free radical. ROS at reflow
following ischemia may increase peroxidation of mitochondrial
membranes and metabolic enzyme activities to prevent the recovery
of heart metabolism and functional parameters. The decrease of free
oxygen radical toxicity by free radical scavengers at the time of
reflow improves the recovery of high-energy phosphate contents,
indicating an association between oxygen free radical production
and the impairment of myocardial energy metabolism during
reperfusion (23).
The second mechanism of the protective action of EP
in the heart may involve its own metabolism (24). EP is an important component of the
energy chain in mitochondria and may restore oxidative metabolism.
Furthermore, EP has a low molecular weight, which provides enough
mobility to penetrate into cellular compartments, including the
mitochondrial cytosol. It is possible that the protective effects
are associated with other oxidizable energy substrates such as
glucose (25).
To confirm the proposed mechanism of action of EP
attributed to glycolytic substrate augmentation, tissue ATP levels
were assayed. Excess exogenous pyruvate may liberate nicotinamide
adenine dinucleotide and increase the proximal glycolytic pathway
generation of ATP. Myocardial oxidative injury was diminished with
EP. Compared with other inferential assays of free radical injury,
including measuring MDA levels, the lipid peroxidation assay is a
direct measure of free radical tissue injury. The reduction in
lipid peroxidation in the EP group, when compared with that the
control group, is an indication of reduced free radical injury.
A possible involvement of ROS in pathways promoting
apoptosis is now widely accepted (26). Mitochondria and redox-state changes
appear to have a predominant role in the promotion and expression
of apoptosis (27). The
intracellular changes during ischemia and reperfusion, including
the accumulation of H+ and Ca2+, as well as
the disruption of the mitochondrial membrane potential, result in
the formation of free radicals or ROS. ROS accumulation and the
subsequent activation of proinflammatory pathways are important in
I/R injury (28). The resulting
disturbances of metabolic processes can endanger cell existence due
to the promotion of programmed cell death. The present study
confirmed the presence of an increased number of apoptotic cells in
a cold I/R injury model of isolated rat hearts. EP appeared to
effectively reduce the extent of apoptosis similarly to other
cardioprotective agents, including deferoxamine (29) and carvedilol (30). However, the precise mechanism that
accounts for the reduction of apoptosis by EP requires further
study.
A concentration of 2 mM EP was selected in the
current study as previous studies with a Langendorff model had
demonstrated that this concentration did not affect the basic
cardiac function, but was capable of inhibiting the apoptosis of
cardiac myocytes (4). The timing
of EP administration was designed to enhance the two purported
mechanisms of action, glycolytic substrate augmentation and
antioxidation. However, further studies are warranted. Future
investigations should evaluate the myocardial protective capacity
of EP in other models of myocardial ischemia as a means of
broadening the spectrum of clinical utility. To closely mimic the
typical ischemia that occurs during heart transplantation, an in
vivo animal model should be engaged to appraise the protective
effects of EP in the future. In addition, future studies should
determine whether using higher doses of EP yields even greater
myocardial protective effects. At present, only a limited
dose-response curve of three concentrations of EP spanning 3 logs
has been studied (31). Alternate
routes of administration, particularly intracoronary, should also
be evaluated to search for increased efficacy.
In conclusion, EP significantly preserves cardiac
function, enhances tissue ATP levels, attenuates myocardial
oxidative injury and markedly reduces apoptosis following
myocardial ischemia, as shown in a cardiovascular model of 4 h of
cold cardioplegia and reperfusion.
Acknowledgements
This study was supported by a grant from the Natural
Science Foundation of Hubei Province (no. 2011CDC051).
References
|
1
|
Stringham JC, Southard JH, Hegge J,
Triemstra L, Fields BL and Belzer FO: Limitations of heart
preservation by cold storage. Transplantation. 53:287–294. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Frank A, Bonney M, Bonney S, et al:
Myocardial ischemia reperfusion injury: from basic science to
clinical bedside. Semin Cardiothorac Vasc Anesth. 16:123–132. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jang IS, Park MY, Shin IW, Sohn JT, Lee HK
and Chung YK: Ethyl pyruvate has anti-inflammatory and delayed
myocardial protective effects after regional ischemia/reperfusion
injury. Yonsei Med J. 51:838–844. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo J, Zhang K, Ji Y, et al: Effects of
ethyl pyruvate on myocardial apoptosis and expression of Bcl-2 and
Bax proteins after ischemia-reperfusion in rats. J Huazhong Univ
Sci Technolog Med Sci. 28:281–283. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu X, Cui B, Zhou X, et al: Ethyl pyruvate
reduces myocardial ischemia and reperfusion injury by inhibiting
high mobility group box 1 protein in rats. Mol Biol Rep.
39:227–231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jahania MS, Sanchez JA, Narayan P, Lasley
RD and Mentzer RM Jr: Heart preservation for transplantation:
principles and strategies. Ann Thorac Surg. 68:1983–1987. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
DeBoer LW, Bekx PA, Han L and Steinke L:
Pyruvate enhances recovery of rat hearts after ischemia and
reperfusion by preventing free radical generation. Am J Physiol.
265:H1571–H1576. 1993.PubMed/NCBI
|
|
8
|
Manthorpe M, Cornefert-Jensen F, Hartikka
J, Felgner J, Rundell A, Margalith M and Dwarki V: Gene therapy by
intramuscular injection of plasmid DNA: studies on firefly
luciferase gene expression in mice. Hum Gene Ther. 4:419–431. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stanley PE: Extraction of adenosine
triphosphate from microbial and somatic cells. Methods Enzymol.
133:14–22. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yagi K: A simple fluorometric assay for
lipoperoxide in blood plasma. Biochem Med. 15:212–216. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ochiai K, Zhang J, Gong G, Zhang Y, Liu J,
Ye Y, Wu X, Liu H, Murakami Y, Bache RJ, Ugurbil K and From AH:
Effects of augmented delivery of pyruvate on myocardial high-energy
phosphate metabolism at high workstate. Am J Physiol Heart Circ
Physiol. 281:H1823–H1832. 2001.PubMed/NCBI
|
|
12
|
Mallet RT: Pyruvate: metabolic protector
of cardiac performance. Proc Soc Exp Biol Med. 223:136–148. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Olivencia-Yurvati AH, Blair JL, Baig M and
Mallet RT: Pyruvate-enhanced cardioprotection during surgery with
cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 17:715–720.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fink MP: Ethyl pyruvate: a novel
anti-inflammatory agent. J Intern Med. 261:349–362. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mallet RT, Sun J, Knott EM, Sharma AB and
Olivencia-Yurvati AH: Metabolic cardioprotection by pyruvate:
recent progress. Exp Biol Med (Maywood). 230:435–443.
2005.PubMed/NCBI
|
|
16
|
Aneja R and Fink MP: Promising therapeutic
agents for sepsis. Trends Microbiol. 15:31–37. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cruz RJ Jr, Harada T, Sasatomi E and Fink
MP: Effects of ethyl pyruvate and other α-keto carboxylic acid
derivatives in a rat model of multivisceral ischemia and
reperfusion. J Surg Res. 165:151–157. 2011.
|
|
18
|
Wang Y, Li B, Li Z, Huang S, Wang J and
Sun R: Improvement of hypoxia-ischemia-induced white matter injury
in immature rat brain by ethyl pyruvate. Neurochem Res. 38:742–752.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kung CW, Lee YM, Cheng PY, Peng YJ and Yen
MH: Ethyl pyruvate reduces acute lung injury via regulation of iNOS
and HO-1 expression in endotoxemic rats. J Surg Res. 167:323–331.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Akkoc H, Kelle I, Tunik S, Bahceci S,
Sencar L, Ayaz E, Nergiz Y, Erdinc L and Erdinc M: Protective
effect of ethyl pyruvate on liver injury in streptozotocin-induced
diabetic rats. Acta Gastroenterol Belg. 75:336–341. 2012.PubMed/NCBI
|
|
21
|
Han Y, Englert JA, Yang R, Delude RL and
Fink MP: Ethyl pyruvate inhibits nuclear factor-kappaB-dependent
signaling by directly targeting p65. J Pharmacol Exp Ther.
312:1097–1105. 2005. View Article : Google Scholar
|
|
22
|
Uchiyama T, Delude RL and Fink MP:
Dose-dependent effects of ethyl pyruvate in mice subjected to
mesenteric ischemia and reperfusion. Intensive Care Med.
29:2050–2058. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Raedschelders K, Ansley DM and Chen DD:
The cellular and molecular origin of reactive oxygen species
generation during myocardial ischemia and reperfusion. Pharmacol
Ther. 133:230–255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kao KK and Fink MP: The biochemical basis
for the anti-inflammatory and cytoprotective actions of ethyl
pyruvate and related compounds. Biochem Pharmacol. 80:151–159.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fink MP: Ethyl pyruvate. Curr Opin
Anaesthesiol. 21:160–167. 2008. View Article : Google Scholar
|
|
26
|
Sinha K, Das J, Pal PB and Sil PC:
Oxidative stress: the mitochondria-dependent and
mitochondria-independent pathways of apoptosis. Arch Toxicol.
87:1157–1180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Handy DE and Loscalzo J: Redox regulation
of mitochondrial function. Antioxid Redox Signal. 16:1323–1367.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Frank A, Bonney M, Bonney S, Weitzel L,
Koeppen M and Eckle T: Myocardial ischemia reperfusion injury: from
basic science to clinical bedside. Semin Cardiothorac Vasc Anesth.
16:123–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dobsák P, Siegelova J, Wolf JE, Rochette
L, Eicher JC, Vasku J, Kuchtickova S and Horky M: Prevention of
apoptosis by deferoxamine during 4 hours of cold cardioplegia and
reperfusion: in vitro study of isolated working rat heart model.
Pathophysiology. 9:272002.PubMed/NCBI
|
|
30
|
Yue TL, Ma XL, Wang X, Romanic AM, Liu GL,
Louden C, Gu JL, Kumar S, Poste G, Ruffolo RR Jr and Feuerstein GZ:
Possible involvement of stress-activated protein kinase signaling
pathway and Fas receptor expression in prevention of
ischemia/reperfusion-induced cardiomyocyte apoptosis by carvedilol.
Circ Res. 82:166–74. 1998. View Article : Google Scholar
|
|
31
|
Martin BJ, Valdivia HH, Bünger R, Lasley
RD and Mentzer RM Jr: Pyruvate augments calcium transients and cell
shortening in rat ventricular myocytes. Am J Physiol. 274:H8–H17.
1998.PubMed/NCBI
|