Introduction
Infections have long been known to complicate care
in patients after traumatic injury frequently leading to excess
morbidity and mortality (1).
Intra-abdominal infection is the most common infection in abdominal
trauma patients with an incidence rate of 2–9% (2,3). It
is frequently complicated with acute respiratory distress syndrome,
multiple organ failure, gastrointestinal fistula, abdominal wall
defects and malnutrition (4).
Uncomplicated intra-abdominal infections, including suppurative
appendicitis, can be eliminated simply by surgery with prophylactic
anti-infective drugs. By contrast, patients with severe
intra-abdominal infection (SIAI) that is persistent and complicated
with progressive organ dysfunction require the administration of
antibiotics in addition to surgical intervention.
SIAI refers to intra-abdominal infections
complicated by sepsis and septic shock (5). The mortality rate of SIAI can reach
50% (6) and antibiotic
intervention is required upon onset. Bacteriological and
susceptibility analyses of pus and whole blood may aid the
selection of antibiotics in treating abdominal infection, prior to
which antibiotics should be empirically administered. At present,
antibiotics are empirically administered to SIAI patients based on
the worldwide Study for Monitoring Antimicrobial Resistance Trends
(SMART) and the domestic CHINET bacterial resistance surveillance
(7,8). However, pus derived from the deep
abdominal cavity has seldom been subjected to bacteriology and
susceptibility analysis. Therefore, the present study
retrospectively analyzed the spectrum of bacterial infection and
drug resistance changes of pus in patients with intra-abdominal
trauma and SIAI who were admitted to Jinling Hospital, Nanjing
University School of Medicine (Nanjing, China) between January 2001
and May 2012. The aim of the study was to increase the accuracy of
empirical medication. Considering the poor survival rates of SIAI
patients and the lack of studies investigating the bacterial
cultures of abdominal cavity pus, the results of the present study
are particularly significant for clinical practice.
Materials and methods
General information
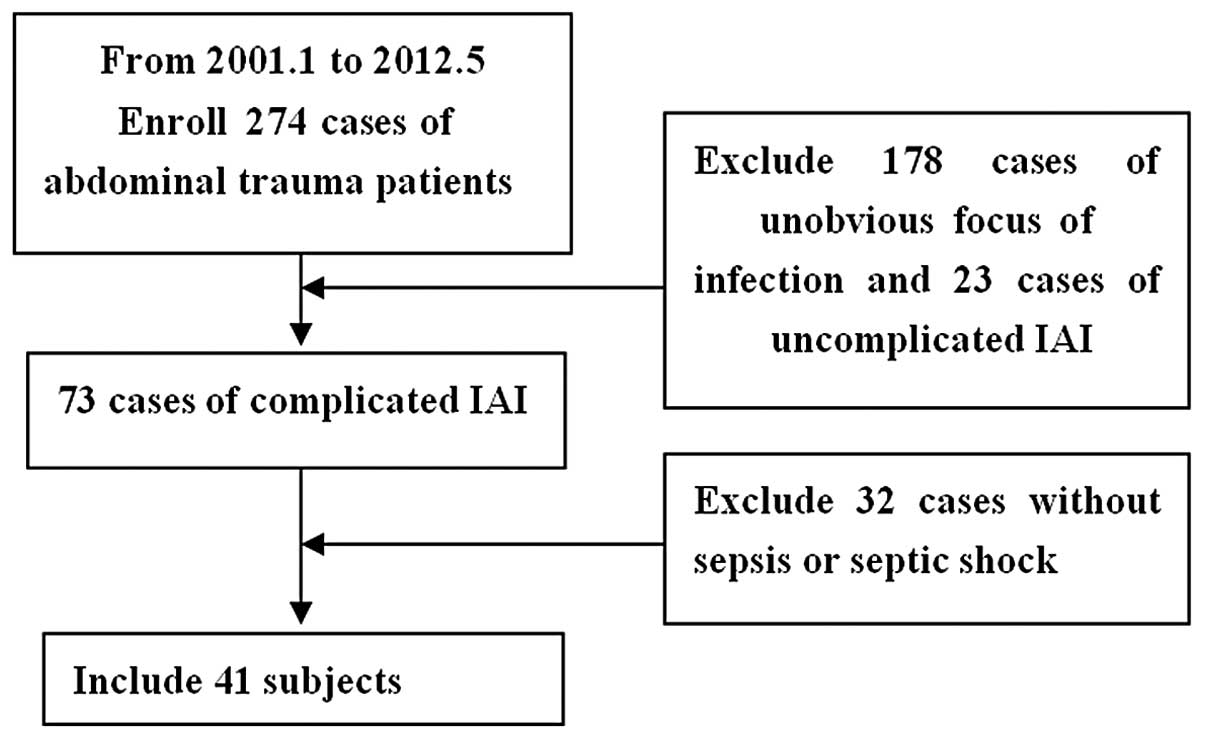
A total of 274 intra-abdominal trauma patients (age,
38.2±19.7 years) who had been enrolled in Nanjing General Hospital
between January 2001 and May 2012 were selected for this study,
including 225 males and 49 females. There were 196 cases of closed
injury and 88 cases of open injury, including 176 traffic
accidents, 50 fall injuries, 32 collision injuries and 16 sharp
injury cases. Patients were treated between 0.5 and 24 h following
the trauma. A total of 191 patients were admitted to the emergency
room immediately following trauma, of which 48 cases were
complicated with shock. A total of 41 out of the 96 intra-abdominal
infection cases suffered from SIAI. The detailed injury statuses
are summarized in Table I. This
clinical study is approved by the ethic committee of the Jinling
Hospital Nanjing University. The collection of all the clinical
specimen are under the authorization of the patients or the
patients’ families.
 | Table IStatuses of the 274 abdominal injury
patients. |
Table I
Statuses of the 274 abdominal injury
patients.
| Injury type | Cases, n |
|---|
| Simple abdominal wall
injury | 31 |
| Abdominal viscera and
retroperitoneal injuriesa | 219 |
| Hepatorrhexis | 49 |
| Splenic rupture | 77 |
| Renal contusion | 23 |
| Pancreatic
injury | 68 |
| Gastric and duodenal
injuries | 53 |
| Small intestine
rupture | 24 |
| Colon rupture | 10 |
| Complicated brain,
chest, bone and urinary system injuries | 24 |
Screening of subjects
Patients were diagnosed with SIAI if the
intra-abdominal infections were complicated with sepsis and/or
septic shock (Fig. 1).
Systemic inflammatory response syndrome (SIRS) was
diagnosed if patients had two or more of the following symptoms: i)
a body temperature of >38°C or <36°C; ii) a heart rate >90
bpm; iii) a respiratory rate >20 breaths/min or a
PaCO2 value <4.26 kPa (32 mmHg); and iv) a white
blood cell count of >12×109/l or
<4×109/l or a stab granulocyte count of >0.10. The
diagnostic criteria of sepsis were in accordance with those for
SIRS with definite evidence of infection (9).
Septic shock was diagnosed in patients with a
systolic blood pressure of <90 mmHg or whose systolic blood
pressure had decreased by ≥40 mmHg on the basis of the original
value, with or without symptoms associated with poor tissue
perfusion, including acidosis, oliguria or acute consciousness
disorders (10).
Sample collection
All samples were collected from the patients during
surgery or in intensive care. From the deep abdominal cavity, ≥1 ml
pus (gallbladder bile and bile in the gallbladder wall or common
bile duct were not included) was collected using a disposable
sterile syringe, which was quickly sealed in a sampling tube. The
samples were sent to a laboratory within 2 h for aerobic and
anaerobic cultures.
Pathogenic examination and susceptibility
determination
Abdominal pus samples were routinely cultured in a
BACTEC 9120 automated blood culture system (BD Diagnostics, Sparks,
MS, USA) which raised an alarm when cases testing positive for
bacteria were identified. Samples yielding positive results were
subjected to susceptibility tests using the Kirby-Bauer disk
diffusion susceptibility method, according to the National
Committee for Clinical Laboratory Standards (2011) (11). Diameters of the zones of complete
inhibition (as judged by the unaided eye), including the diameter
of the disk, were measured. Zone margins were considered as the
area exhibiting no marked or visible growth that is was possible to
detect by the unaided eye. The results of the susceptibility tests
were reported as susceptible, intermediate or resistant.
Gram-positive and -negative bacteria were identified using the
Vitek-32 microbial identification system and analytical profile
index strips purchased from BioMérieux (Lyon, France). Control
strains, including standard Staphylococcus aureus (S.
aureus; ATCC25923), Escherichia coli (E. coli;
ATCC25922) and Pseudomonas aeruginosa (P. aeruginosa;
ATCC27853), were provided by the Quality Control Center of Jiangsu
Province (Lianyungang, China). WHONET 5.4 software developed by WHO
Collaborating Centre for Surveillance of Antimicrobial Resistance
based at the Brigham and Women’s Hospital in Boston was used to
analyze laboratory findings.
Results
Types and distribution of pathogenic
bacteria
From the 41 SIAI patients, 123 positive pus samples
were collected (100%) from which 297 strains were isolated,
including 131 strains of Gram-positive bacteria (44.1%) and 159
strains of Gram-negative bacteria (53.5%). In addition, 5 strains
of anaerobic bacteria (1.7%) and 2 strains of fungi (0.6%) were
isolated. E. coli, S. aureus and Klebsiella
pneumoniae (K. pneumoniae) were the most predominant
bacteria. The flora distribution is shown in Table II.
 | Table IIDistribution of 297 pathogenic
microbial strains. |
Table II
Distribution of 297 pathogenic
microbial strains.
| Type of pathogenic
microbe | Strains (n) | Proportion (%) |
|---|
| Gram-positive
bacteria | 131 | 44.1 |
| Staphylococcus
aureus | 66 | 22.2 |
| Enterococcus
faecalis | 28 | 9.4 |
| Enterococcus
faecium | 19 | 6.4 |
| Staphylococcus
epidermidis | 12 | 4.0 |
| Staphylococcus
haemolyticus | 6 | 2.0 |
| Gram-negative
bacteria | 159 | 53.5 |
| Escherichia
coli | 72 | 24.2 |
| Klebsiella
pneumoniae | 34 | 11.4 |
| Pseudomonas
aeruginosa | 21 | 7.1 |
| Stenotrophomonas
maltophilia | 8 | 2.7 |
| Enterobacter
cloacae | 5 | 1.7 |
| Chryseobacterium
indologenes | 3 | 1.0 |
| Burkholderia
cepacia | 3 | 1.0 |
| Acinetobacter
calcoaceticus | 2 | 0.7 |
|
Pneumobacillus | 2 | 0.7 |
| Chryseobacterium
meningosepticum | 1 | 0.3 |
| Acinetobacter
lwoffii | 1 | 0.3 |
| Citrobacter
freundii | 1 | 0.3 |
| Morganella
morganii | 1 | 0.3 |
| Pseudomonas
cepacia | 1 | 0.3 |
| Proteus
vulgaris | 1 | 0.3 |
| Alcaligenes
xylosoxidans | 1 | 0.3 |
| Comamonas
acidovorans | 1 | 0.3 |
| Acinetobacter
baumannii | 1 | 0.3 |
| Anaerobic
bacteria | 5 | 1.7 |
| Bacteroides
fragilis | 1 | 0.3 |
| Bacteroides
ovatus | 1 | 0.3 |
| Bacteroides
thetaiotaomicron | 1 | 0.3 |
| Bacteroides
distasonis | 1 | 0.3 |
| Bacteroides
vulgatus | 1 | 0.3 |
| Fungi | 2 | 0.6 |
|
Saccharomycetes | 1 | 0.3 |
| Candida
albicans | 1 | 0.3 |
Susceptibility of pathogenic
bacteria
Pathogenic bacteria are prone to resistance against
a number of antibiotics. Gram-negative bacteria exhibited the
highest susceptibility to imipenem, but were resistant to
cephalosporins. E. coli was highly susceptible to
cefoperazone (91%) and imipenem (98%), while K. pneumoniae
was highly susceptible to imipenem (100%) and amikacin (79%).
However, >67% of P. aeruginosa strains tolerated imipenem
and were treated most effectively by a quinolone antibiotic
ciprofloxacin (90%). Gram-positive cocci, which were generally not
susceptible to cephalosporins, exhibited 100% susceptibility to
teicoplanin and linezolid. S. aureus was susceptible to
vancomycin (100%) and Enterococcus faecalis (E.
faecalis) was particularly susceptible to teicoplanin and
linezolid without drug-resistant strains (Table III).
 | Table IIIPercentages of R, I and S strains of
five main pathogenic bacteria to common antibiotics. |
Table III
Percentages of R, I and S strains of
five main pathogenic bacteria to common antibiotics.
| E. coli (72
strains), % | S. aureus
(66 strains), % | K.
pneumoniae (34 strains), % | E. faecalis
(28 strains), % | P.
aeruginosa (21 strains), % |
|---|
|
|
|
|
|
|
|---|
| Antibiotics | R | I | S | R | I | S | R | I | S | R | I | S | R | I | S |
|---|
| Amikacin | 11 | 8 | 81 | 22 | 5 | 73 | 12 | 9 | 79 | 64 | 4 | 32 | 19 | 5 | 76 |
| Gentamicin | 60 | 2 | 38 | 33 | 5 | 62 | 26 | 6 | 68 | 75 | 7 | 18 | 24 | 14 | 62 |
| Ampicillin | 49 | 22 | 29 | 39 | 6 | 55 | 100 | 0 | 0 | 43 | 7 | 50 | 100 | 0 | 0 |
| Piperacillin | 83 | 17 | 0 | 91 | 0 | 9 | 100 | 0 | 0 | - | - | - | 86 | 14 | 0 |
| Cefazolin | 61 | 7 | 32 | 36 | 3 | 61 | 100 | 0 | 0 | - | - | - | - | - | - |
| Cefuroxime | 61 | 3 | 36 | 28 | 5 | 67 | 100 | 0 | 0 | - | - | - | - | - | - |
| Cefotaxime | 58 | 3 | 39 | 95 | 5 | 0 | 32 | 6 | 62 | - | - | - | 48 | 38 | 14 |
| Ceftazidime | 58 | 6 | 36 | - | - | - | 29 | 6 | 65 | - | - | - | 19 | 19 | 62 |
| Cefoperazone | 4 | 5 | 91 | - | - | - | 24 | 3 | 73 | - | - | - | 90 | 10 | 0 |
| Aztreonam | - | - | - | - | - | - | - | - | - | - | - | - | 90 | 10 | 0 |
| Imipenem | 1 | 1 | 98 | - | - | - | 0 | 0 | 100 | - | - | - | 67 | 5 | 28 |
| Ciprofloxacin | 58 | 11 | 31 | 94 | 6 | 0 | 23 | 12 | 65 | 57 | 18 | 25 | 5 | 5 | 90 |
| Piperacillin | 83 | 6 | 11 | - | - | - | 12 | 15 | 73 | - | - | - | 24 | 0 | 76 |
| Paediatric compound
sulfamethoxazole | - | - | - | 67 | 6 | 27 | - | - | - | - | - | - | 81 | 19 | 0 |
| Vancomycin | - | - | - | 0 | 0 | 100 | - | - | - | 0 | 14 | 86 | - | - | - |
| Erythromycin | - | - | - | 85 | 5 | 10 | - | - | - | 29 | 7 | 64 | - | - | - |
| Penicillin | - | - | - | 100 | 0 | 0 | - | - | - | - | - | - | - | - | - |
| Oxacillin | - | - | - | 100 | 0 | 0 | - | - | - | - | - | - | - | - | - |
| Clindamycin | - | - | - | 85 | 6 | 9 | - | - | - | - | - | - | - | - | - |
| Phosphonomycin | - | - | - | 36 | 0 | 64 | - | - | - | - | - | - | - | - | - |
| Teicoplanin | - | - | - | 0 | 0 | 100 | - | - | - | 0 | 0 | 100 | - | - | - |
| Linezolid | - | - | - | 0 | 0 | 100 | - | - | - | 0 | 0 | 100 | - | - | - |
Discussion
Currently, SIAI patients are empirically
administered antibiotics at an early stage, based on international
SMART research and the domestic CHINET bacterial resistance
surveillance on collected samples from the respiratory system
(46.9%, e.g. sputum), urine (19.9%), blood (11.9%), pus (5.2%),
sterile body fluids (4.0%), genital tract secretions (1.7%), feces
(1.2%) and others (8.2%) (7). To
date, studies on the bacterial culture and drug resistance of pus
in the deep abdominal cavity remain scarce. In the present study,
Gram-negative bacteria (53.5%) primarily contributed to SIAI, in
which E. coli (24.2%) and K. pneumoniae (11.4%)
predominated. The results are consistent with those of a previous
SMART study that analyzed patients from 14 centers in six countries
in the Asia-Pacific region (12),
in which Gram-negative enterobacteria accounted for 82% of cases of
intra-abdominal infection (E. coli, 43%; K.
pneumoniae, 20%). In addition, the resistance rate of E.
coli to ceftazidime was only 17.5% in the aforementioned study,
but this was elevated to 58% in the present study. The
susceptibilities of E. coli to imipenem in the two studies
exceeded 98%. Therefore, the analysis and review of the local
bacteria distribution and susceptibility results of abdominal pus
is crucial.
Intra-abdominal infection, which refers to an
infection of an organ in the abdominal cavity, with the exception
of peritonitis, may be divided into uncomplicated and complicated
infections (13). Uncomplicated
intra-abdominal infections are infections of only one organ with
intact anatomical structure. By contrast, complicated infections,
which are intrinsically secondary intra-abdominal infections,
represent intra-abdominal abscesses or peritonitis following the
invasion of pathogenic bacteria into the abdominal cavity from
involved organs. Complicated intra-abdominal infections are often
associated with intra-abdominal visceral perforation, ischemic
gangrene and penetrating injury. It is possible to recover the
majority of uncomplicated intra-abdominal infections by surgery
without conventional antibiotic treatment, with the exception of
prophylactic antibiotics. However, complicated intra-abdominal
infections require treatment combining surgical protocols with
anti-infective agents.
SIAI, as a complicated intra-abdominal infection,
mainly manifests as diffuse peritonitis or multiple intra-abdominal
and peritoneal abscesses, including severe pancreatitis, hollow
organ perforation and anastomotic fistula. SIAI is commonly
accompanied by apparent sepsis and intra-abdominal infection due to
the invasion of numerous bacteria and toxins into the blood
circulation, which can thus be referred to as sepsis of abdominal
origin (incidence rate, 10%) (14). In addition, patients with SIAI are
extremely vulnerable to acute respiratory distress syndrome and
acute renal failure. In the present study, 39 out of 41 SIAI
patients underwent continuous renal replacement therapy (95.1%) and
33 cases received tracheotomy for ventilator-assisted respiration
(80.4%). Encountering uncontrollable infection sources, patients
may succumb to constant or recurrent septic shock owing to the
continuous release of bacteria and toxins into the blood. Thus,
50–70% of patients eventually succumb to multiple organ failure
following respiratory and renal functional damage, as well as
successive intestinal and hepatic dysfunction (15). A retrospective cohort study
investigating secondary intra-abdominal infections verified that
inappropriate initial treatment is likely to result in the failure
of clinical treatment for SIAI, thus affecting the prognosis
adversely (16). Notably, it is
improper to excessively administer antibiotics at the outset of
treatment.
Considering the abrupt onset, low survival rate and
disunified antibiotic intervention of SIAI, as well as the lack of
relevant bacteriology and drug resistance studies, in the present
study the Department of General Surgery, as a national trauma
rescue center, successfully intervened in SIAI patient treatment by
culturing, analyzing and identifying associated bacteria. In the
present study, E. coli and K. pneumoniae in patients
with SIAI moderately tolerated cephalosporins by producing
extended-spectrum β-lactamases (17). In addition, a small number of K.
pneumoniae strains yield highly productive AmpC β-lactamase
enzymes (18), which renders them
highly resistant to third-generation cephalosporins that have been
widely applied in clinical practice. Furthermore, undesirable
inducible enzymes may be generated by Enterobacter,
Citrobacter, Serratia and Morganella bacteria
due to incautious administration of third-generation cephalosporins
(19). The present study
demonstrates that E. coli (98%) and K. pneumoniae
(100%) were highly susceptible to imipenem, allowing this
antibiotic to be administered with priority in the treatment of
Gram-negative bacterial infection. In addition, the wide
application of the third-generation cephalosporin brings is the
increasing trend of the Gram-positive bacteria. In the present
study, the significantly greater incidence of Gram-positive
bacteria (44.1%) compared with that observed in the CHINET
bacterial drug resistance surveillance in 2010 (28.4%) (7) may be associated with the high
proportion of open abdominal cavities (36/41, 87.8%). In addition,
a higher number of patients in the present study underwent
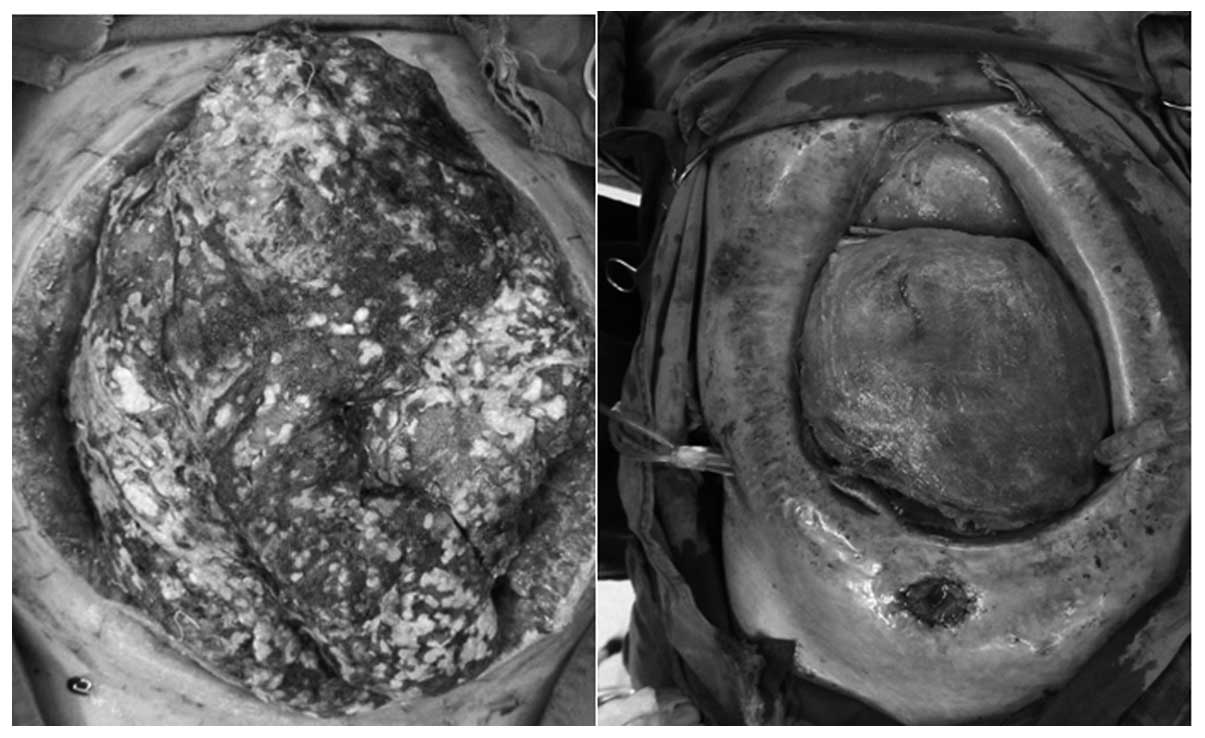
abdominal surgeries. For example, one patient successively
underwent five peritoneal irrigation and drainage procedures, as
well as open abdominal surgeries, two abdominal gauze packing and
removal surgeries and one enterostomy (Fig. 2). The resultant 18 strains of
bacteria isolated from nine pus samples consisted of five strains
of E. coli (27.8%), four strains of S. aureus
(22.2%), four strains of E. faecalis (22.2%), three strains
of K. pneumoniae (16.7%), one strain of P. aeruginosa
(5.6%) and one of Acinetobacter baumannii (5.6%). Frequent
open abdominal surgeries may directly increase the number of
Gram-positive cocci in the pus culture.
Recently, third-generation cephalosporins have been
combined with ornidazole, due to the coexistence of anaerobic and
aerobic bacteria in severe or complicated intra-abdominal
infections. These pathogenic bacteria may become highly resistant
to common antibiotics, triggering refractory or secondary
infections. With regard to previous studies, summarizing local
bacteriology and susceptibility results provides clinical guidance
for dealing with drug-resistant bacteria worldwide.
In summary, initial empirical antibiotic therapy
should be modified based on susceptibility analysis results. In
addition, patients with SIAI should be administered the most potent
antibiotics immediately rather than the most commonly used
antibiotics. Finally, it is critical to remove the sources of
infection and to prevent intraoperative and postoperative bacterial
contaminations in order to improve the therapeutic effects of
eligible antibiotics.
References
|
1
|
Murray CK, Hinkle MK and Yun HC: History
of infections associated with combat-related injuries. J Trauma.
64(3 Suppl): S221–S231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dellinger EP, Oreskovich MR, Wertz MJ, et
al: Risk of infection following laparotomy for penetrating
abdominal injury. Arch Surg. 119:20–27. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goins WA, Rodriguez A, Joshi M and Jacobs
D: Intra-abdominal abscess after blunt abdominal trauma. Ann Surg.
212:60–65. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Croce MA, Fabian TC, Stewart RM, et al:
Correlation of abdominal trauma index and injury severity score
with abdominal septic complications in penetrating and blunt
trauma. J Trauma. 32:380–388. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nathens AB, Rotstein OD and Marshall JC:
Tertiary peritonitis: clinical features of a complex nosocomial
infection. World J Surg. 22:158–163. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moore LJ, Moore FA, Jones SL, et al:
Sepsis in general surgery: a deadly complication. Am J Surg.
198:868–874. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu DM, Wang F, Hu FP, et al: CHINET 2009
surveillance of bacterial resistance in China. Chin J Infect
Chemother. 11:321–329. 2011.(In Chinese).
|
|
8
|
Paterson DL, Rossi F, Baquero F, et al: In
vitro susceptibilities of aerobic and facultative Gram-negative
bacilli isolated from patients with intra-abdominal infections
worldwide: the 2003 Study for Monitoring Antimicrobial Resistance
Trends (SMART). J Antimicrob Chemother. 55:965–973. 2005.
View Article : Google Scholar
|
|
9
|
No authors listed. American College of
Chest Physicians/Society of Critical Care Medicine Consensus
Conference: Definitions for sepsis and organ failure and guidelines
for the use of innovative therapies in sepsis. Crit Care Med.
20:864–874. 1992. View Article : Google Scholar
|
|
10
|
Dellinger RP, Levy MM, Carlet JM, et al:
Surviving Sepsis Campaign: International guidelines for management
of severe sepsis and septic shock: 2008. Intensive Care Med.
34:17–60. 2008. View Article : Google Scholar
|
|
11
|
Clinical and Laboratory Standards
Institute (CLSI). Performance standards for antimicrobial
susceptibility testing; Twenty-first informational supplement: CLSI
M100-S21. CLSI/NCCIS; Wayne, PA, USA: pp. 1–172. 2011
|
|
12
|
Hsueh PR, Snyder TA, Dinubile MJ, et al:
2004 Asia-Pacific SMART Team: In vitro susceptibilities of aerobic
and facultative Gram-negative bacilli isolated from patients with
intra-abdominal infections in the Asia-Pacific region: 2004 results
from SMART (Study for Monitoring Antimicrobial Resistance Trends).
Int J Antimicrob Agents. 28:238–243. 2006.
|
|
13
|
Solomkin JS, Hemsell DL, Sweet R, et al:
Infectious Diseases Society of America and the food and Drug
Administration: Evaluation of new anti-infective drugs for the
treatment of intraabdominal infections. Clin Infect Dis. 15(Suppl
1): S33–S42. 1992. View Article : Google Scholar
|
|
14
|
Pieracci FM and Barie PS: Management of
severe sepsis of abdominal origin. Scand J Surg. 96:184–196.
2007.PubMed/NCBI
|
|
15
|
Malangoni MA: Evaluation and management of
tertiary peritonitis. Am Surg. 66:157–161. 2000.
|
|
16
|
Sturkenboom MC, Goettsch WG, Picelli G, et
al: Inappropriate initial treatment of secondary intra-abdominal
infections leads to increased risk of clinical failure and costs.
Br J Clin Pharmacol. 60:438–443. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tumbarello M, Sanguinetti M, Montuori E,
et al: Predictors of mortality in patients with bloodstream
infections caused by extended-spectrum-β-lactamase-producing
Enterobacteriaceae: importance of inadequate initial antimicrobial
treatment. Antimicrob Agents Chemother. 51:1987–1994. 2007.
|
|
18
|
Yan JJ, Ko WC, Jung YC, et al: Emergency
of Klebsiella pneumoniae isolates producing inducible DHA-1
beta-lactamase in a university hospital in Taiwan. J Clin
Microbiol. 40:3121–3126. 2002.
|
|
19
|
Vandewoude KH, Hoste EA and Colardyn F:
Antibiotic resistance and exposure to different generation
cephalosporins. Crit Care Med. 28:2678–2679. 2000. View Article : Google Scholar : PubMed/NCBI
|