Introduction
Liver resection is the only curative treatment for a
number of patients with primary or secondary liver tumors (1). Portal vein occlusion is widely used
to induce liver hypertrophy in the future remnant liver (FRL) prior
to major liver resection (2). Two
strategies are available to induce hypertrophy of the liver: Portal
vein ligation (PVL) and portal vein embolization (PVE). Although a
number of clinicians consider PVE to be superior to PVL, previous
studies have shown that PVL is as effective as PVE in inducing
hypertrophy to the volume of the remnant liver (3,4).
Preoperative portal vein occlusion by PVE or PVL is an effective
method to increase the volume of the FRL. While the use of PVE and
PVL is increasing, there is growing evidence that PVE and PVL
stimulate not only the growth of the FRL, but also affect tumor
size in occluded and non-occluded liver segments (5,6).
The prevalence of cirrhosis in patients with
hepatocellular carcinoma (HT) is between 80 and 90%, while 10–20%
of HT cases develop in patients without cirrhosis (7). At present, it is commonly accepted
that PVL and PVE are generally safe procedures that have few side
effects in non-cirrhotic patients (8). Sakai et al (6) demonstrated that PVL accelerates tumor
growth in ligated lobes, but not contralateral lobes. However, to
date, there have been no studies specifically demonstrating the
effect of PVE or PVL on liver tumor growth and regeneration in
cirrhotic liver lobes. Thus, in the present study, a rat model of
PVL was used to determine the effects of ligation on HT growth and
liver regeneration. In addition, the association between cirrhosis
severity and tumor growth was evaluated in ligated and non-ligated
lobes.
Materials and methods
Animal preparation
Male Wistar rats weighing 350–400 g were purchased
from the Center for Animal Experiment/Animal Bio-safety Level III
Laboratory of Wuhan University (Wuhan, China) and the Animal
Facility of Nancy University (Nancy, France). The Research
Committees of the two universities approved the animal experimental
procedures. All the rats received standardized care in accordance
with the National Institutes of Health Guidelines for Ethical
Animal Research. Animals were maintained in an animal experimental
room at a temperature of 25±5°C under a 12-h light/dark cycle.
Study protocol
A total of 45 rats were randomly divided into three
groups. The PVL group consisted of normal rats that received PVL
(n=15). The HT group consisted of rats with tumors that did not
receive PVL (n=15) and the HT + PVL group comprised rats with
tumors that received PVL (n=15). Rats in the HT and HT + PVL groups
were fed a diet containing diethylnitrosamine (DEN; Sigma Aldrich,
St. Louis, MO, USA; 95 mg/kg body weight/week) for 12 weeks to
induce hepatocellular carcinomas. Rats that survived with evident
HTs were selected for further study. Surgery was performed when the
administration of DEN was completed. PVL was performed in the PVL
and HT + PVL groups, while the rats in the HT group received
sham-operation. Blood (at days 0, 1, 2 and 7) and liver (at day 14)
samples were collected for analysis following surgery. Tumor size
was measured using positron emission tomography (PET) scans 7 days
prior to PVL, as well as at day 7 and 14 following PVL. Ligated and
non-ligated liver lobes were also measured at day 14 following PVL.
The tumor growth rate and ratios of non-ligated lobe weight to
total body weight and non-ligated lobe weight to ligated lobe
weight were calculated.
PVL
Rats were anesthetized via inhalation of
isoflurane/O2 (Baxter International, Inc., Munich,
Germany). Following a midline laparotomy, the liver was removed
from the ligaments. A double running suture was performed on
sham-operated rats. The selective PVL was performed on the middle
and left lobes (lobes 1–3) under a microscope. Then, the middle and
left lobes (lobes 1–3) were defined as ligated lobes, while the
right lobes were defined as non-ligated lobes (lobes 4–7). The
corresponding portal veins of the liver were ligated with a 7-0
Fumalen following careful dissection of the hepatic artery.
Portography was performed prior to and following the selective PVL
to visualize the liver anatomy and to demonstrate portal occlusion
of the appropriate liver segments.
Blood analysis
Blood samples were obtained from the femoral vein
and centrifuged at 3,000 × g for 10 min at 4°C. Blood serum was
stored at −20°C prior to analysis. Alanine transaminase (ALT) and
total bilirubin (TBI) levels were measured using a TBA-2000FR
System (Toshiba Corporation, Tokyo, Japan).
Evaluation of tumor size and liver
weight
Tumor size was measured using PET 7 days prior to
PVL and at day 7 and 14 following PVL. Rats were sacrificed 2 weeks
following PVL and the weights of the ligated and non-ligated lobes
were measured using a laboratory microscale (Sartorius AG,
Goettingen, Germany). The ratios of non-ligated lobe weight to
total body weight and non-ligated lobe weight to ligated lobe
weight were calculated. The tumor growth rate was calculated using
the following formula: Growth rate = (TV2 − TV1)/TV1, where TV1 was
the tumor volume prior to PVL and TV2 was the tumor volume
following PVL.
Histological examination
Morphological examination was performed before and
14 days after PVL. Liver biopsy specimens were obtained from the
rats prior to PVL for histological evaluation and all the rats were
sacrificed at day 14 following PVL for histological evaluation.
Under a light microscope, three observational fields were randomly
selected in each specimen and were blindly evaluated by two
pathologists following randomization. Liver tissue was assessed in
each case using a modified Knodell scoring system via four main
aspects on hematoxylin and eosin (H&E) stained sections:
Periportal and bridging necrosis, intralobular degeneration, focal
necrosis, portal inflammation and fibrosis.
Statistical analysis
Data are expressed as the mean ± SEM. Comparisons
among mean values were performed by one- or two-way analysis of
variance. Statistical analysis was conducted using SPSS software
8.0 (SPSS, Inc., Chicago, IL, USA) and a two-tailed value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
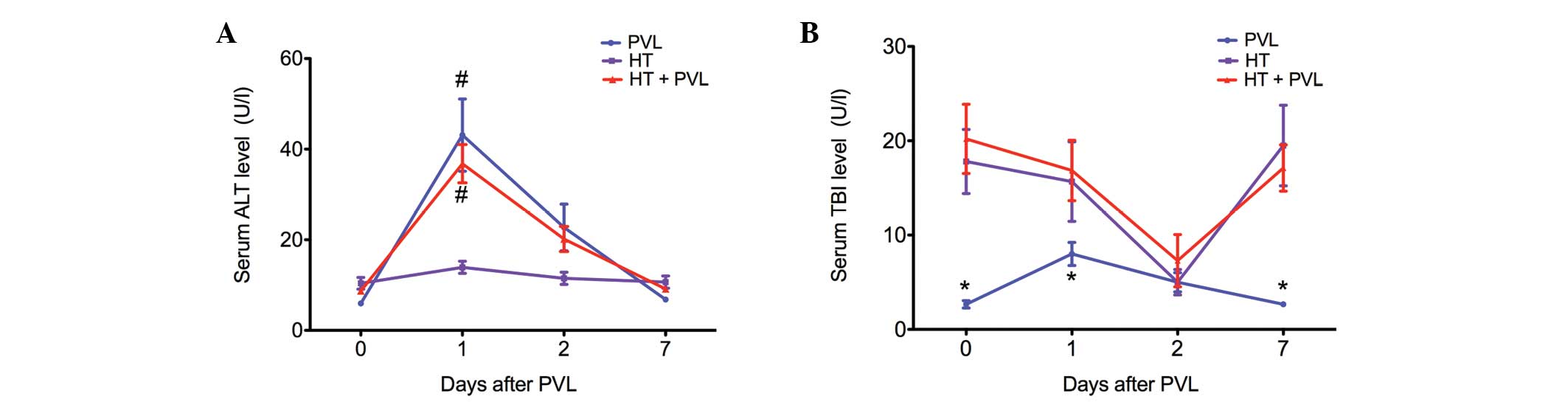
Changes in ALT and TBI serum levels
Serum levels of ALT were assessed as a measure of
hepatocyte necrosis, while TBI levels were analyzed to determine
whether the biliary tract was injured during the PVL surgery
(Fig. 1). In the PVL and HT + PVL
groups, serum ALT levels were significantly increased at day 1 and
2 following PVL (P<0.05). However, TBI levels in the PVL and HT
+ PVL groups did not increase until 2 days after PVL surgery. In
addition, TBI levels in the HT group were significantly higher as
compared with those in the PVL group at day 0, 1 and 7 (P<0.05).
However, there was no statistically significant difference in TBI
levels between the HT and HT + PVL groups (P>0.05).
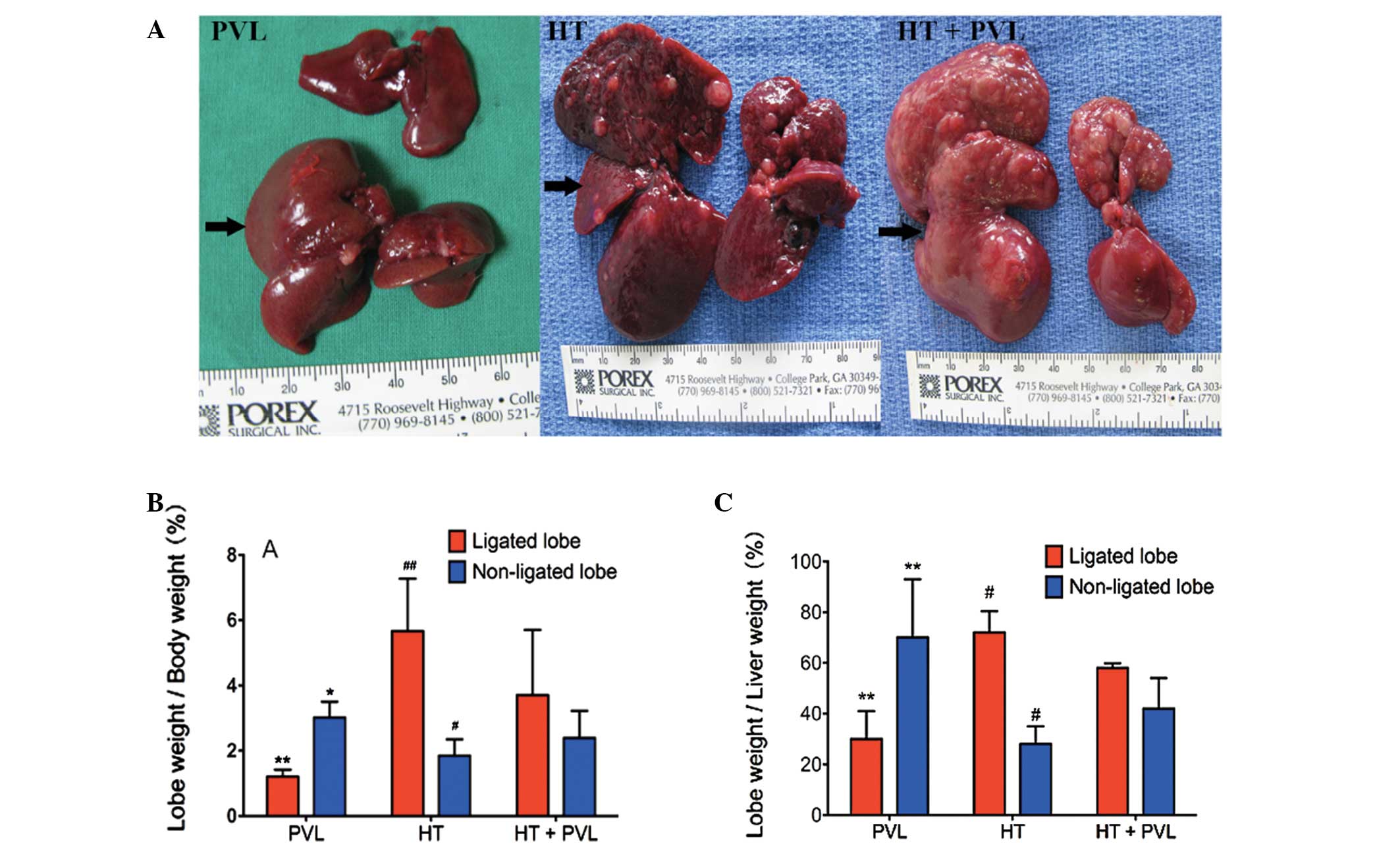
Liver growth and regeneration following
PVL
To evaluate liver growth and regeneration following
PVL, ratios between non-ligated or ligated lobe weights to total
body weight and non-ligated lobe weight to total liver weight were
measured two weeks following PVL surgery. As shown in Fig. 2, the ratio of ligated lobe weight
to total body weight in the HT + PVL group was significantly higher
as compared with the PVL group (P<0.01), while the ratio of
non-ligated lobe weight to total body weight in the HT + PVL group
was significantly lower as compared with the PVL group (P<0.05).
In addition, the ratio of ligated lobe weight to total liver weight
in the HT + PVL group was significantly lower as compared with the
PVL group (P<0.01). Compared with the non-ligated lobes in the
HT + PVL group, the ratios of lobes 4–7 to total body weight and to
total liver weight were significantly lower in the HT group
(P<0.05).
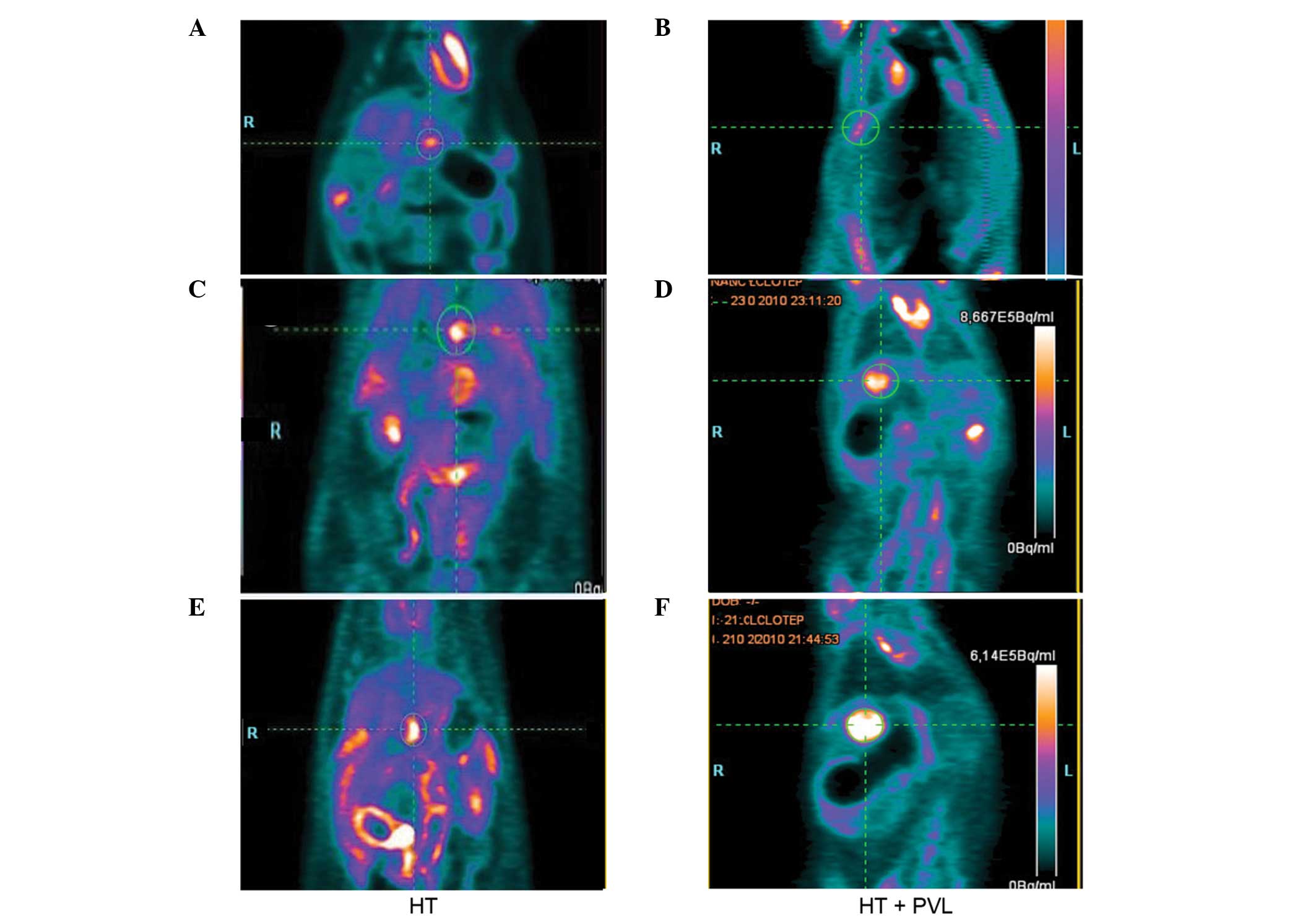
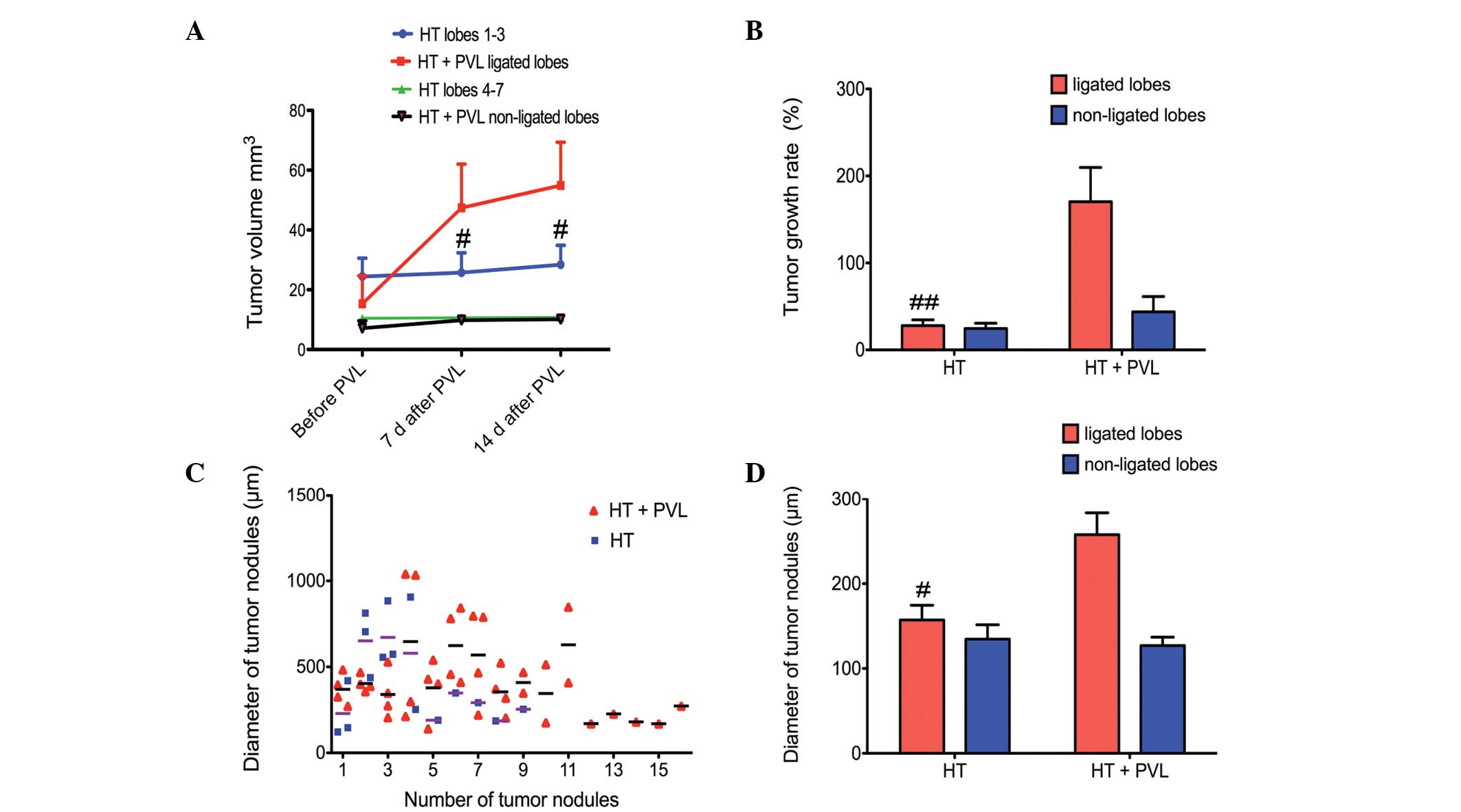
Tumor growth following PVL
Tumor size was evaluated by PET scans prior to PVL
and at day 7 and 14 following PVL surgery (Fig. 3). With regard to the non-ligated
lobes, tumor size in the HT + PVL group was similar to that of
lobes 4–7 in the HT group (P>0.05; Fig. 4). However, tumor size in the
ligated lobes of the HT + PVL group significantly increased
following PVL surgery and was significantly higher when compared
with the lobes 1–3 of the HT group (P<0.05; Fig. 4A). The tumor growth rate in the
ligated lobes of the HT + PVL group was significantly higher than
that of lobes 1–3 in the HT group (P<0.01; Fig. 4B). There was no significant
difference in the tumor growth rate between the non-ligated lobes
of the HT + PVL group and lobes 4–7 of the HT group (P>0.05).
The number of tumor nodules was counted in the HT and HT + PVL
groups. In addition, the diameters of the tumor nodules were
measured under a light microscope. The average diameter of the
tumor nodules in the ligated lobes of the HT + PVL group was
significantly higher when compared with lobes 1–3 of the HT group
(P<0.05; Fig. 4C and D).
However, there was no significant difference between lobes 4–7 of
the HT and HT + PVL groups.
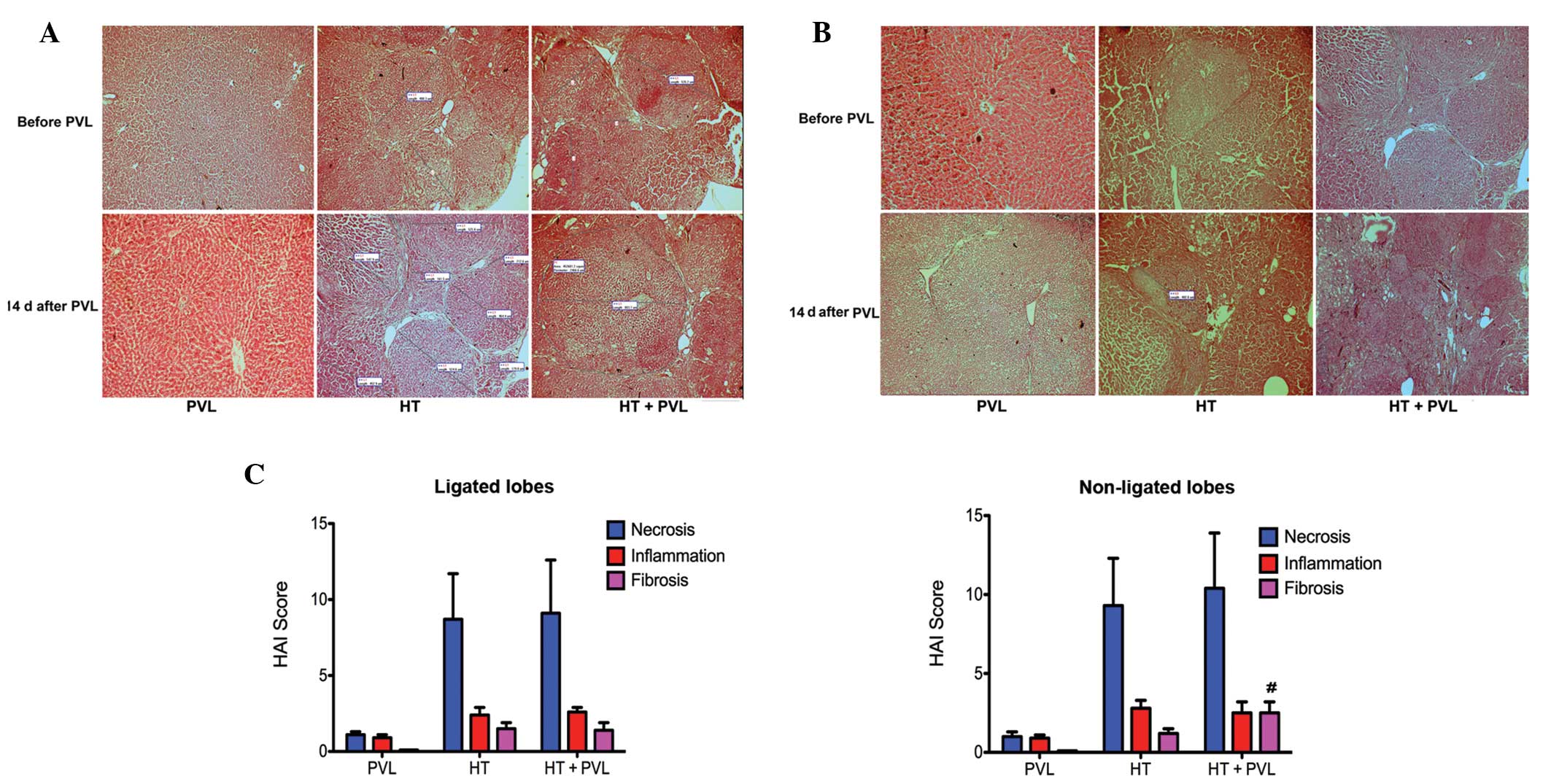
Histological evaluation in ligated and
non-ligated lobes
Following administration of DEN for 12 weeks, the
cirrhosis severity varied among the rats in the HT + PVL and HT
groups. Liver tissue was assessed in each case with H&E stained
sections. Fig. 5 shows the liver
histology of the rats prior to and two weeks following PVL. In the
HT and HT + PVL groups, numerous tumor nodules were observed in the
ligated and non-ligated lobes under a light microscope (Fig. 5A and B). Varying degrees of
necrosis, intralobular degeneration, portal inflammation and
fibrosis were observed in the samples from the HT and HT + PVL
groups prior to PVL surgery. Histological evaluation prior to PVL
revealed no injuries in the liver of the rats in the PVL group.
Hypertrophy was induced by PVL in the non-ligated lobes of the PVL
and HT + PVL groups. Following PVL surgery, connective tissues
accumulated and reticulin fibers spread radially throughout the
liver in the ligated lobes of the HT + PVL group. Compared with the
HT group, the number of infiltrating inflammatory cells in the
liver insignificantly increased in the HT + PVL group and the
deposition of fibrous components around the portal area also
increased. According to the Knodell index, hepatic fibrosis in the
non-ligated lobes of the HT + PVL group was more apparent than that
in the lobes 4–7 of the HT group (P<0.05; Fig. 5C). These results indicate that PVL
promotes the onset of hepatic fibrosis during hypertrophy formation
in non-ligated cirrhotic lobes.
Discussion
In the current study, the effects of PVL on tumor
growth and liver regeneration were evaluated in ligated and
non-ligated cirrhotic liver lobes. The changes in serum ALT and TBI
levels indicated that PVL was successfully conducted. The results
demonstrated that hypertrophy in non-ligated lobes was apparent in
normal and HT rats. In addition, the tumor growth rate in the
ligated lobes increased following PVL surgery, however, in the
non-ligated lobes, there were no marked changes following surgery.
The liver regeneration rate in non-ligated lobes and degeneration
rate in ligated lobes was much higher in the normal rats (PVL
group) than in the HT rats (HT + PVL group). Furthermore, PVL
promoted the onset of hepatic fibrosis during hypertrophy formation
in the non-ligated cirrhotic lobes.
At the beginning of the 20th century,
non-ligated lobe regeneration was recognized following portal
branch ligation. As previously reported, PVL can be achieved safely
without causing mortality and is an effective method to induce
hypertrophy (3,5). In the present study, PVL surgery
successfully induced hypertrophy in normal and HT rats. However,
liver regeneration in the normal rats was much more apparent when
compared with the HT rats. Significantly, histological evaluation
revealed that the increased contralateral lobes primarily consisted
of fibrous tissue and tumor nodules in the cirrhotic livers
following PVL. Therefore, hypertrophy in cirrhotic liver lobes may
be considered as non-functional.
Compensatory hyperplasia is possibly stimulated by
hepatotrophic substances that are contained in portal blood flow or
by increased blood flow in the non-occluded portal vein branch
(9,10). In addition, it is commonly accepted
that liver regeneration depends predominantly on the proliferation
of hepatocytes (11–13). As previously reported, the
incidence of cirrhosis or fibrosis is high in primary liver cancer
(14). Liver cirrhosis is
characterized by diffuse disorganization of the normal hepatic
structure of regenerative nodules and fibrotic tissue.
Consequently, decreased numbers of hepatocytes may result in a
lower regenerative ability. In addition, cirrhosis leads to portal
hypertension and hyperdynamic circulation that can have widespread
effects in the body (15).
Endothelial dysfunction is generally observed among cirrhotic
patients with portal hypertension (16). However, a previous study
demonstrated that inductive angiocrine signals from the sinusoidal
endothelium are required for liver regeneration (17). Other studies have also reported
that vascular endothelial growth factor promotes liver regeneration
by increasing the proliferation of hepatocytes (18,19).
There are a number of indications from clinical and
experimental studies that, despite liver atrophy, tumors in ligated
lobes do not shrink in size, but rather show acceleration of growth
(2,6,8,20).
The observations of the present study are consistent with these
studies that have demonstrated increased tumor growth in ligated
lobes following PVL. A previous study demonstrated that accelerated
tumor growth appeared to be a result of increased growth factor
expression (6). Following PVL
surgery, the expression levels of tumor necrosis factor (TNF)-α and
interleukin (IL)-6 were significantly higher in the ligated lobes
compared with the non-ligated lobes. TNF-α and IL-6 have been
implicated as important contributors to liver growth and
regeneration (11,21). Increased hepatocyte growth factor
(HGF) and epidermal growth factor (EGF) levels may be an additional
explanation for the accelerated tumor growth due to the stimulatory
effects that HGF and EGF exhibit on tumor cells (6,22).
The present study has several limitations. Firstly,
a rat model was used to investigate the effects of PVL on tumor
growth and liver regeneration. Compared with humans, rats differ
with regard to anatomy and physiology. Secondly, the present study
did not offer any insight into the potential mechanisms that
contribute to the histological changes in the non-ligated cirrhotic
liver lobes. Thus, further studies are required to investigate the
underlying mechanisms.
In conclusion, the results of the present study
support the hypothesis that PVL accelerates tumor growth in ligated
lobes, but not in contralateral lobes. In addition, the results
indicate that PVL induces liver regeneration in cirrhotic liver
lobes with lower efficiency than in non-cirrhotic lobes.
Hypertrophy in the contralateral cirrhotic lobes is predominantly a
consequence of hepatic fibrosis. Thus, PVL for cirrhotic liver
lobes should be considered carefully in the future work.
Acknowledgements
The authors thank Dr Xian-Long Zhou for reviewing
the study and providing critical comments.
References
|
1
|
Clavien PA, Petrowsky H, DeOliveira ML and
Graf R: Strategies for safer liver surgery and partial liver
transplantation. N Engl J Med. 356:1545–1559. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Broering DC, Hillert C, Krupski G, Fischer
L, Mueller L, Achilles EG, Schulte am Esch J and Rogiers X: Portal
vein embolization vs. portal vein ligation for induction of
hypertrophy of the future liver remnant. J Gastrointest Surg.
6:905–913. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Capussotti L, Muratore A, Baracchi F,
Lelong B, Ferrero A, Regge D and Delpero JR: Portal vein ligation
as an efficient method of increasing the future liver remnant
volume in the surgical treatment of colorectal metastases. Arch
Surg. 143:978–982. 2008.PubMed/NCBI
|
|
4
|
Aussilhou B, Lesurtel M, Sauvanet A,
Farges O, Dokmak S, Goasguen N, Sibert A, Vilgrain V and Belghiti
J: Right portal vein ligation is as efficient as portal vein
embolization to induce hypertrophy of the left liver remnant. J
Gastrointest Surg. 12:297–303. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barbaro B, Di Stasi C, Nuzzo G, Vellone M,
Giuliante F and Marano P: Preoperative right portal vein
embolization in patients with metastatic liver disease. Metastatic
liver volumes after RPVE. Acta Radiol. 44:98–102. 2003.PubMed/NCBI
|
|
6
|
Sakai N, Clarke CN, Schuster R, Blanchard
J, Tevar AD, Edwards MJ and Lentsch AB: Portal vein ligation
accelerates tumor growth in ligated, but not contralateral lobes.
World J Gastroenterol. 16:3816–3826. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Severi T, van Malenstein H, Verslype C and
van Pelt JF: Tumor initiation and progression in hepatocellular
carcinoma: risk factors, classification, and therapeutic targets.
Acta Pharmacol Sin. 31:1409–1420. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hayashi S, Baba Y, Ueno K, Nakajo M, Kubo
F, Ueno S, Aikou T, Komokata T, Nakamura N and Sakata R:
Acceleration of primary liver tumor growth rate in embolized
hepatic lobe after portal vein embolization. Acta Radiol.
48:721–727. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harada H, Imamura H, Miyagawa S and
Kawasaki S: Fate of the human liver after hemihepatic portal vein
embolization: cell kinetic and morphometric study. Hepatology.
26:1162–1170. 1997.PubMed/NCBI
|
|
10
|
Kusaka K, Imamura H, Tomiya T and Makuuchi
M: Factors affecting regeneration after right portal vein
embolization. Hepatogastroenterology. 51:532–535. 2004.PubMed/NCBI
|
|
11
|
Michalopoulos GK: Liver regeneration. J
Cell Physiol. 213:286–300. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alison MR, Islam S and Lim S: Stem cells
in liver regeneration, fibrosis and cancer: the good, the bad and
the ugly. J Pathol. 217:282–298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Palmes D and Spiegel HU: Animal models of
liver regeneration. Biomaterials. 25:1601–1611. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liver Cancer Study Group of Japan. Primary
liver cancer in Japan. Clinicopathologic features and results of
surgical treatment. Ann Surg. 211:277–287. 1990.PubMed/NCBI
|
|
15
|
Kim MY, Baik SK and Lee SS: Hemodynamic
alterations in cirrhosis and portal hypertension. Korean J Hepatol.
16:347–352. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rockey DC and Chung JJ: Reduced nitric
oxide production by endothelial cells in cirrhotic rat liver:
endothelial dysfunction in portal hypertension. Gastroenterology.
114:344–351. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ding BS, Nolan DJ, Butler JM, James D,
Babazadeh AO, Rosenwaks Z, Mittal V, Kobayashi H, Shido K, Lyden D,
Sato TN, Rabbany SY and Rafii S: Inductive angiocrine signals from
sinusoidal endothelium are required for liver regeneration. Nature.
468:310–315. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Taniguchi E, Sakisaka S, Matsuo K,
Tanikawa K and Sata M: Expression and role of vascular endothelial
growth factor in liver regeneration after partial hepatectomy in
rats. J Histochem Cytochem. 49:121–130. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oe H, Kaido T, Mori A, Onodera H and
Imamura M: Hepatocyte growth factor as well as vascular endothelial
growth factor gene induction effectively promotes liver
regeneration after hepatectomy in Solt-Farber rats.
Hepatogastroenterology. 52:1393–1397. 2005.
|
|
20
|
Kokudo N, Tada K, Seki M, Ohta H, Azekura
K, Ueno M, Ohta K, Yamaguchi T, Matsubara T, Takahashi T, Nakajima
T, Muto T, Ikari T, Yanagisawa A and Kato Y: Proliferative activity
of intrahepatic colorectal metastases after preoperative
hemihepatic portal vein embolization. Hepatology. 34:267–272. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fausto N, Campbell JS and Riehle KJ: Liver
regeneration. Hepatology. 43(2 Suppl 1): S45–S53. 2006. View Article : Google Scholar
|
|
22
|
Harun N, Nikfarjam M, Muralidharan V and
Christophi C: Liver regeneration stimulates tumor metastases. J
Surg Res. 138:284–290. 2007. View Article : Google Scholar : PubMed/NCBI
|