Introduction
Surgical resection is the only possible curative
treatment for gastric cancer, however, this treatment is limited to
stage I early gastric cancer cases. Neoadjuvant chemotherapy for
the treatment of gastric cancer was initially reported by Wikle
et al in 1989. Since then, numerous studies have
demonstrated the safety and potency of this treatment, which
functions to reduce the pathological stage and improve the surgical
resection rate, particularly the R0 resection rate
(1). In addition, neoadjuvant
chemotherapy has been shown to reduce postoperative recurrence and
metastasis, as well as improve the prognoses of patients with
gastric cancer (1,2). Postoperative local recurrence and
peritoneal metastasis of advanced gastric cancer are important
factors that affect patient prognosis, with intraperitoneal
metastasis being the most frequent outcome and cause of mortality
in advanced gastric cancer. Studies have shown that for advanced
gastric cancer with penetrated serosa, neoadjuvant chemotherapy
reduces intraperitoneal recurrence and metastasis, thus, increases
the overall survival rate of patients (3). In addition, hyperthermic
intraperitoneal perfusion chemotherapy has been shown to
effectively eliminate cancer cells that escape to the peritoneal
cavity, thus, preventing peritoneal local recurrence and metastasis
(4). In the present study,
preoperative neoadjuvant chemotherapy was combined with
postoperative hyperthermic intraperitoneal perfusion chemotherapy
for the treatment of advanced gastric cancer.
Materials and methods
Ethics
The study was approved by the Medical Ethics
Committee of the Cangzhou Central Hospital (Cangzhou, China) and
informed consent was provided by all patients.
Clinical data and grouping
A total of 192 patients that had been diagnosed with
advanced gastric cancer and that had undergone surgery between
January 2006 and January 2010 at the Department of Oncological
Surgery, Cangzhou Central Hospital, were enrolled in the study.
Inclusion criteria firstly included a confirmed diagnosis of
gastric cancer by gastroscopy biopsy and histopathological
examinations, with the Tumor, Node and Metastasis classification
identifying the tumors as IIIA or IIIB, according to the American
Joint Committee on Cancer staging system (5), without the presence of hepatic,
pulmonary, cerebral or bone metastasis. Secondly, the tumors were
evaluated to be stage IIIA or IIIB by endoscopic ultrasound (EUS)
and computed tomography (CT) scans that revealed at least one
measurable lesion. Thirdly, patients were required to be aged
between 18 and 75 years and have a Karnofsky Performance Status
score of ≥60. Finally, patients had not undergone chemotherapy
prior to enrollment and no surgical or chemotherapeutic
contradictions were present in the preoperative examinations.
Patients were excluded from the study if they had residual gastric
cancer or had undergone a laparotomy. The patients were randomly
divided into four groups (Tables I
and II). The control group
comprised 48 cases (males, 21; females, 27; age, 39–72 years;
average age, 56 years) of which 28 cases were classified as stage
IIIA and 20 cases were classified as stage IIIB. In addition, 16
cases had highly or moderately differentiated adenocarcinomas, 25
cases had poorly or undifferentiated adenocarcinomas and 7 cases
had mucinous adenocarcinoma or mucinous cell carcinoma. Surgical
treatment without postoperative chemotherapy was performed in this
group. The neoadjuvant chemotherapy group comprised 48 cases
(males, 19; females, 29; age, 41–69 years; average age, 55 years)
of which 29 cases were classified as stage IIIA and 19 cases were
classified as stage IIIB. In addition, 15 cases had highly or
moderately differentiated adenocarcinomas, 21 cases had poorly or
undifferentiated adenocarcinomas and 12 cases had mucinous
adenocarcinoma or mucinous cell carcinoma. Preoperative neoadjuvant
chemotherapy and surgical treatment were performed in this group,
but without postoperative hyperthermic intraperitoneal perfusion
chemotherapy. The hyperthermic intraperitoneal perfusion
chemotherapy group comprised 48 cases (males, 22; females, 26; age,
39–70 years; average age, 53 years) of which 25 cases were
classified as stage IIIA and 23 cases were classified as stage
IIIB. In addition, 14 cases had highly or moderately differentiated
adenocarcinomas, 22 cases had poorly or undifferentiated
adenocarcinomas and 12 cases had mucinous adenocarcinoma or
mucinous cell carcinoma. Surgical treatment and postoperative
hyperthermic intraperitoneal perfusion chemotherapy were performed
in this group, but without neoadjuvant chemotherapy. The joint
group comprised 48 cases (males, 20; females, 28; age, 42–68 years;
average age, 55 years) of which 27 cases were classified as stage
IIIA and 21 cases were classified as stage IIIB. In addition, 18
cases had highly or moderately differentiated adenocarcinomas, 21
cases had poorly or undifferentiated adenocarcinomas and 9 cases
had mucinous adenocarcinoma or mucinous cell carcinoma.
Preoperative neoadjuvant chemotherapy, surgical treatment and
hyperthermic intraperitoneal perfusion chemotherapy were performed
in this group. Differences among the clinical data of the four
groups exhibited no statistical significance (P>0.05), but had
comparability (Tables I and
II).
 | Table IComparison of gastric cancer pathology
types in each group. |
Table I
Comparison of gastric cancer pathology
types in each group.
| Groups | Moderately/well
differentiated adenocarcinoma |
Poorly/undifferentiated
adenocarcinoma | Mucinous
adenocarcinoma or mucinous cell carcinoma | χ2 | P-value |
|---|
| Control | 16 | 25 | 7 | | |
| Neoadjuvant
chemotherapy | 15 | 21 | 12 | | |
| Hyperthermic
intraperitoneal perfusion chemotherapy | 14 | 22 | 12 | | |
| Joint | 18 | 21 | 9 | 2.84 | 0.83 |
 | Table IIGastric cancer stages of each
group. |
Table II
Gastric cancer stages of each
group.
| Groups | Stage IIIA | Stage IIIB | χ2 | P-value |
|---|
| Control | 28 | 20 | | |
| Neoadjuvant
chemotherapy | 29 | 19 | | |
| Hyperthermic
intraperitoneal perfusion chemotherapy | 25 | 23 | | |
| Joint | 27 | 21 | 0.74 | 0.86 |
Therapeutic project
Neoadjuvant chemotherapy was performed with 135
mg/m2 paclitaxel administered via an intravenous drip on
day 1, 20 mg/m2 cisplatin administered via an
intravenous drip between day 1 and 5 and 0.8–1.0 g tegafur
administered per day via an intravenous drip between day 1 and 5.
The course was repeated every 3 weeks. In the neoadjuvant
chemotherapy group, the efficacy was evaluated by clinical
conditions, EUS and CT scans every 2 weeks, according to the
response evaluation criteria in solid tumors (6). If the evaluation determined the
treatment to be effective or stable, the regimen continued to the
end of the fourth course, 3 weeks after which surgery was
performed. Furthermore, following surgery, an additional three
courses of treatment with the original regimen were administered.
By contrast, if tumor progression occurred, immediate surgical
treatment was performed. This was followed by four courses of a
different regimen called ‘ECF’, which contained 50 mg/m2
epirubicin and 60 mg/m2 cisplatin administered via an
intravenous drip on day 1 and 600 mg/m2 fluorouracil
administered via an intravenous drip between day 1 and 3.
Postoperative hyperthermic intraperitoneal perfusion chemotherapy
started on day 1 or 2 following surgery, according to the recovery
status of the patient following the radical resection of the
gastric cancer. This chemotherapy was performed for 90 min per day
for four consecutive days. In the neoadjuvant chemotherapy and
joint groups, the surgical treatment usually started 3 weeks
following the last course of neoadjuvant chemotherapy. In the
control and neoadjuvant chemotherapy groups, systemic chemotherapy
was performed between week 2 and 3 following surgery. While in the
hyperthermic intraperitoneal perfusion chemotherapy and joint
groups, chemotherapy did not start until 1 month following
surgery.
Intraoperative placement of hyperthermic
perfusion catheters
During the radical resection of gastric cancer,
silicone hyperthermic perfusion catheters (Jilin Morestep Medical
Device Co., Ltd., Changchun, China) were placed at the bilateral
paracolic sulcus, diaphragmatic surface of the liver and the
splenic recess separately. The catheters had side pores on the
front end, and inside and outside diameters of 8 and 10 mm,
respectively. An additional drainage catheter was placed at the
upper abdomen and exited at the paraumbilical region.
Hyperthermic perfusion chemotherapy
Chemotherapy was conducted using an RHL-2000B
Chemo-hyperthermia perfusion system (Jilin Morestep Medical Device
Co., Ltd.). Treatment started on day 1 or 2 following surgery,
according to the recovery status of the patient. Chemotherapy was
performed for 90 min per day for four consecutive days. On day 1
and 4, the intraperitoneal hyperthermic perfusate consisted of 60
mg/m2 cisplatin and 3,000 ml normal saline, while on day
2 and 3, the perfusate consisted of 0.75 g fluorouracil and 3,000
ml normal saline. In addition, 10 mg dexamethasone and 10 ml
lidocaine (2%) were routinely added to the perfusate in order to
reduce peritoneal reactions. The perfusion machine, circulation
pump and heater were powered at 38°C, which was reached prior to
therapy. Next, the circulatory pipes were connected to the
abdominal drainage catheters via a two-in-three-out manner, through
which the hyperthermic perfusate was infused into the abdominal
cavity. The temperature of the perfusate was then elevated to and
stabilized at 41–43°C using a temperature control system that
lasted for 90 min. During hyperthermic perfusion, the patients
underwent electrocardiogram monitoring to facilitate real-time
adjustment of the perfusion machine according to the situation. The
patient also routinely received intramuscular injections of
dolantin and diazepam to improve tolerance. Following therapy,
~1,000 ml hyperthermic perfusate was left in the abdominal cavity
and the remaining perfusate was drained.
Evaluation of efficiency and adverse
reactions
Alimentary tract reactions and marrow function were
evaluated according to the World Health Organization’s standard
toxicity assessment. In addition, regular re-examinations,
including gastroscopy, chest and abdominal CT scans and tumor
biomarkers, were conducted to obtain information on intraperitoneal
recurrence within 2 years and the 1- or 3-year survival rates.
Statistical analysis
SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA)
was used to conduct statistical analysis of the experimental data.
Quantitative data were analyzed using the χ2 and
Fisher’s exact tests. Progression-free survival and overall
survival rates were calculated using the Kaplan-Meier method.
Logrank tests were used to analyze the significance of difference
among the progression-free and overall survival rates of the four
groups, while the t-test was employed for the comparison of the
2-year progression-free survival and 1- and 3-year survival rates
of the control group. P<0.05 was considered to indicate a
statistically significant difference.
Results
Adverse reactions and complications
No patient succumbed during surgery. There was not a
marked difference between the occurrence rates of I–II degree
myelosuppression, III–IV degree myelosuppression, I–II degree
nausea and III–IV degree nausea and vomiting among the four groups
(P>0.05; Table III).
 | Table IIIComparison of adverse events in each
group. |
Table III
Comparison of adverse events in each
group.
| Parameter | Grade I–II
myelosuppression | Grade III–IV
myelosuppression | Grade I–II nausea and
vomiting | Grade III–IV nausea
and vomiting |
|---|
| Control | 25 | 1 | 18 | 0 |
| Neoadjuvant
chemotherapy | 27 | 2 | 21 | 1 |
| Hyperthermic
intraperitoneal perfusion chemotherapy | 26 | 1 | 23 | 1 |
| Joint | 30 | 3 | 25 | 2 |
| χ2 | 1.19 | | 2.25 | |
| P-value | 0.76 | 0.84 | 0.52 | 0.9 |
Comparison of efficiency in each
group
There were 1, 2, 1 and 0 cases lost during the
follow-ups in the control, neoadjuvant chemotherapy, hyperthermic
intraperitoneal perfusion chemotherapy and joint groups,
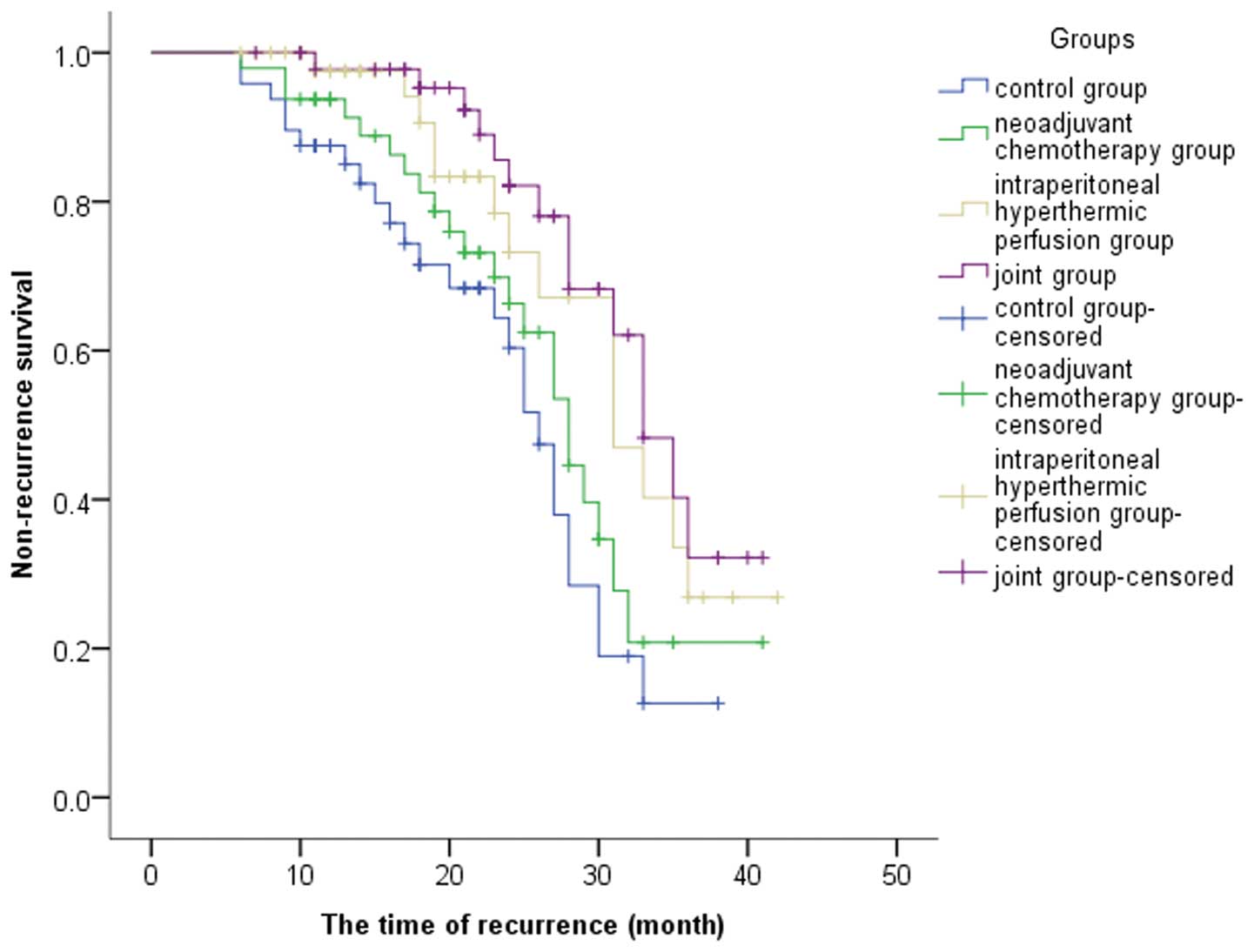
respectively. The median progression-free survival times were 26,
28, 31 and 33 months in the control, neoadjuvant chemotherapy,
hyperthermic intraperitoneal perfusion chemotherapy and joint
groups, respectively; the difference was statistically significant
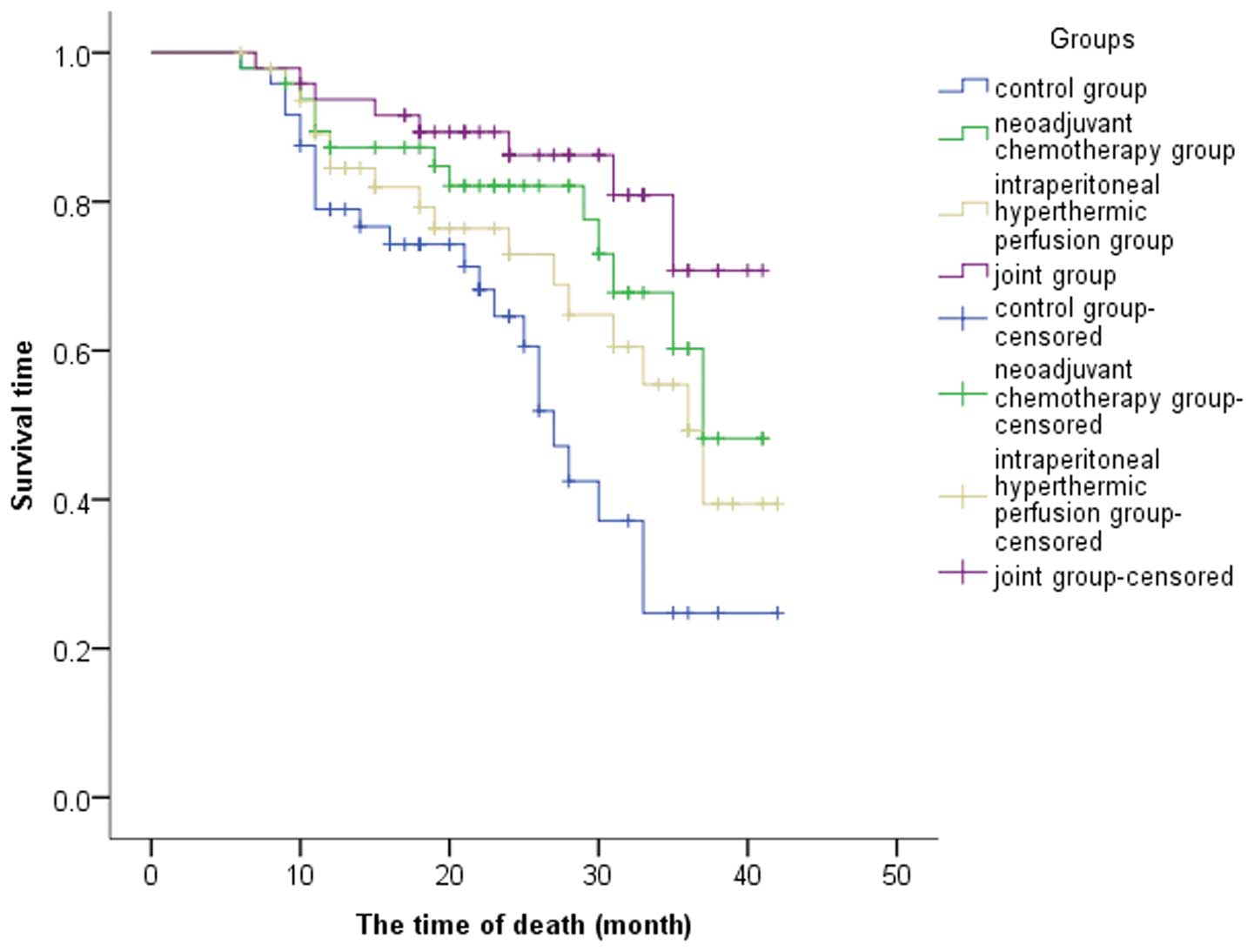
(χ2, 14.63; P<0.001; Fig. 1).
There were 16, 11, 8 and 6 cases that exhibited
recurrence within 2 years in the control, neoadjuvant chemotherapy,
hyperthermic intraperitoneal perfusion chemotherapy and joint
groups, respectively. Thus, the recurrence-free 2-year survival
rates in the corresponding groups were 66.67, 77.08, 83.33 and
87.5%, respectively. Compared with the control group, the
recurrence-free 2-year survival rate of the joint group was
significantly lower (P=0.04; Table
IV).
 | Table IVComparison of recurrence and survival
rates in each group. |
Table IV
Comparison of recurrence and survival
rates in each group.
| Groups | Recurrence rate in 2
years, % | 1-year survival rate,
% | 3-year survival rate,
% |
|---|
| Control | 33.33 (16/48) | 79.16 (38/48) | 35.41 (17/48) |
| Neoadjuvant
chemotherapy | 22.92 (11/48) | 87.50 (42/48) | 62.50 (30/48) |
| Hyperthermic
intraperitoneal perfusion chemotherapy | 16.67 (8/48) | 85.41 (41/48) | 58.33 (28/48)a |
| Joint | 12.50 (6/48)a | 93.75 (45/48)a | 75.00 (36/48)b |
Comparison of the mortality rates in each
group
Median survival times were 27, 33, 32 and 36 months
in the control, neoadjuvant chemotherapy, hyperthermic
intraperitoneal perfusion chemotherapy and joint groups,
respectively; the differences among the four groups were
statistically significant (χ2, 10.37; P=0.001; Fig. 2). The 1- and 3-year survival rates
were 79 and 25% in the control group, 87.3 and 60.3% in the
neoadjuvant chemotherapy group, 84.5 and 49.3% in the hyperthermic
intraperitoneal perfusion chemotherapy group and 93.7 and 70.8% in
the joint group, respectively. The 1-year survival rate of the
joint group was significantly higher when compared with the control
group and the difference was statistically significant (P=0.03).
However, no statistically significant differences were observed
among the 1-year survival rates of the neoadjuvant chemotherapy,
hyperthermic intraperitoneal perfusion chemotherapy and control
groups (P>0.05; Table IV).
However, when compared with the control group, the 3-year survival
rates of the three treatment groups were significantly higher
(P=0.002).
Discussion
Clinically, advanced gastric cancer is most commonly
observed in Chinese patients (7).
Due to the anatomical features of the stomach, advanced gastric
cancer is prone to local recurrence and distal metastasis.
Furthermore, when the tumor penetrates the serosa and results in
peritoneal implantation, the recurrence and metastasis rates are
markedly elevated, rendering a considerably lower 5-year survival
rate. Therefore, a favorable outcome is difficult to achieve
through a surgical approach alone. The emergence of neoadjuvant
chemotherapy has improved the prospects of patients that were
previously inoperable since the chemotherapy shrinks the tumor and
lowers the clinical staging, which is beneficial for the resection
of local lesions. In addition, theoretically, neoadjuvant
chemotherapy can pretreat the possible micro-metastasis
preoperatively. Hyperthermic intraperitoneal perfusion chemotherapy
can effectively eliminate cancer cells that have escaped to the
peritoneal cavity, thus, preventing peritoneal local recurrence and
metastasis. In previous years, numerous studies have shown that
neoadjuvant and hyperthermic intraperitoneal perfusion
chemotherapies can delay the recurrence and metastasis of gastric
cancer without increasing adverse reactions, thus, improving the
median progression-free survival times and overall survival rates,
(4,8–10).
In the present study, no patients succumbed during
surgery in any of the four groups. The myelosuppression and
alimentary tract adverse reactions were predominantly grade I–II
and were successfully treated without affecting the courses of
chemotherapy. No statistically significant differences were
observed in the occurrence of adverse reactions and complications
that were controlled with proper medication. In the follow-up,
statistically significant differences were observed among the
progression-free survival rates of the four groups. Compared with
the control group, the 2-year progression-free survival rate of the
joint group was significantly lower. According to the UK NCRI MAGIC
trial, 503 patients with resectable gastric cancer were randomly
divided into a preoperative chemotherapy + surgery + postoperative
chemotherapy group and a surgery group. The results revealed that
the total response and 5-year survival rates of the former group
were significantly different when compared with the control group
(11). In the French FFCD trial,
224 operable patients were divided into a perioperative
5-fluorouracil plus cisplatin chemotherapy group (113 cases) and a
surgery group. The results revealed there to be significant
differences in the 5-year disease-free survival and overall
survival rates between the two groups (12). Therefore, the two previous studies
indicate that neoadjuvant chemotherapy can ameliorate patient
prognosis. In the present study, paclitaxel, cisplatin and tegafur
were combined to perform neoadjuvant chemotherapy for advanced
gastric cancer. There was no statistically significant difference
in preoperative staging for each patient enrolled in this trial and
every patient underwent the whole course of chemotherapy. The
results were similar to those of the MAGIC and French FFCD trials
and demonstrated that in the neoadjuvant chemotherapy and joint
groups, the median survival times were improved and the local
recurrence rates were reduced.
Contemporary hyperthermic intraperitoneal perfusion
chemotherapy is usually performed once during surgery, thus, the
effectiveness of the procedure may be impaired by the limited
treatment time. In the current study, four to five drainage
catheters were embedded intraoperatively, making the perfusion
procedure more fluent and convenient. Following surgery, early
hyperthermic intraperitoneal perfusion chemotherapy was performed
once a day for 4 days. This regimen was selected as, theoretically,
more tumor cells were able to be drained out of the peritoneal
cavity, enhancing the efficacy of the intraperitoneal
chemotherapeutic drugs (13).
The mechanism behind recurrence in advanced gastric
cancer requires further elucidation. It is generally hypothesized
that the phenomenon is associated with the following aspects
(14). Firstly, intraperitoneal
free cancer cells are important initiators of recurrence in the
peritoneal cavity. Secondly, intraoperative injury of the surface
of intraperitoneal organs facilitates the implantation and spread
of intraperitoneal free cancer cells. Finally, the stress of
anesthesia and surgery results in the immunity of the patient being
suppressed following surgery, rendering the escape of
intraperitoneal free cancer cells from the surveillance of the
immune system. The mechanisms underlying the prevention effects of
hyperthermic intraperitoneal perfusion chemotherapy on
postoperative recurrence and metastasis in the peritoneal cavity
are as follows (15). Firstly, a
large amount of circulatory perfusate dilutes the concentration of
intraperitoneal free cancer cells and rinses the free cancer cells
out of the peritoneal cavity. Secondly, hyperthermia exhibits a
synergic effect with chemotherapy, which is possibly a result of
hyperthermia enhancing the transportation of tumor cells and the
absorbance of platinum-based chemotherapeutic drugs. Thus, the
cancer cells are sensitized to chemotherapy. Thirdly, hyperthermia
causes the denaturation of surface proteins on cancer cells,
altering the permeability of the cytomembrane and enhancing the
absorbance of drugs and their anticancer effect (16). Finally, the hyperthermic perfusate
is absorbed by the peritoneum and is transported to the liver via
the portal vein. This eliminates the cancer cells located within
the portal vein and prevents hepatic metastasis. A meta-analysis
revealed that hyperthermic intraperitoneal perfusion chemotherapy
improves the overall survival rate of patients, but increases the
risk of complications, including myelosuppression (17). However, in the present study, no
statistically significant differences were observed in the
occurrence of adverse reactions among the four groups. Compared
with the control group, the 3-year survival rates of the joint and
hyperthermic intraperitoneal perfusion chemotherapy groups were
significantly higher (P<0.05). Therefore, these groups had an
improved prognosis, which is in accordance with the results of a
previous study by Wang et al (9). In addition, Votanopoulos et al
found that hyperthermic intraperitoneal perfusion chemotherapy
improved the survival rate of elderly patients (18).
In conclusion, the present study demonstrated that
the occurrence rate of adverse reactions and complications in the
joint group was not statistically different from those of the other
three groups. The follow-up study indicated that the joint group
exhibited a significantly lower 2-year postoperative recurrence
rate and a higher overall survival rate. Therefore, neoadjuvant
chemotherapy combined with hyperthermic intraperitoneal perfusion
chemotherapy improves progression-free survival and overall
survival rates, and is a safe, effective and feasible treatment for
resectable advanced gastric cancer. However, the number of cases
enrolled in the present study was small. Although the time span was
large, the follow-up time was only 3 years, which may have resulted
in the efficacy of the combined therapy not being fully exhibited.
In addition, the 5-year survival rate remains uninvestigated.
Therefore, further follow-up studies are required. In conclusion,
neoadjuvant chemotherapy combined with hyperthermic intraperitoneal
perfusion chemotherapy for the treatment of advanced gastric cancer
has been shown to be well tolerated with improved compliance and
efficiency.
Acknowledgements
The authors thank the doctors and nurses in the
Department of Oncology, Cangzhou Central Hospital, for their
support during the study.
References
|
1
|
Yoshikawa T, Rino Y, Yukawa N, et al:
Neoadjuvant chemotherapy for gastric cancer in Japan: a standing
position by comparing with adjuvant chemotherapy. Surg Today.
44:11–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fujitani K: Overview of adjuvant and
neoadjuvant therapy for resectable gastric cancer in the East. Dig
Surg. 30:119–129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Webb CA, Weyker PD, Moitra VK and Raker
RK: An overview of cytoreductive surgery and hyperthermic
intraperitoneal chemoperfusion for the anesthesiologist. Anesth
Analg. 116:924–931. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen SS, Yang XC, Chi F, et al: A phase II
study of preoperative chemotherapy with modified FOLFOX6 followed
by surgery and postoperative chemoradiation in patients with
localized gastric adenocarcinoma. Oncol Res. 20:327–332. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ahn HS, Lee HJ, Hahn S, et al: Evaluation
of the seventh American Joint Committee on Cancer/International
Union Against Cancer Classification of gastric adenocarcinoma in
comparison with the sixth classification. Cancer. 116:5592–5598.
2010. View Article : Google Scholar
|
|
6
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar
|
|
7
|
Zhu X and Li J: Gastric carcinoma in
China: Current status and future perspectives (Review). Oncol Lett.
1:407–412. 2010.PubMed/NCBI
|
|
8
|
Tsuburaya A, Nagata N, Cho H, et al: Phase
II trial of paclitaxel and cisplatin as neoadjuvant chemotherapy
for locally advanced gastric cancer. Cancer Chemother Pharmacol.
71:1309–1314. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang JP, Li CP and Chao Y: Hyperthermic
intraperitoneal chemotherapy: another choice for advanced gastric
cancer? J Chin Med Assoc. 76:415–416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsilimparis N, Bockelmann C, Raue W, et
al: Quality of life in patients after cytoreductive surgery and
hyperthermic intraperitoneal chemotherapy: is it worth the risk?
Ann Surg Oncol. 20:226–232. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chua YJ and Cunningham D: The UK NCRI
MAGIC trial of perioperative chemotherapy in resectable gastric
cancer: implications for clinical practice. Ann Surg Oncol.
14:2687–2690. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ychou M, Boige V, Pignon JP, et al:
Perioperative chemotherapy compared with surgery alone for
resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD
multicenter phase III trial. J Clin Oncol. 29:1715–1721. 2011.
View Article : Google Scholar
|
|
13
|
Hager ED, Dziambor H, Höhmann D, et al:
Intraperitoneal hyperthermic perfusion chemotherapy of patients
with chemotherapy-resistant peritoneal disseminated ovarian cancer.
Int J Gynecol Cancer. 11(Suppl 1): 57–63. 2001. View Article : Google Scholar
|
|
14
|
Dicken BJ, Bigam DL, Cass C, et al:
Gastric adenocarcinoma: review and considerations for future
directions. Ann Surg. 241:27–39. 2005.PubMed/NCBI
|
|
15
|
Nissan A, Stojadinovic A, Garofalo A, et
al: Evidence-based medicine in the treatment of peritoneal
carcinomatosis: Past, present, and future. J Surg Oncol.
100:335–344. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bozzetti F, Yu W, Baratti D, et al:
Locoregional treatment of peritoneal carcinomatosis from gastric
cancer. J Surg Oncol. 98:273–276. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang JY, Xu YY, Sun Z, et al: Comparison
different methods of intraoperative and intraperitoneal
chemotherapy for patients with gastric cancer: a meta-analysis.
Asian Pac J Cancer Prev. 13:4379–4385. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Votanopoulos KI, Newman NA, Russell G, et
al: Outcomes of cytoreductive surgery (CRS) with hyperthermic
intraperitoneal chemotherapy (HIPEC) in patients older than 70
years; survival benefit at considerable morbidity and mortality.
Ann Surg Oncol. 20:3497–3503. 2013.
|