Introduction
Acquired long QT syndrome (LQTS) is a potentially
fatal medical condition that can be exacerbated by a wide range of
antiarrhythmic drugs, particularly those in class III (1,2).
Ibutilide is a new class III antiarrhythmic drug that functions to
recover atrial flutter and atrial fibrillation, but may cause
torsades de pointes (TdP). The drug works by prolonging the
duration of the action potential, particularly under conditions of
hypokalemia and hypomagnesemia (3,4).
Ibutilide prolongs repolarization by enhancing the persistent
Na+ current and suppressing the Ikr current (5).
Several studies have indicated that amplified
transmural and transseptal dispersion of repolarization (TDR) is
essential for the development of TdP in congenital and acquired
LQTS (6–9). The amplified TDR exacerbates
differential refractoriness across the myocardial wall, inducing
early afterdepolarization (EAD) and R-on-T extrasystole, which
initiates and maintains re-entrant circuits (10).
TDR is a result of significant heterogeneity in the
expression of ion channels among various cell types in the
ventricular wall (10). Such
intrinsic electrophysiological heterogeneity is diminished due to
the existence of gap junctions. Gap junctions permit the movement
of small molecules along the electrochemical gradient and thus
assist the electrical synchronization of adjacent myocytes,
reducing TDR. Enhancing gap junction coupling is considered to
result in the reduction of TDR and thus provide an antiarrhythmic
effect, particularly in LQTS. Antiarrhythmic peptide 10 (AAP10) is
a gap junction opener (11) that
inhibits ventricular arrhythmia, particularly TdP, in various
congenital LQTS models mimicked by drugs in animal experiments
(12,13).
The aim of the present study was to determine
whether AAP10 inhibits ibutilide-induced TdP, a type of
drug-induced LQTS. In addition, the mechanism of TdP was explored
with the aim of providing a safe method for the use of
ibutilide.
Materials and methods
Study approval
All experiments involving animals were approved by
the Institutional Animal Care and Use Committee of Tongji Medical
College (Wuhan, China).
Arterially perfused rabbit left
ventricular wedge preparations
Arterially perfused rabbit left ventricular wedges
were prepared by a standard technique. Japanese white rabbits,
provided by the Wuhan Institute of Biological Products (Wuhan,
China), were anesthetized with 35–40 mg/kg sodium pentobarbital
(i.v.) and anticoagulated with heparin. The hearts were quickly
excised and submerged in cold (4°C) cardioplegic solution (mmol/l):
NaCl, 109; KCl, 24; NaH2PO4, 0.9;
NaHCO3, 20; CaCl2, 1.8; MgSO4,
0.5; and glucose, 5.5. The left circumflex branch of the coronary
artery was cannulated and perfused with the same cardioplegic
solution. Unperfused areas of the left ventricle, easily identified
by the reddish appearance due to the existence of unflushed
erythrocytes, were removed.
Electrophysiological observations of the
wedge preparations
Cannulated preparations were placed in a small
heated tissue bath and arterially perfused with Tyrode’s solution
(mmol/l): NaCl, 129.0; KCl, 4.0; NaH2PO4,
0.9; NaHCO3, 20; CaCl2, 1.8;
MgSO4, 0.5; and glucose, 5.5; buffered with 95%
O2 and 5% CO2. The temperature was maintained
at 35.7±0.2°C and the perfusion pressure was maintained at 35–45
mmHg by a peristaltic pump. A bipolar concentric silver electrode
fixed to the surface of the endocardium provided a continuous
single stimulus with a basic cycle length (BCL) of 2,000 msec. Two
Ag/AgCl electrodes were placed on the two sides of the wedge and
were used to record the pseudo-electrocardiograms. Transmembrane
action potentials were recorded simultaneously by floating glass
microelectrodes (direct current resistance, 10–20 MΩ) filled with
2.7 M KCl.
Study protocols
Japanese white rabbits (weight, 2–2.5 kg) of either
gender, were randomly divided into the five following groups:
Control, hypokalemia and hypomagnesemia (hypo), ibutilide,
ibutilide and hypo and AAP10 groups. The control group (n=9) were
perfused with Tyrode’s solution. The hypo group (n=9) were perfused
with a modified reduced-potassium and reduced-magnesium Tyrode’s
solution (mmol/l): NaCl, 131.0; KCl, 2.0;
NaH2PO4, 0.9; NaHCO3, 20;
CaCl2, 1.8; MgSO4, 0.25; and glucose, 5.5.
The ibutilide group (n=9) were perfused with Tyrode’s solution for
1 h, which was followed by the addition of 2 mg/l ibutilide (Anhui
BBCA Pharmaceutical Co., Ltd., Anhui, China). The development of
spontaneous and programmed electrical stimulation (PES)-induced TdP
was observed, as well as the EAD and changes in the QT interval and
Tp-e. The ibutilide and hypo group (n=9) were perfused with
ibutilide dissolved in modified reduced-potassium and
reduced-magnesium Tyrode’s solution. The AAP10 group (n=9) were
perfused with 500 nM AAP10 (Chinese Peptide Co., Hangzhou, China)
for 15 min, which was followed by the addition of a solution
containing 500 nM AAP10 and 2 mg/l ibutilide in modified
reduced-potassium and reduced-magnesium Tyrode’s solution.
The QT interval was defined as the time from the
onset of QRS to the point at which the final downslope of the T
wave crossed the isoelectric line. TDR was measured by Tp-e (from
the peak to the end of the T wave). The Tp-e and QT interval ratio
were measured in the same beats.
Western blotting
The ventricular wedge preparations were removed from
the tissue bath following the completion of PES and immediately
frozen at −80°C. The frozen tissues were pulverized with a mortar
and pestle that had been cooled in liquid nitrogen. The samples
were then homogenized in a lysis buffer containing 30 mM Tris (pH
7.4), 150 mM NaCl, 1% nonylphenoxypolyethoxyethanol, 0.25% sodium
deoxycholate, 1 mM EDTA, 0.1 mM phenylmethylsulfonyl fluoride, 1
μg/ml aprotinin, 1 μg/ml pepstatin and 1 μg/ml leupeptin.
Homogenates were cleared by centrifugation at 10,000 × g for 30 min
at 4°C.
Lysate samples containing 50 μg total protein were
resolved by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and immunoblotted. GAPDH protein was used as a
control to ensure equal protein loading. Primary antibody
incubations were performed overnight at 4°C using mouse monoclonal
antibodies to measure the non-phosphorylated component of connexin
43 or total connexin 43 (Cx43; 1:1,000 dilution; Zymed
Laboratories, Inc., San Francisco, CA, USA). After washing, the
membranes were incubated with horseradish peroxidase-conjugated
goat anti-mouse secondary antibodies (1:1,000 dilution; Novogen,
Inc., Madison, WI, USA), treated with chemiluminescence reagent
(Pierce Biotechnology, Inc., Rockford, IL, USA) and exposed to
X-ray film. Immunoreactivity was quantified by densitometric
analysis with Image-Pro Plus 6.0 software (Media Cybernetics Inc.,
Rockville, MD, USA). The quantity of Cx43 was defined by the band
density corresponding to the Cx43 protein normalized against
GAPDH.
Statistical analysis
Statistical analysis was performed using the
Student’s t-test or one-way analysis of variance. Fisher’s exact
test was used for comparing event incidences, including the
occurrence of EAD, R-on-T extrasystole and TdP. All values are
expressed as mean ± SEM, unless otherwise noted. P<0.05 was
considered to indicate a statistically significant difference.
Statistical analyses were performed with SPSS for Windows (Version
13.0, SPSS, Inc., Chicago, IL, USA).
Results
Effect of ibutilide on cardiac
electrophysiological parameters
As with other class III antiarrhythmic drugs, the
action potential and QT interval were significantly prolonged by
ibutilide at a BCL of 2,000 msec. In the ibutilide group, the QT
interval was increased from 303±19 to 498±68 msec (P<0.001, vs.
control), Tp-e was increased from 50±7 to 116±24 msec (P<0.01,
vs. control) and the Tp-e/QT ratio was increased from 0.16±0.02 to
0.23±0.04 (P<0.01, vs. control). The results show that ibutilide
markedly increased the Tp-e interval compared with the QT interval
(Table I).
 | Table IChanges in the electophysiological
parameters of the various groups (n=9 per group). |
Table I
Changes in the electophysiological
parameters of the various groups (n=9 per group).
| Groups | QT interval msec | Tp-e msec | Tp-e/QT |
|---|
| Control | 303±19b | 50±7b | 0.16±0.02b |
| Hypo | 318±27b | 62±15b |
0.19±0.04c |
| Ibutilide | 498±68a | 116±24b | 0.23±0.04a |
| Ibutilide and
hypo | 611±168 | 190±79 | 0.31±0.08 |
| AAP10 | 459±52a | 93±31b | 0.20±0.05b |
The incidence of EAD was 3/9 (P=0.10, vs. control)
and was accompanied by R-on-T extrasystole. No spontaneous TdP or
other ventricular arrhythmias were observed in the ibutilide group
(Table II).
 | Table IIIncidence of arrhythmia in the
various groups (n=9 per group). |
Table II
Incidence of arrhythmia in the
various groups (n=9 per group).
| | | TdP |
|---|
| | |
|
|---|
| Groups | EAD | R-on-T | SPO | PES | Total |
|---|
| Control | 0 | 0 | 0 | 0 | 0 |
| Hypo | 0 | 0 | 0 | 2 | 2 |
| Ibutilide | 3 | 3 | 0 | 0 | 0 |
| Ibutilide and
hypo | 4 | 4 | 4 | 3 | 7 |
| AAP10 | 3 | 3 | 1 | 0 | 1a |
Changes in electrophysiological
parameters in the ibutilide and hypo group
When ibutilide was perfused under conditions of
hypokalemia and hypomagnesemia with a BCL of 2,000 msec, the
prolongation of the QT interval was greater than that with
ibutilide alone. The QT interval extended to 611±168 msec
(P<0.01, vs. ibutilide group), while Tp-e was also extended to
190±79 msec (P<0.001, vs. ibutilide group). The Tp-e/QT ratio
increased to 0.31±0.08 (P<0.01, vs. ibutilide group; Table I).
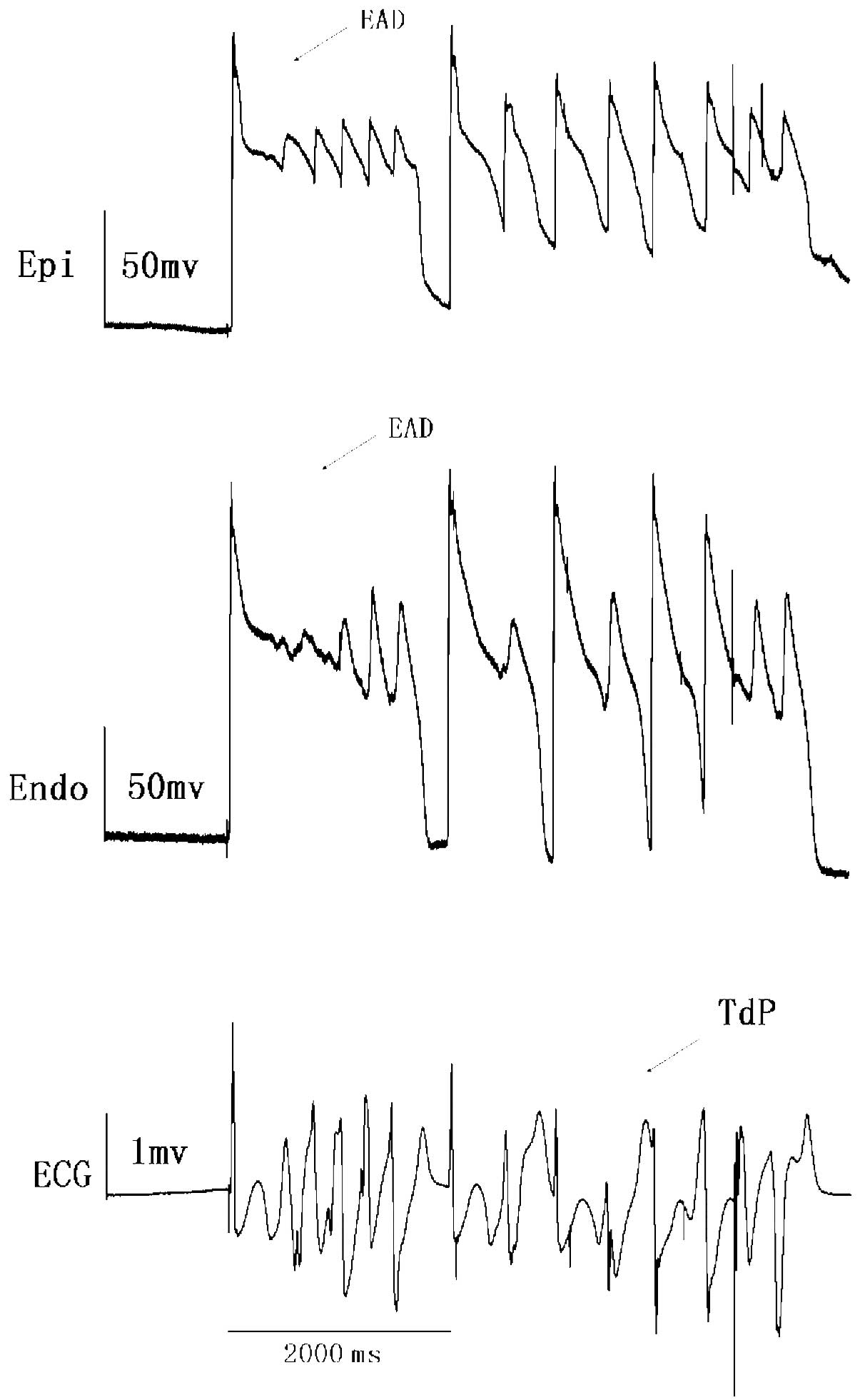
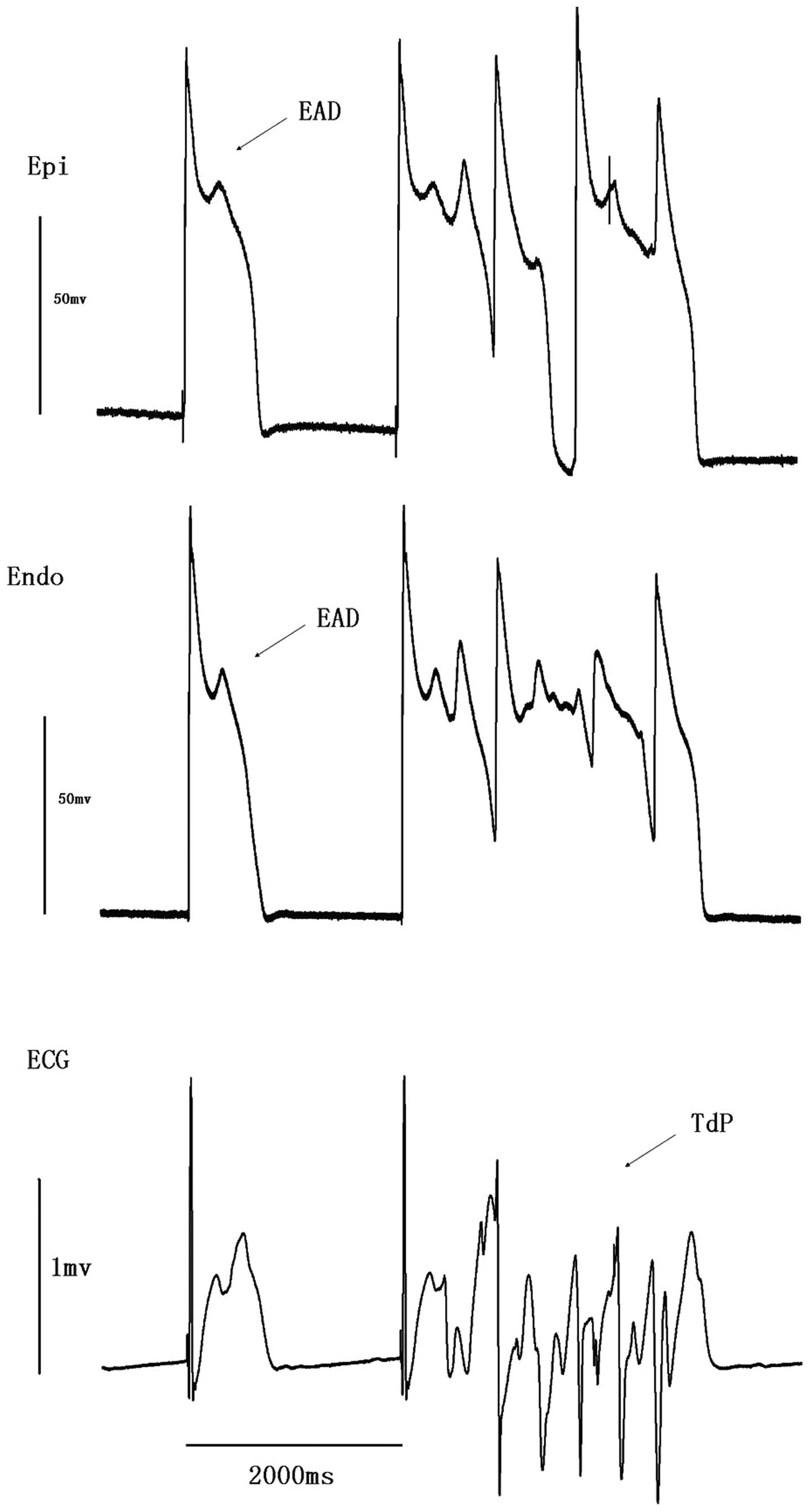
EAD was observed in four preparations (n=9) while
TdP occurred in seven (P<0.01, vs. ibutilide group; Table II). TdP in four preparations was
spontaneous (Fig. 1 and 2), while in the other three preparations
TdP was induced by PES. Spontaneous TdP was always followed by EAD
and R-on-T extrasytole.
Effect of AAP10 on the QT interval, Tp-e
and the incidence of EAD, R-on-T extrasystole and TdP
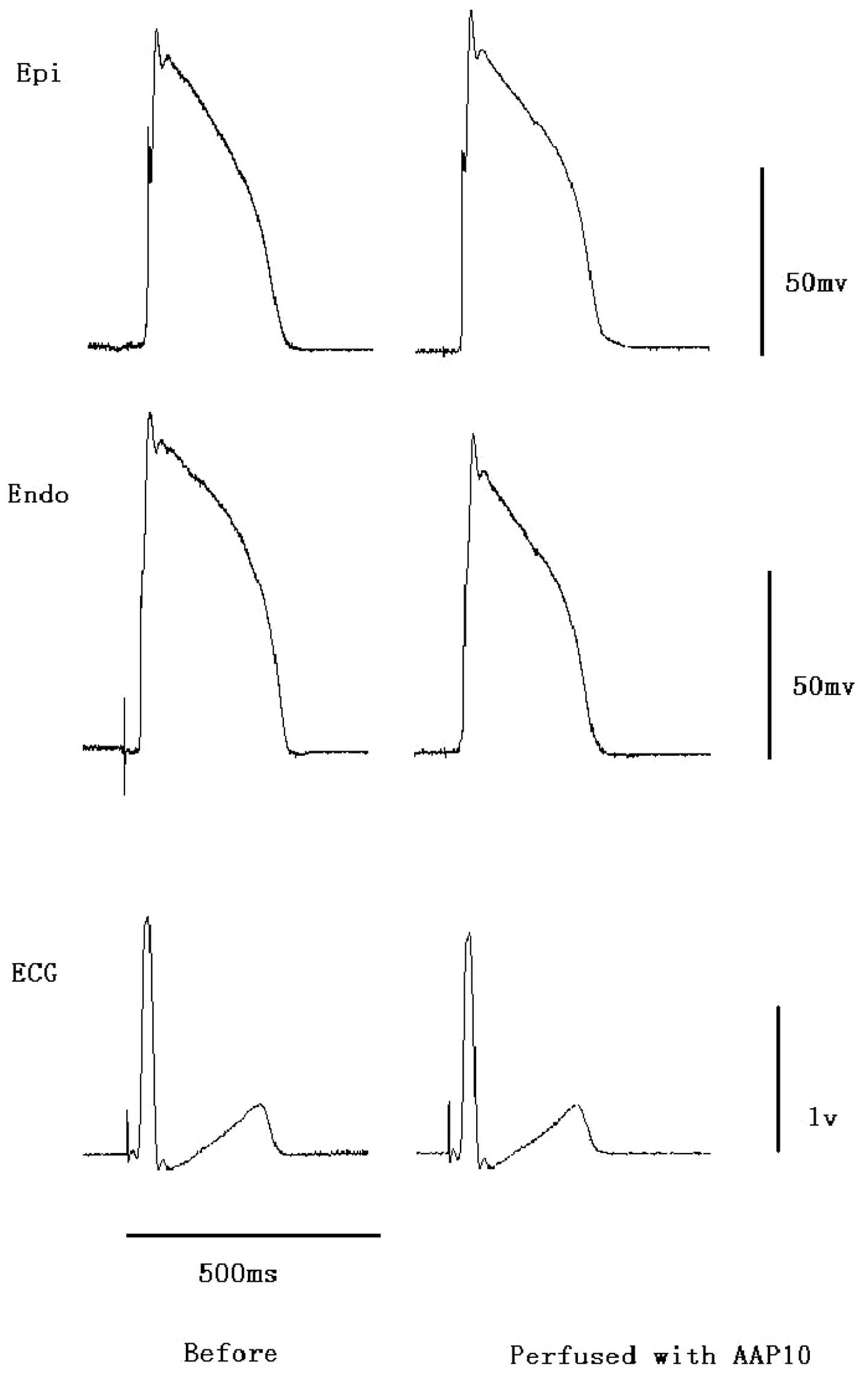
Prior to the administration of ibutilide, perfusion
with AAP10 at a concentration of 500 nM did not significantly
affect the QT interval, action potential duration, Tp-e or QRS
duration (Fig. 3). However, the
prolonged action potentials, QT intervals and Tp-e induced by
ibutilide under conditions of hypokalemia and hypomagnesemia were
reversed by AAP10 (Fig. 4 and
Table I). The QT interval
decreased to 459±52 msec (P<0.01, vs. ibutilide and hypo group),
Tp-e decreased to 93±31 msec (P<0.001, vs. ibutilide and hypo
group) and Tp-e/QT decreased to 0.20±0.05 (P<0.001, vs.
ibutilide and hypo group). The reduction in Tp-e/QT showed that the
reduction in Tp-e was greater that than in the QT interval.
EAD and R-on-T extrasystole were observed in 3
preparations (n=9), while TdP occurred in 1 preparation (P<0.01,
vs. ibutilide and hypo group; Table
II).
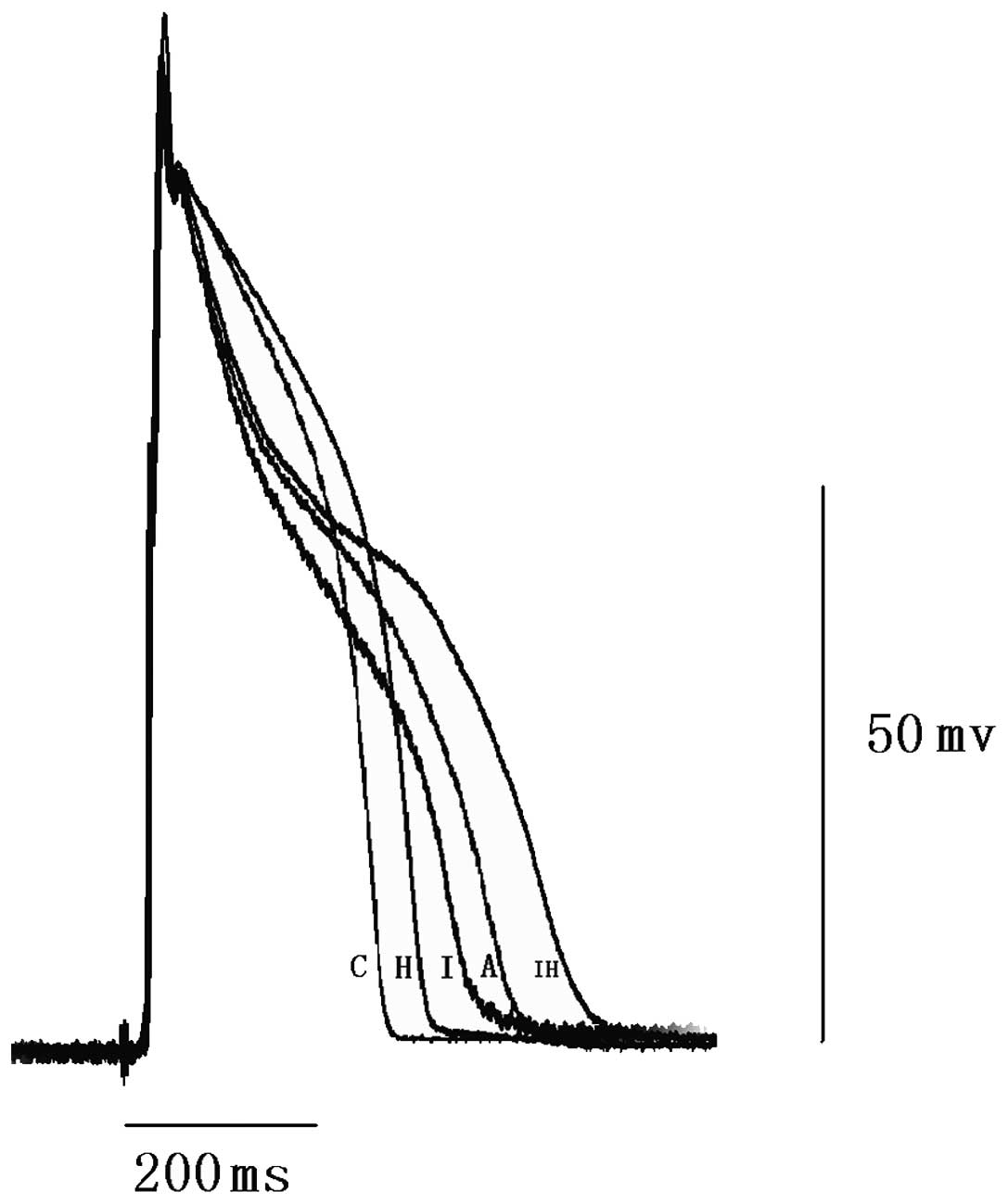
Changes in the phosphorylation levels of
Cx43 serine 368
Comparisons between the serine 368
non-phosphorylated Cx43 and total Cx43 expression profiles in the
various groups are shown in Fig.
5. There were no significant differences in total Cx43
expression levels among the various groups. The level of Cx43 with
non-phosphorylated serine 368 increased in the ibutilide and hypo
group compared with that in the control group. This indicates that
the majority of serine 368 residues in Cx43 molecules localized in
gap junctions in the normal myocardium are phosphorylated. AAP10 at
500 nM attenuated the increase in non-phosphorylated residues in
the ibutilide and hypo group. This indicates that AAP10 can reverse
the dephosphorylation of serine 368 in Cx43, which is consistent
with AAP10 reducing the incidence of arrhythmia.
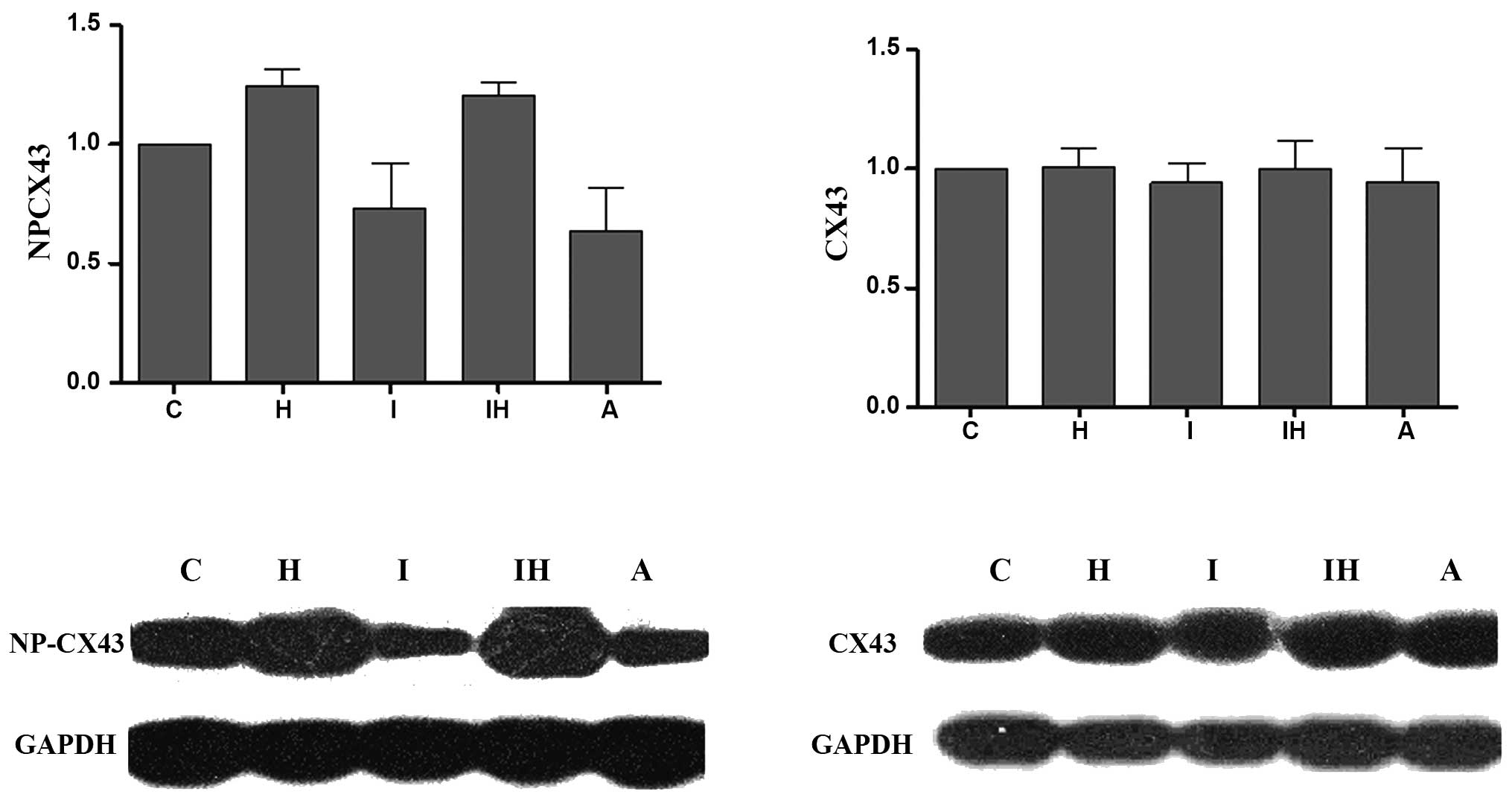 | Figure 5Western blotting results and
quantitative densitometric analysis of non-phosphorylated Cx43 and
total Cx43. Signals were quantified by densitometry and normalized
against GAPDH. There are no significant differences among the
control and other groups for total Cx43. However, the
non-phosphorylated Cx43 level is significantly increased in the
ibutilide and hypo group (P<0.05, vs. control). AAP10 at a
concentration of 500 nM significantly attenuated the increase in
non-phosphorylated Cx43 (P<0.05, vs. ibutilide and hypo group).
C, control group; H, hypo group; I, ibutilide group; A, AAP10
group; IH, ibutilide and hypo group; AAP10, antiarrythmic peptide
10; hypo, hypokalemia and hypomagnesemia; Cx43, connexin 43. |
Discussion
Prolonging the QT interval with drugs can result in
a predisposition to TdP, which may degenerate into ventricular
fibrillation and cause sudden mortality (14). Drug-induced TdP may be more common
than initially hypothesized (15).
A number of medicines lead to drug-induced TdP, including class IA
and III antiarrhythmic drugs, antibiotics and antidepressants
(16).
Atrial fibrillation is one of the most common
arrhythmias, particularly with increasing age. The incidence has
been shown to reach 10% among individuals >80 years-old
(17). Ibutilide is a new class
III antiarrhythmic drug that has been widely used in recent years
to treat cardioversion atrial fibrillation and atrial flutter in
clinical practice. The cardioversion rate with ibutilide is
significantly higher than with other antiarrhythmic drugs,
particularly for atrial flutter cardioversion (18). However, several clinical trials
have reported adverse effects, including non-sustained and
sustained polymorphic ventricular tachycardia (VT). A meta-analysis
of five studies found the occurrence of TdP with ibutilide to be
1.7% and the incidence of polymorphic VT with ibutilide to be
higher than with sotalol (3).
Thus, in-hospital cardiac monitoring is recommended when ibutilide
infusion is initiated (1).
Animal studies have indicated that ibutilide
produces a greater degree of TDR, as well as a higher incidence of
EAD and TdP in cardiomyopathic dogs compared with acute
atrioventricular block (19). The
present study identified that Tp-e and Tp-e/QT also increased in
normal rabbit wedge preparations when perfused with ibutilide. The
incidence of TdP increased under conditions of hypokalemia and
hypomagnesemia, a characteristic of drug-induced LQTS.
The pharmacological effects of ibutilide are
Ikr-blockade and persistent Na+ current activation.
However, the heterogeneity of Ikr distribution and the persistent
Na+ current channels between myocardial cells leads to
an increased TDR across the ventricular wall. Several studies have
indicated that amplified TDR is the primary basis of the mechanism
essential for the development of TdP (20–23).
The increased TDR creates a vulnerable window for the development
of re-entry. The reduction in net repolarizing current also results
in a predisposition to developing EAD-induced triggered activity in
myocardial cells, which provides the extrasystole that triggers TdP
when it falls within the vulnerable window (24–26).
The studies by Yan et al (24–26)
and a number of previous studies suggest that Tp-e/QT disturbance
may be a new index for predicting heart arrhythmia (27–31).
This method is superior to TDR, to a certain extent, since it
corrects the impact of the QT interval for TDR.
In the present study, the Tp-e/QT ratio was
increased by ibutilide resulting in TdP. AAP10 was found to
decrease Tp-e/QT and suppress ibutilide-induced TdP. Our studies
have shown that gap junctions play an important role in TDR
(12–13). Through opening gap junctions the
TDR is reduced to a certain extent, resulting in enhanced ion
exchange between cells. In the model of congenital LQTS mimicked by
drugs, increasing the coupling of gap junctions resulted in the
inhibition of TdP and the reduction of TDR, through strengthening
the ion exchange between various cells (12,13).
Gap junctions are electrical and chemical channels
located between adjacent myocardial cells and are the major
constituent of intercalated discs. The main gap junction protein in
ventricular muscle is Cx43. AAP10 is a synthetic peptide with
antiarrhythmic effects that functions by opening gap junctions
(32). The coupling and uncoupling
of gap junctions is primarily regulated by the phosphorylation of
Cx43 (33). AAP10 binds to a
G-protein-dependent membrane receptor and enhances the
phosphorylation of serine 368 in Cx43 by activating protein kinase
C (34). In the present study, the
immunoblotting results revealed that the expression levels of
non-phosphorylated Cx43 were much higher in the ibutilide and hypo
group compared with the control. However, in the presence of AAP10,
the dephosphorylation of serine 368 was decreased, which
corresponds with reductions in Tp-e and Tp-e/QT and the incidence
of arrhythmia. These results indicate that well-coupled gap
junctions may function as a brake to limit increases in TDR under
conditions of LQTS.
The present study indicated that AAP10 decreases the
incidence of ibutilide-induced VT. The combined application of
antiarrhythmic drugs has become increasingly studied in recent
years. By balancing the various pharmacological mechanisms of
antiarrhythmic drugs, the combined application of antiarrhythmic
drugs may well offset side-effects, including proarrhthmia, without
affecting the antiarrhythmic function.
The present study demonstrated that the gap junction
enhancer, AAP10, significantly reduces Tp-e and Tp-e/QT and
prevents TdP by inhibiting the dephosphorylation of Cx43 in
ibutilide-induced LQTS. The study also indicated that the
administration of AAP10 with a class III antiarrhythmic drug may be
a novel approach for the treatment of arrhythmias while avoiding
proarrhythmia.
Acknowledgements
The study was supported by grants from the National
Natural Science Foundation of China (nos. 30770879 and
30973601).
References
|
1
|
Roden DM: Drug-induced prolongation of the
QT interval. N Engl J Med. 350:1013–1022. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yamada S, Kuga K and Yamaguchi I: Torsade
de pointes induced by intravenous and long-term oral amiodarone
therapy in a patient with dilated cardiomyopathy. Jpn Circ J.
65:236–238. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kowey PR, VanderLugt JT and Luderer JR:
Safety and risk/benefit analysis of ibutilide for acute conversion
of atrial fibrillation/flutter. Am J Cardiol. 78:46–52. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zeltser D, Justo D, Halkin A, Prokhorov V,
Heller K and Viskin S: Torsade de pointes due to noncardiac drugs:
most patients have easily identifiable risk factors. Medicine
(Baltimore). 82:282–290. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Naccarelli GV, Lee KS, Gibson JK and
VanderLugt J: Electrophysiology and pharmacology of ibutilide. Am J
Cardiol. 78:12–16. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sicouri S, Glass A, Ferreiro M and
Antzelevitch C: Transseptal dispersion of repolarization and its
role in the development of Torsade de Pointes arrhythmias. J
Cardiovasc Electrophysiol. 21:441–447. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Antzelevitch C: Ionic, molecular, and
cellular bases of QT-interval prolongation and torsade de pointes.
Europace. 9(Suppl 4): iv4–iv15. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Raviña T, Raviña P and Gutierrez J:
Acquired long QT syndrome: risperidone-facilitated triggered
activity and Torsades de Pointes during complete AV block. I. Int J
Cardiol. 116:416–420. 2007.PubMed/NCBI
|
|
9
|
Wang L: Congenital long QT syndrome: 50
years of electrophysiological research from cell to bedside. Acta
Cardiol. 58:133–138. 2003.PubMed/NCBI
|
|
10
|
Antzelevitch C, Shimizu W, Yan GX, et al:
The M cell: its contribution to the ECG and to normal and abnormal
electrical function of the heart. J Cardiovasc Electrophysiol.
10:1124–1152. 1999.PubMed/NCBI
|
|
11
|
Aonuma S, Kohama Y, Akai K, Komiyama Y,
Nakajima S, Wakabayashi M and Makino T: Studies on heart. XIX
Isolation of an atrial peptide that improves the rhythmicity of
cultured myocardial cell clusters. Chem Pharm Bull (Tokyo).
28:3332–3339. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Quan XQ, Bai R, Liu N, Chen BD and Zhang
CT: Increasing gap junction coupling reduces transmural dispersion
of repolarization and prevents torsade de pointes in rabbit LQT3
model. J Cardiovasc Electrophysiol. 18:1184–1189. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Quan XQ, Bai R, Lu JG, et al:
Pharmacological enhancement of cardiac gap junction coupling
prevents arrhythmias in canine LQT2 model. Cell Commun Adhes.
16:29–38. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hondeghem LM: QT and TdP. QT: an
unreliable predictor of proarrhythmia. Acta Cardiol. 63:1–7. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abdon NJ, Herlitz J and Bergfeldt L:
Drug-induced cardiac arrest maybe more common than believed.
Lakartidningen. 107:521–522. 524–525. 2010.(In Swedish).
|
|
16
|
Postema PG, Neville J, de Jong JS, Romero
K, Wilde AA and Woosley RL: Safe drug use in long QT syndrome and
Brugada syndrome: comparison of website statistics. Europace.
15:1042–1049. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Benjamin EJ, Wolf PA, D’Agostino RB,
Silbershatz H, Kannel WB and Levy D: Impact of atrial fibrillation
on the risk of death: the Framingham Heart Study. Circulation.
98:946–952. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vos MA, Golitsyn SR, Stangl K, et al: The
Ibutilide/Sotalol Comparator Study Group: Superiority of ibutilide
(a new class III agent) over DL-sotalol in converting atrial
flutter and atrial fibrillation. Heart. 79:568–575. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hsieh MH, Chen YJ, Lee SH, Ding YA, Chang
MS and Chen SA: Proarrhythmic effects of ibutilide in a canine
model of pacing induced cardiomyopathy. Pacing Clin Electrophysiol.
23:149–156. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Antzelevitch C: M cells in the human
heart. Circ Res. 106:815–817. 2010. View Article : Google Scholar
|
|
21
|
Shimizu W, McMahon B and Antzelevitch C:
Sodium pentobarbital reduces transmural dispersion of
repolarization and prevents torsades de pointes in models of
acquired and congenital long QT syndrome. J Cardiovasc
Electrophysiol. 10:154–164. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weissenburger J, Nesterenko VV and
Antzelevitch C: Transmural heterogeneity of ventricular
repolarization under baseline and long QT conditions in the canine
heart in vivo: torsades de pointes develops with halothane but not
pentobarbital anesthesia. J Cardiovasc Electrophysiol. 11:290–304.
2000. View Article : Google Scholar
|
|
23
|
Pu J, Zhang C, Quan X, et al: Effect of
potassium aspartate and magnesium on ventricular arrhythmia in
ischemia-reperfusion rabbit heart. J Huazhong Univ Sci Technolog
Med Sci. 28:517–519. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yan GX and Antzelevitch C: Cellular basis
for the electrocardiographic J wave. Circulation. 93:372–379. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yan GX, Rials SJ, Wu Y, Liu T, Xu X,
Marinchak RA and Kowey PR: Ventricular hypertrophy amplifies
transmural repolarization dispersion and induces early
afterdepolarization. Am J Physiol Heart Circ Physiol.
281:H1968–H1975. 2001.PubMed/NCBI
|
|
26
|
Yan GX, Shimizu W and Antzelevitch C:
Characteristics and distribution of M cells in arterially perfused
canine left ventricular wedge preparations. Circulation.
98:1921–1927. 1998. View Article : Google Scholar
|
|
27
|
Gupta P, Patel C, Patel H, Narayanaswamy
S, Malhotra B, Green JT and Yan GX: T(p-e)/QT ratio as an index of
arrhythmogenesis. J Electrocardiol. 41:567–574. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Anttonen O, Väänänen H, Junttila J,
Huikuri HV and Viitasalo M: Electrocardiographic transmural
dispersion of repolarization in patients with inherited short QT
syndrome. Ann Noninvasive Electrocardiol. 13:295–300. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu HR, Vlaminckx E, Van de Water A,
Rohrbacher J, Hermans A and Gallacher DJ: In-vitro experimental
models for the risk assessment of antibiotic-induced QT
prolongation. Eur J Pharmacol. 577:222–232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu T, Brown BS, Wu Y, Antzelevitch C,
Kowey PR and Yan GX: Blinded validation of the isolated arterially
perfused rabbit ventricular wedge in preclinical assessment of
drug-induced proarrhythmias. Heart Rhythm. 3:948–956. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Inoue M, Shimizu M, Ino H, et al: Q-T peak
dispersion in congenital long QT syndrome: possible marker of
mutation of HERG. Circ J. 67:495–498. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dhein S, Manicone N, Müller A, Gerwin R,
Ziskoven U, Irankhahi A, Minke C and Klaus W: A new synthetic
antiarrhythmic peptide reduces dispersion of epicardial activation
recovery interval and diminishes alterations of epicardial
activation patterns induced by regional ischemia. A mapping study.
Naunyn Schmiedebergs Arch Pharmacol. 350:174–184. 1994. View Article : Google Scholar
|
|
33
|
Müller A, Gottwald M, Tudyka T, Linke W,
Klaus W and Dhein S: Increase in gap junction conductance by an
antiarrhythmic peptide. Eur J Pharmacol. 327:65–72. 1997.PubMed/NCBI
|
|
34
|
Lampe PD, TenBroek EM, Burt JM, Kurata WE,
Johnson RG and Lau AF: Phosphorylation of connexin43 on serine368
by protein kinase C regulates gap junctional communication. J Cell
Biol. 149:1503–1512. 2000. View Article : Google Scholar : PubMed/NCBI
|