Introduction
Acute kidney injury (AKI) is a common critical
illness and has demonstrated an increasing trend in its incidence
(1). AKI with chronic kidney
disease (CKD) is the third reason leading to CKD, following acute
tubular necrosis and prerenal AKI (2). The Project to Improve Care in Acute
Renal Disease (PICARD) in the United States showed that 30% of
patients with AKI were patients with AKI and CKD (3), suggesting that the incidences of AKI
and AKI with CKD are increasing annually, as well as increasing in
children. Hospital and pediatric intensive care unit
(PICU)-acquired prerenal AKI rates appear to have increased
>9-fold between 1982 and 2004 (4), most likely due to the increasing use
of more invasive management techniques and a higher illness
severity of critically ill children. AKI in children is usually
caused by renal ischemia, nephrotoxic drugs and sepsis (5). Henoch-Schönlein purpura nephritis
(HSPN) is one of the most common types of CKD in children, and may
lead to renal insufficiency (6–8). In
addition, CKD is one of the factors causing AKI in Chinese
children. However, the occurrence of AKI with HSPN (A-on-C) is
rarely reported.
Neutrophil gelatinase-assiciated lipocalin (NGAL) is
a member of the lipocalin superfamily of proteins that has been
extensively studied in AKI. NGAL is one of the most robustly
expressed proteins in the kidney following ischemic or nephrotoxic
injury in animals (9–13) and humans (14–17).
A previous study showed that NGAL was released from tubular cells
following various injuring stimuli (18). NGAL has now been validated as an
early predictive biomarker of AKI in cardiopulmonary bypass
(19), kidney transplantation
(16,20), diarrhea-associated hemolytic uremic
syndrome (21) and contrast
nephropathy (22). However, the
expression of NGAL in the serum, urine and renal tissues of
children with HSPN and A-on-C, has yet to be elucidated.
Kidney injury molecule-1 (KIM-1) is a sensitive
marker of the presence of tubular damage (23). KIM-1 is upregulated in
dedifferentiated proximal tubule cells following ischemic or
nephrotoxic AKI in animal models, and a proteolytically processed
domain may be detected in the urine (24). Tubular KIM-1 expression is
significantly associated with tubulointerstitial damage and
inflammation (25). In
experimental and human kidney disease, increased urinary KIM-1
levels are strongly correlate with tubular KIM-1 expression,
showing that urinary KIM-1 levels may be a valuable biomarker for
the presence of tubulointertitial damage (26). However, the expression of KIM-1 in
the serum, urine and renal tissues of children with HSPN and A-on-C
has not yet been investigated.
In the present study, it was hypothesized that the
expression of serum and urine NGAL and KIM-1 was significantly
increased in patients with A-on-C, with expression in the renal
tubules. In addition, it was hypothesized that the urine NGAL and
KIM-1 levels negatively correlated with glomerular filtration rate
(GFR), although not with urine protein. The study, examined the
changes in NGAL and KIM-1 levels in the serum, urine and renal
tissues of children with A-on-C, and investigated the correlation
between NGAL/KIM-1 and GFR/urine protein levels.
Materials and methods
Patients and laboratory data
A prospective single-center evaluation was performed
of the serum, urinary and renal NGAL and KIM-1 levels in a cohort
of children admitted to the Department of Pediatric Nephrology,
Shengjing Hospital of China Medical University (Shenyang, China)
between January 2010 and October 2011. Patients were included if
they were diagnosed with A-on-C, according to the Pediatric Risk,
Injury, Failure, Loss and End-Stage Renal Disease (pRIFLE) criteria
(Table I) (27), or with HSPN with nephrotic-range
proteinuria, and divided into two groups; group HSPN and group
A-on-C. Patients were excluded if they had a known diagnosis of
CKD, including dialysis or transplantation, or a recent urinary
tract infection. The caregivers of the patients provided informed
written consent. The protocol and consent forms were approved by
the Shengjing Hospital Institutional Review Board prior to study
commencement (no. 20110819).
 | Table IpRIFLE AKI criteria. |
Table I
pRIFLE AKI criteria.
| GFR | Urine output |
|---|
| Risk ‘R’ | Decreased by 25% | <0.5 ml/kg/h for 8
h |
| Injury ‘I’ | Decreased by 50% | <0.5 ml/kg/h for
16 h |
| Failure ‘F’ | Decreased by 75% or
<35 ml/min/1.73 m2 | <0.3 ml/kg/h for
24 h or anuric for 12 h |
All relevant data, including age, gender, weight,
hemoglobin (Hb), serum creatinine (SCr), cystatin C (CysC), serum
β2-macroglobulin (β2-MG), albumin, urinary β2-MG and urine protein
levels, were recorded for each of the patients. GFR, also known as
estimated creatinine clearance (GFR), was calculated using the
original Schwartz formula (28),
as opposed to the updated Schwartz formula used in the Chronic
Kidney Disease in Children study. This was due to the fact that the
original formula was used to validate the pRIFLE AKI classification
system (27).
Blood and urinary biomarker
assessment
Blood and 5-ml urine samples were collected from
each participating patient for the analysis of NGAL and KIM-1
levels using an ELISA (R&D Systems, Minneapolis, MN, USA), in
accordance with the manufacturer’s instructions. The samples were
centrifuged at 3,000 rpm for 15 min (Beckman, Brea, CA, USA), and
the supernatant was then decanted into 1-ml aliquots and stored at
−80°C prior to assay. Urinary Cr was measured using a quantitative
colorimetric assay kit (Sigma, St. Louis, MO, USA) to normalize the
urine samples.
Immunohistochemical staining
The NGAL and KIM-1 protein levels in groups HSPN and
A-on-C were examined using immunohistochemical staining. The tissue
was fixed in 4% formaldehyde and then embedded in paraffin blocks.
Slides of kidney tissues measuring 4 μm were routinely prepared for
the immunohistochemical analysis of the NGAL and KIM-1 protein
levels. A streptavidin-biotin complex (SABC) immunohistochemical
assay (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was
performed and rabbit anti-human NGAL antibodies (Santa Cruz
Biotechnology, Inc.), which were diluted by a ratio of 1:50, were
used as primary antibodies. Based on the SABC staining technique,
brownish yellow granules in the cytoplasm indicated positive cells.
In addition, an SABC immunohistochemical assay using rabbit
anti-human KIM-1 antibodies (Santa Cruz Biotechnology, Inc) as the
primary antibodies was performed, with the antibodies diluted by a
ratio of 1:160. Brownish yellow granules in cytoplasm indicated
positive cells.
Results were analyzed using an image analysis system
(MetaMorph® Imaging System; Universal Imaging
Corporation, West Chester, PA, USA). As the average integral
optical density value increased, the expression intensity of the
product with a positive reaction became stronger, indicative of
higher protein expression.
Statistical analysis
Statistical analyses were performed using SPSS 10.0
statistical software (SPSS, Inc., Chicago, IL, USA). All data are
presented as the mean ± standard deviation). A t-test was performed
for intergroup comparisons. P<0.05 was considered to indicate a
statistically significant difference. Pearson or Spearman
correlation coefficients were used to assess the correlations
between estimated GFR (eGFR) and other variables.
Results
Demographics and laboratory data
In total, 25 patients, with an average age of
8.58±2.15 years, were enrolled in the study. Among them, 16
patients had HSPN (group HSPN), while 9 patients had A-on-C (group
A-on-C). The renal tissues of 10 patients in group HSPN and 6
patients in group A-on-C were examined using immunohistochemistry.
The demographics and laboratory data are shown in Table II. The expression of serum CysC,
β2-MG, SCr and urine β2-MG in group A-on-C was significantly higher
than that in group HSPN, and the expression of NGAL and KIM-1 in
the serum and urine of the patients in group A-on-C was also
significantly higher than that in group HSPN.
 | Table IIDemographic, clinical and laboratory
data of the study population. |
Table II
Demographic, clinical and laboratory
data of the study population.
| Parameter | Group A-on-C | Group HSPN | t-value | P-value |
|---|
| Gender, M/F | 3/6 | 7/9 | - | - |
| Age, years | 7.55±1.42 | 9.50±2.75 | 1.96 | 0.062 |
| Weight, kg | 28.72±9.28 | 34.62±13.52 | 1.16 | 0.258 |
| Hb, g/la | 110.44±6.62 | 127.94±6.82 | 6.22 | <0.001 |
| SCr, μmol/la | 91.22±16.34 | 47.31±10.92 | 8.07 | <0.001 |
| CysC, mg/la | 2.53±0.86 | 0.80±1.56 | 7.92 | <0.001 |
| Albumin, g/l | 32.51±4.93 | 37.73±3.69 | 2.77 | 0.016 |
| Serum β2-MG,
mg/la | 5.79±3.22 | 1.63±0.28 | 5.23 | <0.001 |
| GFR, ml/min/1.73
m2a | 68.60±11.78 | 129.56±8.21 | 15.23 | <0.001 |
| Urine β2-MG,
mg/la | 14.01±18.92 | 0.61±0.31 | 2.88 | 0.008 |
| Urine protein,
g/d | 3.18±1.50 | 3.35±1.73 | 0.24 | 0.813 |
| Serum NGAL,
ng/mla | 312.82±33.17 | 211.16±34.63 | 7.15 | <0.001 |
| Urine NGAL,
ng/mla | 1627.61±470.83 | 412.91±52.36 | 10.39 | <0.001 |
| Serum KIM-1,
pg/mla | 147.91±68.15 | 9.28±1.39 | 8.27 | <0.001 |
| Urine KIM-1,
pg/mla | 329.21±57.36 | 18.18±9.07 | 8.02 | <0.001 |
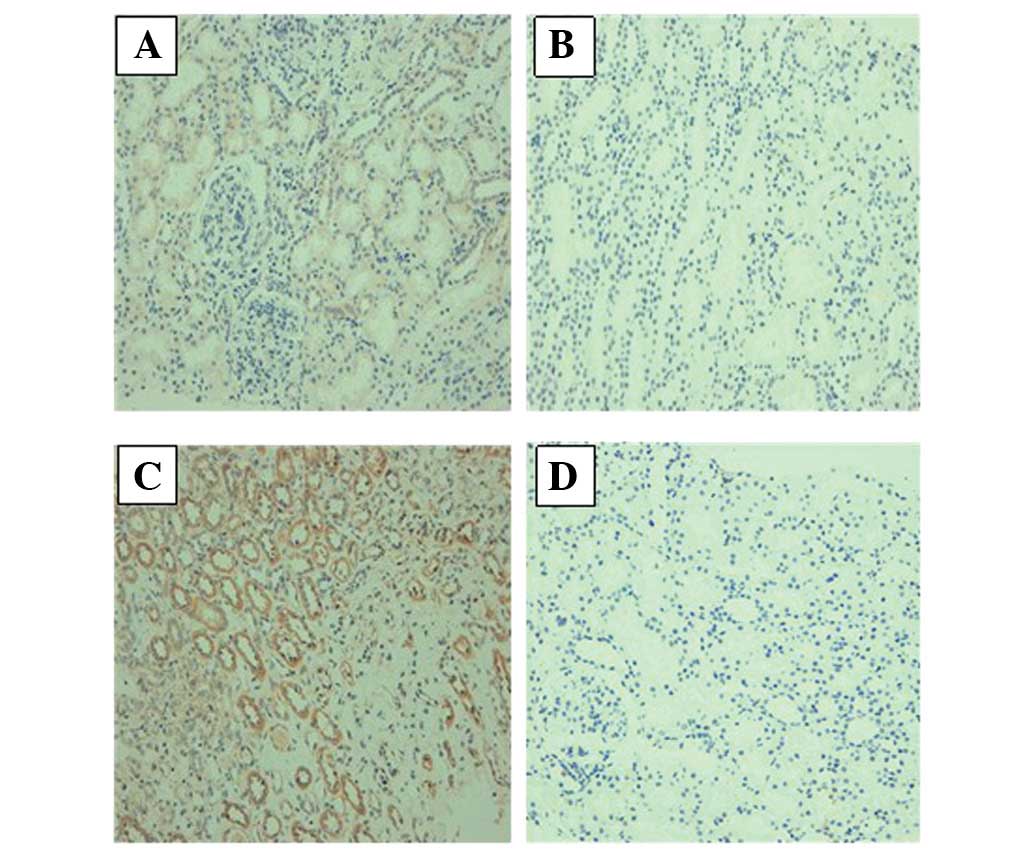
Expression of NGAL and KIM-1 in the
kidney
The NGAL and KIM-1 protein levels were examined
using immunohistochemical staining. The results showed that the
NGAL and KIM-1 proteins were expressed in renal tubular epithelial
cells, and that they were mainly expressed in the proximal tubule,
not the distal convoluted tubule or the collecting duct. The
expression of NGAL protein in group A-on-C was significantly higher
than that in group HSPN. In addition, KIM-1 protein expression was
low in group HSPN, while the expression of KIM-1 was positive in
group A-on-C (Fig. 1). The results
of the quantitative analysis of NGAL and KIM-1 expression are shown
in Table III.
 | Table IIIRenal expression of NGAL and KIM-1 in
the two patient groups. |
Table III
Renal expression of NGAL and KIM-1 in
the two patient groups.
| Parameter | Group A-on-C
(n=6) | Group HSPN
(n=10) | t-value | P-value |
|---|
| NGALa | 0.47±0.11 | 0.03±0.10 | 13.417 | 0.00 |
| KIM-1a | 0.64±0.14 | 0.03±0.14 | 13.875 | 0.00 |
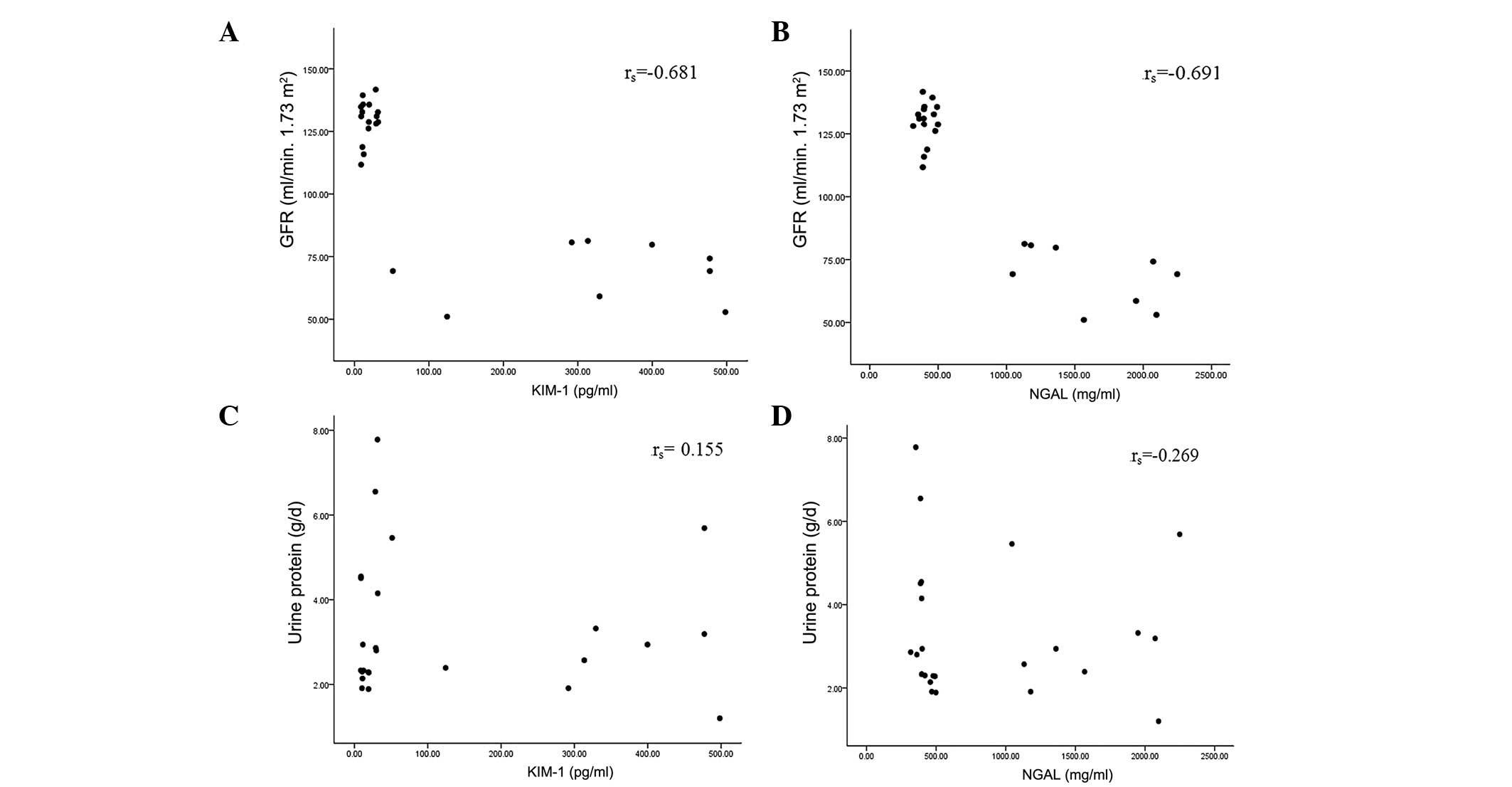
Correlation between urine NGAL/KIM-1
levels and GFR, and NGAL/KIM-1 levels and urine protein
Pearson or Spearman correlation coefficients were
used to assess the correlations between urine NGAL/KIM-1 levels and
GFR, and urine NGAL/KIM-1 levels and urine protein. The results
showed that urine NGAL and KIM-1 were negatively correlated with
GFR; however, there was no significant correlation between urine
NGAL/KIM-1 levels and urine protein (Fig. 2).
Discussion
AKI has become increasingly important in nephrology,
particularly in the field of critical kidney diseases. Among adult
patients with AKI, patients with AKI and CKD account for ~30%
(29). Clinical studies concerning
A-on-C in children are rare (30).
HSPN is one of the most common types of CKD in children, and may
lead to renal insufficiency. However, A-on-C is rarely reported. In
the present study, a number of children with HSPN already had AKI
when they were admitted to hospital due to infection, hypovolemia
or routine urine abnormalities. The differences between the early
AKI biomarkers, NGAL and KIM-1, in the serum, urine and kidney of
children with HSPN and those with A-on-C have not yet been
reported. In addition, there have been no studies concerning the
use of NGAL and KIM-1 levels to predict A-on-C, or concerning the
correlation between NGAL/KIM-1 levels and GFR/urine protein.
Accurate biomarkers that are able to rapidly detect
AKI in children with CKD are likely to be particularly valuable, as
baseline SCr values are often unavailable to calculate the required
relative increase in SCr to diagnose AKI. In the present study, the
expression of serum CysC, β2-MG and SCr and urinary β2-MG and SCr
in group A-on-C was significantly higher than that in group HSPN,
which was consistent with the expression of NGAL and KIM-1.
However, there were no significant differences in urine protein
levels between the two groups. A previous study showed that the
urine protein levels of children with HSPN was the independent risk
factor of prognosis (31). The
results of the present study showed that there were no significant
differences in urine protein levels between patients with A-on-C
and patients with HSPN alone, suggesting that the level of urine
protein is not the direct cause of AKI, and may only be used to
assess the long-term prognosis. The expression of serum and urine
NGAL and KIM-1 in group A-on-C was significantly higher than that
in group HSPN, suggesting that NGAL and KIM-1 proteins were highly
expressed in patients with A-on-C, and that these proteins may be
able to be used as predictive factors for AKI and CKD. The serum
CysC and serum and urine β2-MG levels were also increased in
patients with A-on-C, and may therefore be combined with NGAL and
KIM-1 to predict AKI and CKD. With the pRIFLE formula, it was
revealed that while the SCr level was normal in three children, the
serum and urine NGAL and KIM-1 levels were significantly increased.
This suggested that the increase in serum and urine NGAL and KIM-1
levels occurred prior to the increase in SCr, and may therefore be
used to predict AKI and CKD.
From the pathological results, it was demonstrated
that NGAL and KIM-1 proteins were expressed in renal tubular
epithelial cells, and that the expression of NGAL and KIM-1
proteins in group A-on-C was significantly higher than that in
group HSPN. In group HSPN, the NGAL and KIM-1 proteins were not
observed in the tubular cells or glomerulus, which contrasted with
the high expression in group A-on-C. This indicated that tubular
injury had occurred in children with HSPN, which then led to AKI,
and that in patients with HSPN alone. The results also showed that
NGAL and KIM-1 were primarily expressed in the proximal tubule, not
the distal convoluted tubule or the collecting duct, which was
consistent with the pathological observations of AKI.
In this study, it was demonstrated that urine NGAL
and KIM-1 levels were negatively correlated with GFR, although not
with urine protein levels. This suggested that urine NGAL and KIM-1
levels may not be significantly increased in children with HSPN
without AKI, even when high levels of urine protein exist. However,
in children with A-on-C suffering from infection or hypovolemia,
the urine NGAL and KIM-1 levels are likely to be significantly
increased. Therefore, the level of urine protein may be associated
with the long-term prognosis in patients with HSPN, not the
occurrence of AKI. The results suggested that the increase in urine
NGAL and KIM-1 levels was associated with AKI, not the CKD or urine
protein levels, which was consistent with a previous study
(32).
The limitations of the present study were as
follows: i) The samples of AKI were small, with no extremely
serious cases requiring renal replacement therapy and no
classification of AKI; ii) the renal biopsy cases were small, which
affected NGAL and KIM-1 protein expression following tubular
injury; iii) the study was a single center study, and therefore a
further large-scale multi-center clinical study is required to
confirm the results.
In conclusion, the expression of serum and urine
NGAL and KIM-1 is significantly increased in children with A-on-C.
In addition, the results of the present study demonstrated that the
levels of serum and urine NGAL and KIM-1 were increased only when
AKI occurred in the children, and that NGAL and KIM-1 levels were
negatively correlated with GFR. This suggested that changes in NGAL
and KIM-1 levels may be used to diagnose A-on-C in children.
Acknowledgements
This study was supported by the Natural Science Fund
of Liaoning Province.
References
|
1
|
Xue JL, Daniels F, Star RA, et al:
Incidence and mortality of acute renal failure in Medicare
beneficiaries, 1992 to 2001. J Am Soc Nephrol. 17:1135–1142. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liaño F and Pascual J: Epidemiology of
acute renal failure: A prospective, multicenter, community-based
study. Madrid Acute Renal Failure Study Group. Kidney Int.
50:811–818. 1996.PubMed/NCBI
|
|
3
|
Pisoni R, Wille KM and Tolwani AJ: The
epidemiology of severe acute kidney injury: from BEST to PICARD, in
acute kidney injury: new concepts. Nephron Clin Pract.
109:c188–c191. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vachvanichsanong P, Dissaneewate P, Lim A
and McNeil E: Childhood acute renal failure: 22-year experience in
a university hospital in southern Thailand. Pediatrics.
118:e786–e791. 2006.PubMed/NCBI
|
|
5
|
Hui-Stickle S, Brewer ED and Goldstein SL:
Pediatric ARF epidemiology at a tertiary care center from 1999 to
2001. Am J Kidney Dis. 45:96–101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ronkainen J, Ala-Houhala M, Huttunen NP,
Jahnukainen T, Koskimies O, Ormälä T and Nuutinen M: Outcome of
Henoch-Schonlein nephritis with nephrotic-range proteinuria. Clin
Nephrol. 60:80–84. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shenoy M, Bradbury MG, Lewis MA and Webb
NJ: Outcome of Henoch-Schönlein purpura nephritis treated with
long-term immunosuppression. Pediatr Nephrol. 22:1717–1722.
2007.PubMed/NCBI
|
|
8
|
Coppo R, Andrulli S, Amore A, et al:
Prediction of outcome in Henoch-Schönlein Nephritis in children and
adults. Am J Kidney Dis. 47:993–1003. 2006.PubMed/NCBI
|
|
9
|
Supavekin S, Zhang W, Kucherlapati R, et
al: Differential gene expression following early renal
ischemia/reperfusion. Kidney Int. 63:1714–1724. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mishra J, Ma Q, Prada A, et al:
Identification of neutrophil gelatinase-associated lipocalin as a
novel early urinary biomarker for ischemic renal injury. J Am Soc
Nephrol. 14:2534–2543. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mishra J, Mori K, Ma Q, et al: Neutrophil
gelatinase-associated lipocalin: a novel early urinary biomarker
for cisplatin nephrotoxicity. Am J Nephrolol. 24:307–315. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mishra J, Mori K, Ma Q, et al:
Amelioration of ischemic acute renal injury by neutrophil
gelatinase-associated lipocalin. J Am Soc Nephrol. 15:3073–3082.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Devarajan P, Mishra J, Supavekin S, et al:
Gene expression in early ischemic renal injury: clues towards
pathogenesis, biomarker discovery, and novel therapeutics. Mol
Genet Metab. 80:365–376. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Devarajan P: Cellular and molecular
derangements in acute tubular necrosis. Curr Opin Pediatr.
17:193–199. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nguyen MT, Ross GF, Dent CL, et al: Early
prediction of acute renal injury using urinary proteomics. Am J
Nephrol. 25:318–326. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mishra J, Ma Q, Kelly C, et al: Kidney
NGAL is a novel marker of acute injury following transplantation.
Pediatr Nephrol. 21:856–863. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mishra J, Dent C, Tarabishi R, et al:
Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker
for acute renal injury after cardiac surgery. Lancet.
365:1231–1238. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Davide B, Antonio L, Giuseppe C, et al:
Neutrophil gelatinase-associated lipocalin (NGAL) and progession of
chronic kidney disease. Clin J Am Nephrol. 4:337–344. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dent CL, Ma Q, Dastrala S, et al: Plasma
NGAL predicts acute kidney injury, morbidity and mortality after
pediatric cardiac surgery: a prospective uncontrolled cohort study.
Crit Care. 11:R1272007. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Parikh CR, Jani A, Mishra J, et al: Urine
NGAL and IL-18 are predictive biomarkers for delayed graft function
following kidney transplantation. Am J Transplant. 6:1639–1645.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Trachtman H, Christen E, Cnaan A, et al:
Urinary neutrophil gelatinase-associated lipocalcin in D+HUS: a
novel marker of renal injury. Pediatr Nophrol. 21:989–994.
2006.PubMed/NCBI
|
|
22
|
Hirsch R, Dent C, Pfriem H, et al: NGAL is
an early predictive biomarker of contrast-induced nephropathy in
children. Pediatr Nephrol. 22:2089–2095. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vaidya VS, Ramirez V, Ichimura T, et al:
Urinary kidney injury molecule-1: A sensitive quantitative
biomarker for early detection of kidney tubular injury. Am J
Physiol Renal Physiol. 290:F517–F529. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Z, Humphreys BD and Bonventre JV:
Shedding of the urinary biomarker kidney injury molecule-1 (KIM-1)
is upregulated by MAP kinases and juxtamembrane region. J Am Soc
Nephrol. 18:2702–2714. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van Timmeren MM, van den Heuvel MC, Bailly
V, et al: Tubular kidney injury molecule-1 (KIM-1) in human renal
disease. J Pathol. 212:209–217. 2007.
|
|
26
|
van Timmeren MM, Bakker SJ, Vaidya VS, et
al: Tubular kidney injury molecule-1 in protein overload
nephropathy. Am J Physiol Renal Physiol. 291:F456–F464.
2006.PubMed/NCBI
|
|
27
|
Akcan-Arikan A, Zappitelli M, Loftis LL,
et al: Modified RIFLE criteria in critically ill children with
acute kidney injury. Kidney Int. 71:1028–1035. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schwartz GJ, Brion LP and Spitzer A: The
use of plasma creatinine concentration for estimating glomerular
filtration rate in infants, children and adolescents. Pediatr Clin
North Am. 34:571–590. 1987.PubMed/NCBI
|
|
29
|
Clinical practice guideline for acute
kidney injury. KDIGO (Kidney Disease: Improving Global Outcome).
2:124–138. 2012.
|
|
30
|
Davin JC and Weening JJ: Henoch-Schönlein
purpura nephritis: an update. Eur J Pediatr. 160:689–695. 2001.
|
|
31
|
Lau KK, Suzuki H, Novak J and Wyatt RJ:
Pathogenesis of Henoch-Schönlein purpura nephritis. Pediatr
Nephrol. 25:19–26. 2010.
|
|
32
|
Pitashny M, Schwartz N, Qing X, et al:
Urinary lipocalin-2 is associated with renal disease activity in
human lupus nephritis. Arthritis Rheum. 56:1894–1903. 2007.
View Article : Google Scholar : PubMed/NCBI
|