Introduction
Damaged articular cartilage has a poor capability
for self-repair due to the low cell density (1–3).
Traditional surgical treatments for cartilage defects are
unsatisfactory, and are predominantly hampered by limited resources
(2,4). Showing significant promise as viable
options, tissue-engineering strategies have been widely studied for
cartilage restoration (5).
Scaffolds play a crucial role in cartilage tissue engineering. It
has been reported that scaffolds that have the ability to induce
chondrogenesis are preferably for use in the repair of cartilage
defects (6). In a previous study,
it was shown that the chondrogenic differentiation of bone marrow
mesenchymal stem cells (BMSCs) may be induced by collagen-based
hydrogels in vivo (7).
However, due to limitations in the variety of
material types that have been investigated, the induction of
chondrogenesis by a material alone remains disputable. Whether the
induction is material-driven or self-differentiation requires full
investigation. Furthermore, if the induction is triggered by a
material, the question of whether it is material-dependent must
also be considered. In the present study, certain types of material
that are different from the previously tested hydrogels, namely, a
biphasic calcium phosphate ceramic (BCP), silk fibroin protein
matrix (SFP) and collagen sponge (CS), were applied to further
investigate chondrogenic induction in vivo in order to
evaluate the significance of the material in chondrogenesis.
Materials and methods
Preparation of materials and diffusion
chamber
The in vivo model used to study the
chondrogenic effects of the porous materials was as described
previously (2). BCP, SFP and CS
were generous gifts from Dr B. Li and from Suzhou University
(Suzhou, China), respectively.
The diffusion chamber was made of ultra-high
molecular weight polyethylene, with an outer diameter of 10 mm,
inner diameter of 6 mm and a height of 5 mm. Each chamber was
sealed with a membrane filter (pore size, 0.22 μm) using an
adhesive sealant. Prior to the experiment, only one side of each
chamber was closed. The chambers were sterilized with
γ-irradiation.
BMSC isolation and culture
Under sterile conditions, newborn rabbits were
sacrificed by an overdose of pentobarbital. The bilateral femurs
were dissected with the proximal and distal ends snipped off. The
bone marrow tissue was flushed out using a 1-ml sterile syringe
with minimum essential medium Eagle α modification (α-MEM;
Gibco-BRL, Gaithersburg, MD, USA) containing 10% fetal calf serum
and antibiotics (100 U/ml penicillin and 100 U/ml streptomycin).
Following centrifugation at 800 rpm for 5 min, the cell pellets
were collected and resuspended in fresh culture medium. Cells
(1×105) were cultured in a 25-cm2 culture
flask at 37°C and 5% CO2. The culture medium was changed
every two or three days. The cells of the third passages were
harvested for seeding in the scaffold.
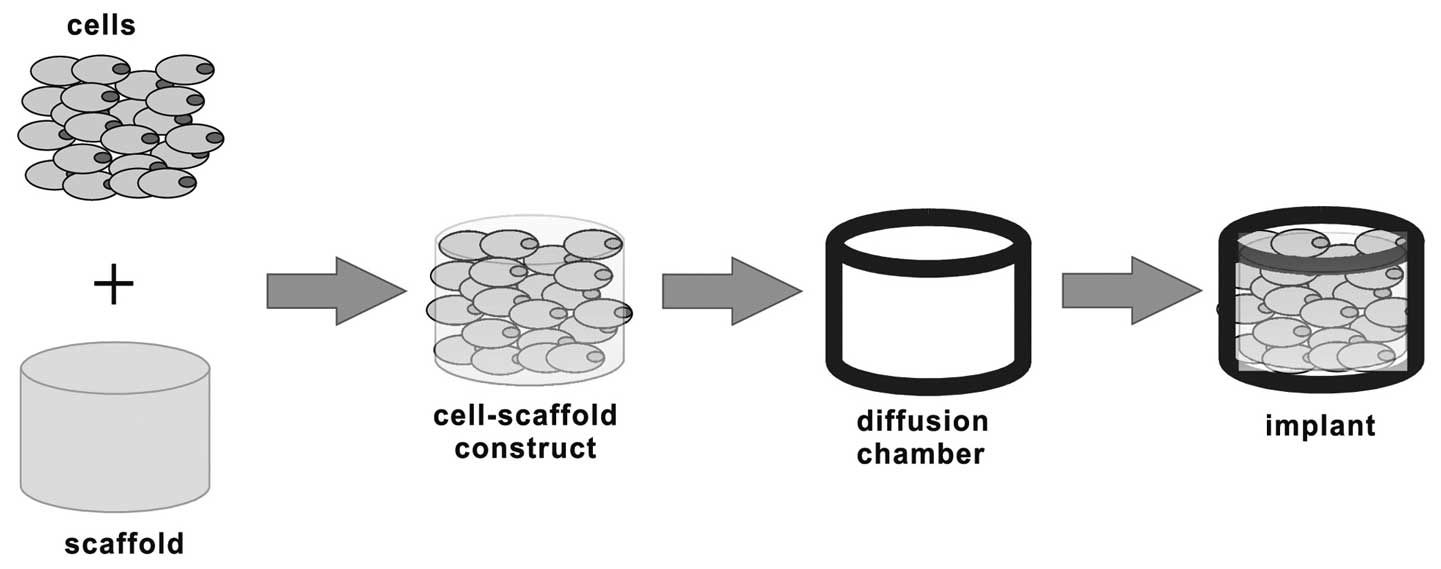
Cell seeding experiment
The samples for implantation were prepared as shown
in Fig. 1. Briefly, when the cells
of the third passage reached 80–90% confluency, they were
trypsinized using 0.25% trypsin and counted using a hemocytometer.
Cell viability was confirmed to be >95% prior to encapsulation.
Following centrifugation, the cell pellets were resuspended and
seeded in BCP, SFP and CS with a cell density of 2×107
cells/cm3. Subsequently, the cell-scaffold composites
were enclosed in the diffusion chambers with the previously open
side sealed by a membrane filter. The filters on the two sides
allow body fluid to pass through while preventing host
invasion.
In vivo experiment
The diffusion chambers carrying the cell-scaffold
composites were surgically inserted subcutaneously into a pocket in
the dorsa of six-month-old rabbits under sodium barbital
anesthesia. The study was conducted in accordance with the US
guidelines for laboratory animal use and care (National Institutes
of Health publication no. 85-23, revised in 1985). Prior to
implantation, leakage of the chambers was carefully checked for and
the chambers that failed the check were discarded. Following
surgery, the wounds were carefully rinsed with 0.9% saline solution
and closed with sutures. All rabbits received ampicillin for two
consecutive postoperative days.
Histological and immunohistochemical
staining
Eight weeks after implantation, the specimens in the
chamber were harvested for histological and immunohistochemical
analyses. The rabbits were sacrificed by an intravenous injection
of euthanasia solution, and the scaffold-cell constructs inside the
chambers were removed for analysis. Breakage of the chamber was
checked for once more. One section of the harvested tissue from the
chamber was fixed immediately in 10% aqueous formalin solution
overnight, and then dehydrated in a gradient ethanol series,
embedded in paraffin and sectioned (5-μm thick). The cross-sections
were stained with hematoxylin and eosin (H&E), safranine-O and
toluidine blue. The type II collagen was immunohistochemically
stained using a monoclonal antibody to type II collagen (no.
AF5710; Acris Antibodies GmbH, Hereford, Germany) according to the
manufacturer’s instructions.
Results
Macroscopic observations
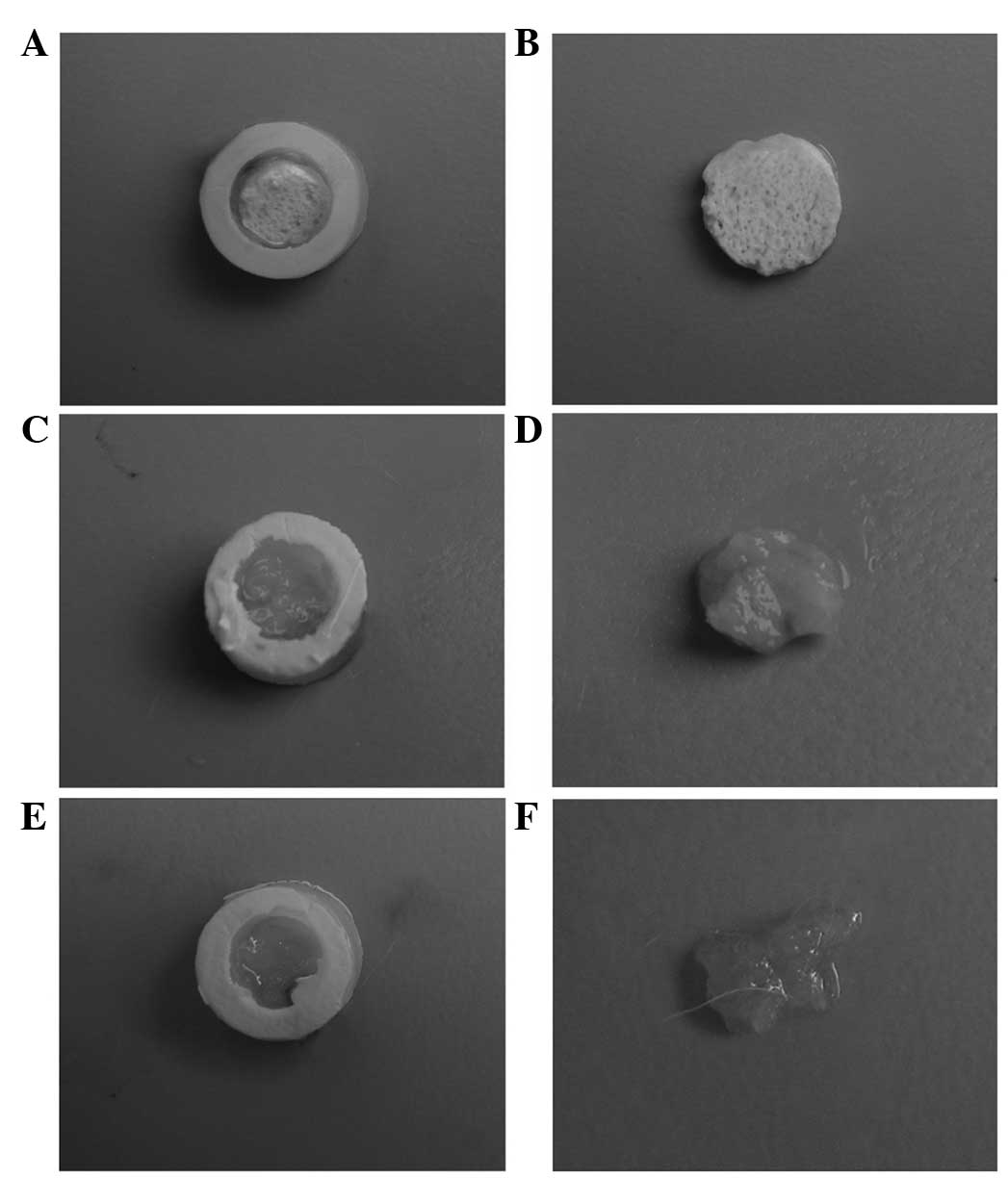
Eight weeks after implantation, the scaffold/cell
constructs inside the chambers were harvested. No leakage of the
chamber and no vascular invasion were observed for any of the
harvested samples, indicating the effectiveness of the diffusion
chamber in avoiding a host reaction. An image of a representative
sample from each group is shown in Fig. 2. In the BCP and SFP groups, a thin,
fibrous tissue, rather than cartilage-like tissue, was formed at
the surface of the scaffold. In the CS group, the constructs had
almost collapsed and no tissue was observed.
Histological and immunohistochemical
staining
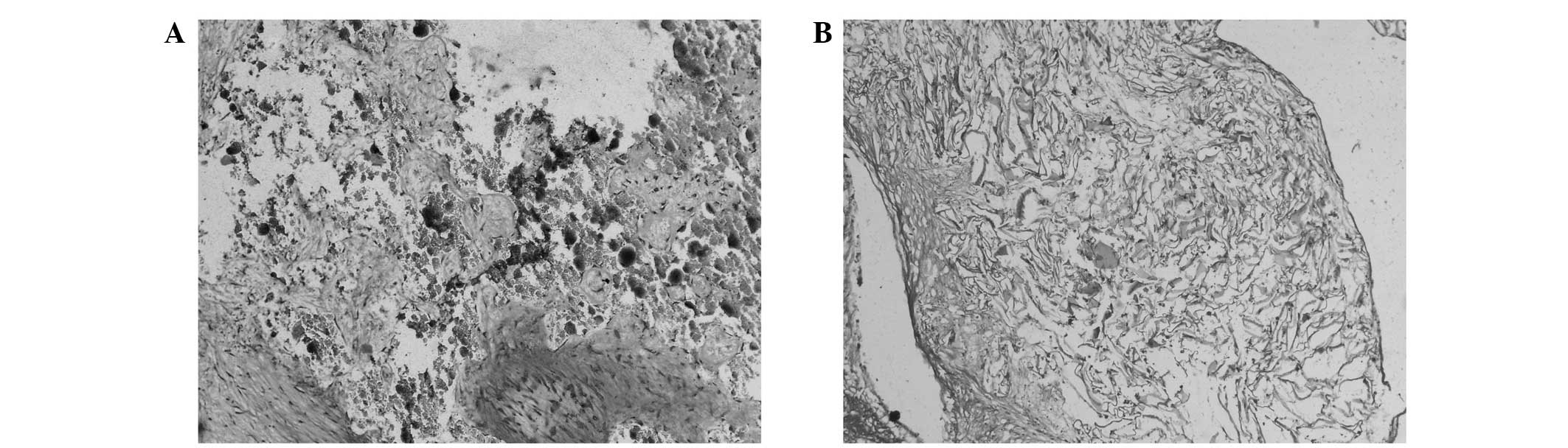
Images representative of the H&E staining in the
BCP and SFP groups are shown in Fig.
3. The BMSCs encapsulated in the BCP and SFP were sparse and
spindle-shaped, with almost no characteristics of chondrocytes. As
for the degradation of the scaffold, it was more marked in the SFP
group than in the BCP group. As no tissue was identified in the CS
group, no H&E staining results were obtained.
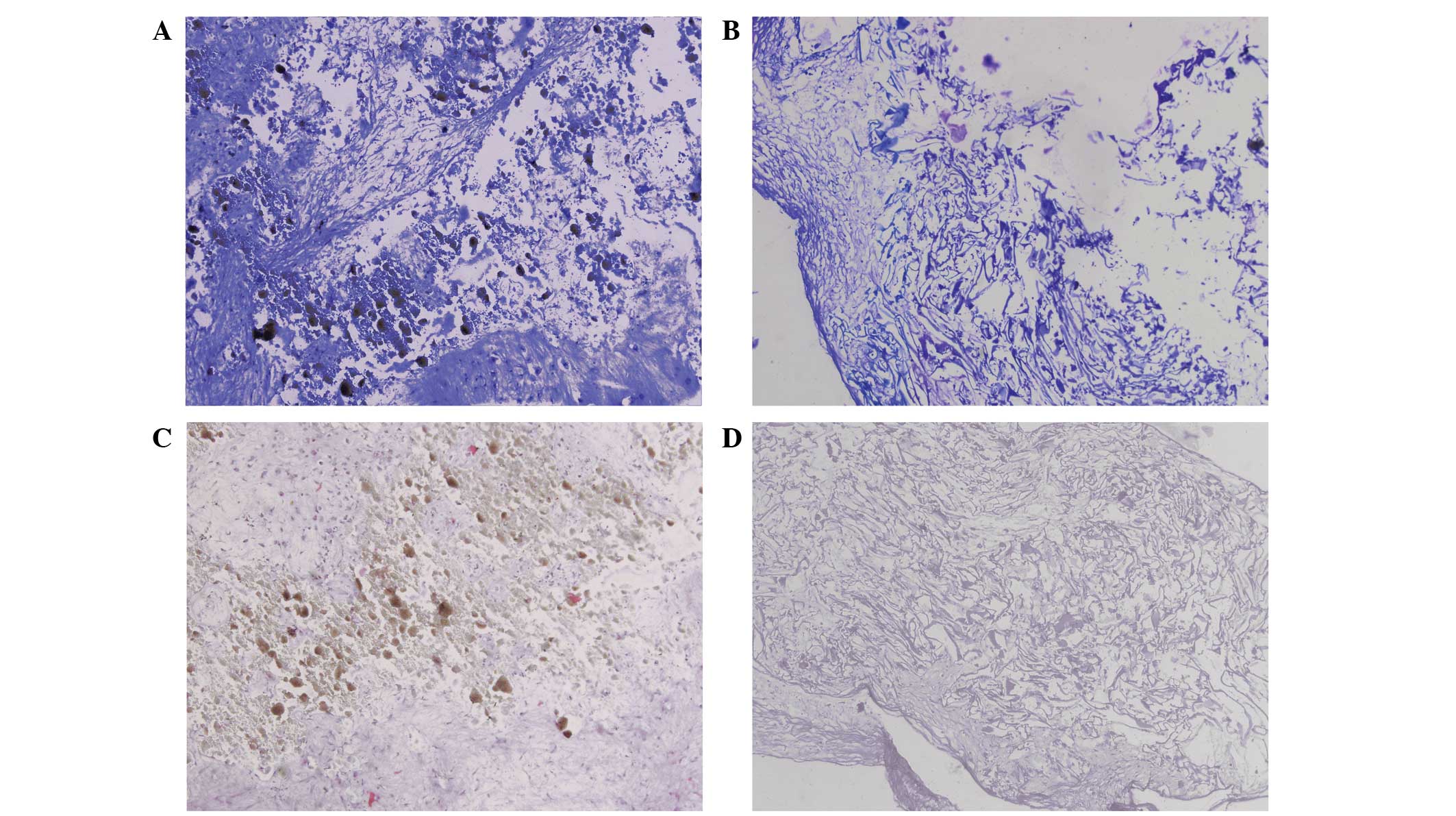
As reflected in the toluidine blue staining assay,
no metachromatic staining of the cell matrix was observed,
indicating that that minimal proteoglycan and glycosaminoglycan
deposition was present in the BCP and SFP groups (Fig. 4A and B). In agreement with the
toluidine blue staining, the immunohistochemical staining of type
II collagen (Fig. 4C and D)
indicated the absence of stained type II collagen for all the
constructs in the BCP and SFP groups. These results suggest that
the BMSCs did not undergo chondrogenic differentiation when loaded
in the BCP or SFP.
Discussion
The present study showed that the BCP, SFP and CS
did not initiate chondrogenic differentiation of the BMSCs in
vivo. The histological and immunohistochemical examinations
revealed cells with no characteristics of the chondrocyte phenotype
and there was little expression of aggrecan type II presented in
the BMSC-BCP and -SFP constructs. No tissue was present in the
constructs with CS as the scaffold. The results indicate that no
chondrogenic differentiation of BMSCs occurred when BCP, SFP and CS
were used as scaffolds.
Regarding a previous study in which it was shown
that chondrogenesis is induced by collagen and collagen-alginate
hydrogels, the present study further demonstrated that
chondrogenesis by materials is material-dependent. In the previous
study, collagen-based hydrogels were demonstrated to have the
ability to induce chondrogenesis in vivo without the
addition of any growth factors. This indicated that hydrogels,
instead of porous materials such as BCP, SCF and CS, may provide a
more favorable environment for chondrogenesis, mimicking that of
natural cartilage (8,9). The result was in accordance with the
study by Fujisato et al which demonstrated that a surface
layer of cells seeded in porous materials easily leads to a change
in the phenotype of the cells to flat and amebocyte- or
fibroblast-like (10).
However, the results revealed that the diffusion
chamber system was useful in allowing nutrition and body fluids to
pass through while preventing invasion by host cells, as previously
observed (11). It is of
significance for chondrogenesis that vascular invasion, which may
lead to calcification and bone formation through the process of
endothelial ossification, was effectively avoided by the filter
membrane.
Based on previous findings of chondrogenic induction
by collagen-based hydrogels, the present study provides further
evidence to indicate that chondrogenic induction by materials is
material-dependent. Hydrogels are superior to porous materials in
the induction of chondrogenic differentiation.
Acknowledgements
This study was financially supported by the National
Science and Technology Pillar Program (grant no. 2012BAI42G00), the
National Natural Science Foundation of China (grant no. 81101156)
and the Guangxi Natural Science Foundation (grant no.
2013GXNSFAA019246).
References
|
1
|
Kuroda R, Ishida K, Matsumoto T, et al:
Treatment of a full-thickness articular cartilage defect in the
femoral condyle of an athlete with autologous bone-marrow stromal
cells. Osteoarthritis Cartilage. 15:226–231. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Redman SN, Oldfield SF and Archer CW:
Current strategies for articular cartilage repair. Eur Cell Mater.
9:23–32. 2005.PubMed/NCBI
|
|
3
|
Mobasheri A, Csaki C, Clutterbuck AL,
Rahmanzadeh M and Shakibaei M: Mesenchymal stem cells in connective
tissue engineering and regenerative medicine: applications in
cartilage repair and osteoarthritis therapy. Histol Histopathol.
24:347–366. 2009.PubMed/NCBI
|
|
4
|
Wang Y, Blasioli DJ, Kim HJ, Kim HS and
Kaplan DL: Cartilage tissue engineering with silk scaffolds and
human articular chondrocytes. Biomaterials. 27:4434–4442. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hwang NS, Varghese S and Elisseeff J:
Cartilage tissue engineering. Stem Cell Assays. Vemuri MC: 407.
Humana Press; Totowa, NJ: pp. 351–373. 2007, View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lutolf MP and Hubbell JA: Synthetic
biomaterials as instructive extracellular microenvironments for
morphogenesis in tissue engineering. Nat Biotechnol. 23:47–55.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng L, Fan HS, Sun J, et al:
Chondrogenic differentiation of mesenchymal stem cells induced by
collagen-based hydrogel: an in vivo study. J Biomed Mater Res A.
93:783–792. 2010.PubMed/NCBI
|
|
8
|
Zheng L, Sun J, Chen X, et al: In vivo
cartilage engineering with collagen hydrogel and allogenous
chondrocytes after diffusion chamber implantation in
immunocompetent host. Tissue Eng Part A. 15:2145–2153. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng L, Sun J, Li B, Zhou W, Fan H and
Zhang X: Comparative study of collagen hydrogels modified in two
ways using the model of ectopic cartilage construction with
diffusion-chamber in immunocompetent host. J Appl Biomater Funct
Mater. Jul 30–2012.(Epub ahead of print). View Article : Google Scholar
|
|
10
|
Fujisato T, Sajiki T, Liu Q and Ikada Y:
Effect of basic fibroblast growth factor on cartilage regeneration
in chondrocyte-seeded collagen sponge scaffold. Biomaterials.
17:155–162. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gundle R, Joyner CJ and Triffitt JT: Human
bone tissue formation in diffusion chamber culture in vivo by
bone-derived cells and marrow stromal fibroblastic cells. Bone.
16:597–601. 1995. View Article : Google Scholar : PubMed/NCBI
|