Introduction
Streptococcus sanguinis (S. sanguinis)
is the dominant bacteria in a healthy oral cavity and it is
generally accepted that an antibacterial substance generated by
S. sanguinis has an inhibitory effect on putative
periodontal pathogens. The main mechanism of this effect is the
generation of hydrogen peroxide and bacteriocin, and the latter
mainly functions under anaerobic conditions (1,2). It
has been reported that bacteriocin accumulates in bacterial cells
when S. sanguinis is cultured under anaerobic conditions,
and is not released into the extracellular environment (3,4).
However, antibacterial substances were detected in the culture
medium of S. sanguinis in one particular study (5). The inhibitory effect of the
antibacterial substance from S. sanguinis on the pathogens
of oral candidiasis is unknown.
A previous study showed that there are considerable
quantities of Prevotella intermedia (P. intermedia)
and Porphyromonas gingivalis (P. gingivalis) in the
subgingival plaque of adult patients with moderate or serious
periodontitis (6). These two
bacteria adhere and colonize under the gingiva. Disorganization of
the periodontal supporting tissue and immune response of the body
are initiated by these virulence factors, which play an important
role in the development of periodontal diseases. Numerous studies
have focused on investigating the biological functions of P.
intermedia and P. gingivalis and elucidating their
mechanisms of pathogenesis (7–10).
Candida is a common pathogenic yeast that
induces deep fungal infections (11). The infection rate of Candida
has increased greatly with the wide use of antibiotics, hormones
and immune agents (12). A mixed
infection of multiple Candida species is frequently detected
in the oral cavity of patients with AIDS. The most common type of
mixed infection is reported to be Candida albicans (C.
albicans) complicated by Candida tropicalis (C.
tropicalis), and C. albicans complicated by Candida
glabrata or Candida krusei has also been observed
(13), with C. albicans and
C. tropicalis having the highest pathogenicity.
In the present study, intracellular and exocrine
proteins were extracted from S. sanguinis and, using P.
intermedia, P. gingivalis, C. albicans and C.
tropicalis as indicators, the biological effects of the protein
extracts on pathogenic bacteria and fungi were detected and
analyzed.
Materials and methods
Extraction and purification of the
intracellular and exocrine proteins from S. sanguinis
The standard strain ATCC 10556 of S.
sanguinis was purchased from the State Key Laboratory of Oral
Diseases, West China College of Stomatology, Sichuan University
(Chengdu, China). Following identification and pure culture, the
bacteria were inoculated on Brain Heart Infusion (BHI) culture
medium and anaerobically cultured for 48 h.
Intracellular proteins
The medium of S. sanguinis was
ultracentrifuged at a low temperature following the anaerobic
culture. The bacterial precipitate was collected, washed and
resuspended in phosphate-buffered saline (PBS). The target proteins
were released from the cells by sonication and the supernatant was
collected by centrifugation (12,000 × g, 4°C, 30 min). Solid
ammonium sulfate was slowly added to the supernatant to yield 60%
saturation, salted out for 6 h at 4°C and centrifuged to isolate
the supernatant. Subsequently, the collections were subjected to a
second round of salting out with 70% saturation and, following
centrifugation, the precipitate was dissolved in PBS. The desalting
purification was conducted by chromatography on Sephadex G-25
(Pharmacia, Picastaway, NJ, USA). The collected materials were
dialyzed, condensed, lyophilized and cryopreserved.
Exocrine proteins
The medium of S. sanguinis was
ultracentrifuged (10,000 × g, 4°C, 10 min) following the anaerobic
culture. The supernatant was collected and an equal volume of cold
anhydrous ethanol was added. The mixture was allowed to stand for 6
h at 4°C and then centrifuged. The precipitate was resuspended in
PBS and cryopreserved.
P. intermedia and P.
gingivalis
The standard strains ATCC 25611 and ATCC 33277 of
P. intermedia and P. gingivalis, respectively, were
purchased from Beijing Stomatological Hospital, Capital Medical
University (Beijing, China). The purified and identified P.
intermedia and P. gingivalis were diluted to
1×108 cfu/ml and a mixture of P. intermedia and
P. gingivalis (ratio 1:1) was also prepared.
Minimal inhibitory concentrations (MICs)
of the protein extracts against P. intermedia and P.
gingivalis
The MICs were detected by a well-plate technique.
P. intermedia suspension (20 μl) was placed on a blood agar
plate, spread evenly and two holes were punched with an aseptic
puncher. The diameter of the holes was 5 mm. The agar in the hole
was removed and sealed with melted BHI solid medium on the bottom
(half the depth). Subsequently, 20 μl intracellular or exocrine
S. sanguinis proteins were added to the holes and
anaerobically cultured for 48 h at 37°C. The P. gingivalis
and P. intermedia + P. gingivalis suspensions were
treated by the same method. The MICs of the intracellular S.
sanguinis proteins were determined by a double dilution method.
The proteins were diluted to 0.5, 0.25, 0.125, 0.0625, 0.0313 and
0.0156 g/l with PBS. Subsequently, 20 μl dilutions to the equal
volume of bacterial suspensions were obtained. Following complete
mixing, 20 μl suspensions were spread evenly on the blood agar
plates and anaerobically cultured for 48 h at 37°C. The MIC of the
intracellular proteins of S. sanguinis against the P.
intermedia and P. gingivalis co-culture was observed and
calculated.
Effects of the S. sanguinis
proteins on biofilms formed by P. intermedia and P.
gingivalis
A sterile cover glass with 500 μl P.
intermedia and P. gingivalis mixture was placed into a
six-well plate, allowed to stand for 10 min and then 2 ml BHI
culture medium was slowly added and the mixture was anaerobically
cultured for 48 h at 37°C to ensure that the planktonic P.
intermedia and P. gingivalis were attached.
Intracellular proteins (1 g/l) were slowly added to one well of the
plate, exocrine proteins were slowly added to another well, and an
equal volume of deionized water was added to the last well. The
liquid from each well was carefully removed after 1 h treatment and
the wells were washed with 1 ml PBS. Acridine orange (AO) and
ethidium bromide (EB) were diluted to 100 mg/l with PBS, the
dilutions were mixed and re-diluted 20-fold, added to the wells,
and observed under a confocal laser scanning microscope (Leica
CTR6500; Leica, Wetzlar, Germany). When the live bacterial DNA
combined with AO, bright green fluorescence was observed, and when
the dead bacterial DNA combined with EB, red fluorescence was
observed. Yellow or orange fluorescence indicated the overlap of
live and dead bacteria. Biofilm viability (BV; %) = green
fluorescence/(green fluorescence + red fluorescence) × 100.
C. albicans and C. tropicalis
The standard strains ATCC 10231 and ATCC 13803 of
C. albicans and C. tropicalis, respectively, were
purchased from the National Center for Medical Culture Collections
(Beijing, China). The purified and identified C. albicans
and C. tropicalis were diluted to 1×106 cfu/ml
with RPMI-1640 culture medium, and a mixture of C. albicans
and C. tropicalis (ratio 1:1) was also prepared.
MICs of the protein extracts against C.
albicans and C. tropicalis
The MICs of the S. sanguinis protein extracts
against C. albicans, C. tropicalis and their
co-cultures were detected using a well-plate technique. The
intracellular and exocrine proteins of S. sanguinis were
diluted to 0.25, 0.5 and 1 g/l with PBS. The tests were performed
on three groups. In the first group, C. albicans suspension
(10 μl) was inoculated into a culture tube with 1 ml RPMI-1640 and
300 μl intracellular proteins. In the second group, C.
albicans suspension (10 μl) was inoculated into a culture tube
with 1 ml RPMI-1640 and 300 μl exocrine proteins. Group three was
used as a control without any protein extracts. The test groups
were cultured at 37°C with agitation for 12 h; then they were
sampled and the bacterial culture was counted. The MICs of the
S. sanguinis protein extracts against the C.
tropicalis culture and C. albicans and C.
tropicalis co-culture were observed and calculated using the
same method.
Effects of the S. sanguinis proteins on
biofilms formed by C. albicans and C. tropicalis
The biofilm models of C. albicans and C.
tropicalis were established by the same method as that
described for P. intermedia and P. gingivalis. The
effects of the intracellular and exocrine proteins on the biofilm
models were tested, 500 μl proteins (1 g/l) were added, 500 μl
5.25% chlorhexidine was used as positive control, two techniques
were used: Continuous tomography to determine the thickness of the
biofilms, and dynamic observation of adherent viable/dead bacteria
in the different periods of biofilm formation.
Effects of the intracellular proteins on
the growth of C. albicans and C. tropicalis
The tests were performed on two groups (Table I). Each group was cultured at 37°C
and agitated, and the optical density (OD) value at 600 nm was
tested every 2 h. Growth curves (y-axis, OD value and x-axis,
incubation time) were plotted to assess the proliferation ability
of each group.
 | Table IShorthand and substances of the
groups in each experiment. |
Table I
Shorthand and substances of the
groups in each experiment.
| Group | Intracellular
proteins 1 g/l | C. albicans
suspension | C.
tropicalis suspension | RPMI-1640
medium |
|---|
| Treatment |
| Intracellular
proteins C. albicans | 300 μl | 10 μl | - | 10 ml |
| Intracellular
proteins C. tropicalis | 300 μl | - | 10 μl | 10 ml |
| Intracellular
proteins co-cultures | 300 μl | 5 μl | 5 μl | 10 ml |
| Control |
| C.
albicans | - | 10 μl | - | 10 ml |
| C.
tropicalis | - | - | 10 μl | 10 ml |
| C. albicans
and C. tropicalis | - | 5 μl | 5 μl | 10 ml |
Effects of the intracellular proteins on
the morphology of C. albicans and C. tropicalis
The tests were performed on two groups (Table I). Each group was cultured at 37°C
and agitated for 12 h and histological examination was conducted to
observe the morphology by optical microscopy (OM; SH11/YF-9,
Shenzhen Xingyu Xin Electronics Co., Ltd., Guangdong, China) and
scanning electron microscopy (SEM; Magellan XHR, FEI, USA).
Statistics and analysis
X-test, one-way analysis of variance and logistic
analysis were conducted using SPSS 16.0 to make statistical
analysis. P<0.05 was considered to indicate a statically
significant result.
Results
Effects of the protein extracts on P.
intermedia and P. gingivalis Inhibitory effect
A marked inhibitory effect of the intracellular
proteins on the growth of P. intermedia and P.
gingivalis was observed, while the exocrine proteins appeared
to be inactive. The MIC of the intracellular proteins against the
co-culture of P. intermedia and P. gingivalis was
0.125 g/l (Table II).
 | Table IIInhibitory effect of the bacterial
intracellular and exocrine proteins of S. sanguinis on P.
intermedia and P. gingivalis. |
Table II
Inhibitory effect of the bacterial
intracellular and exocrine proteins of S. sanguinis on P.
intermedia and P. gingivalis.
| Bacteria | Intracellular
proteins | MIC (g/l) | Exocrine
proteins |
|---|
| P.
intermedia | + | 0.1250 | − |
| P.
gingivalis | + | 0.0625 | − |
| P.
intermedia + P. gingivalis | + | 0.1250 | − |
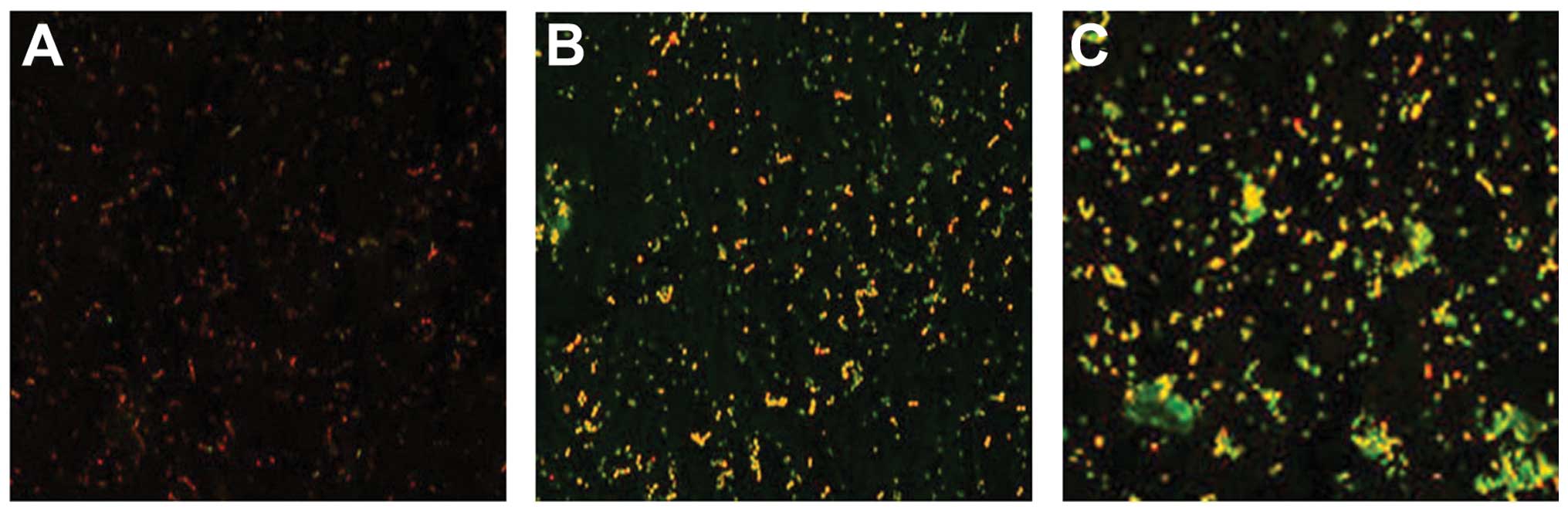
Results tested by confocal laser scanning
microscopy
A significant reduction in the percentage of viable
cells in the P. intermedia and P. gingivalis
co-cultural biofilm following treatment with the intracellular
proteins was observed compared with that of the control biofilm
(P<0.05), while no significant change following treatment with
the exocrine proteins was identified compared with that of the
control group (Table III and
Fig. 1).
 | Table IIIEffects of the intracellular and
exocrine proteins of S. sanguinis on the viability of the
biofilms formed by the P. intermedia and P.
gingivalis co-culture (%, mean ± standard deviation). |
Table III
Effects of the intracellular and
exocrine proteins of S. sanguinis on the viability of the
biofilms formed by the P. intermedia and P.
gingivalis co-culture (%, mean ± standard deviation).
| Measured Item | Intracellular
proteins | Exocrine
proteins | Deionized
water |
|---|
| Biofilm
Viability | 28.33±3.87a | 54.41±4.12 | 56.44±4.79 |
Effects of the protein extracts on C.
albicans and C. tropicalis Inhibitory effect
A significant inhibitory effect of the intracellular
proteins on the growth of C. albicans and C.
tropicalis was observed compared with that of the control
group, while the exocrine proteins did not have a significant
effect. When the concentration of the intracellular proteins was 1
g/l, the number of fungal colonies was significantly different
between the C. albicans, C. tropicalis and their
co-culture groups and the corresponding control group (Table IV).
 | Table IVInhibitory effect of the
intracellular and exocrine proteins of S. sanguinis on C.
albicans and C. tropicalis (106 cfu/ml, mean
± standard deviation). |
Table IV
Inhibitory effect of the
intracellular and exocrine proteins of S. sanguinis on C.
albicans and C. tropicalis (106 cfu/ml, mean
± standard deviation).
| Intracellular
proteins (g/l) | Exocrine proteins
(g/l) | |
|---|
|
|
| |
|---|
| Fungi | 0.25 | 0.5 | 1 | 0.25 | 0.5 | 1 | Control group |
|---|
| C.
albicans | 1.95±0.26 | 1.86±0.31 | 0.72±0.13a | 1.98±0.19 | 1.96±0.09 | 1.98±0.32 | 2.12±0.14 |
| C.
tropicalis | 1.87±0.29 | 1.70±0.36 | 0.72±0.18a | 2.13±0.21 | 1.97±1.03 | 1.83±0.28 | 2.07±0.15 |
| C. albicans + C.
tropicalis | 1.85±0.18 | 1.70±0.34 | 0.69±0.13a | 2.05±0.14 | 1.89±0.22 | 1.85±0.15 | 2.11±0.17 |
Results tested by confocal laser scanning
microscopy
The minimum biofilm thickness of C. albicans
and C. tropicalis was achieved within 12 h in the
intracellular protein groups. The thickness gradually reduced
between 4 and 12 h, and then increased between 12 and 48 h
(Tables V and VI).
 | Table VC. albicans biofilm thickness
at different time points (μm, mean ± standard deviation). |
Table V
C. albicans biofilm thickness
at different time points (μm, mean ± standard deviation).
| Time (h) |
|---|
|
|
|---|
| Group | 4 | 8 | 12 | 24 | 48 |
|---|
| Intracellular
proteins | 33.03±1.87 | 25.55±2.05a | 15.50±41.47a | 30.59±1.85a | 56.55±3.85 |
| Exocrine
proteins | 33.28±2.15 | 41.53±2.13 | 42.24±2.26 | 73.19±2.21 | 94.03±2.66 |
| Chlorhexidine | 35.46±1.92 | 31.55±2.59 | 26.12±1.29 | 24.50±1.76 | 37.38±3.56 |
| Deionized
water | 34.37±2.11 | 40.97±1.22 | 41.11±2.08 | 71.20±2.52 | 96.96±3.60 |
 | Table VIC. tropicalis biofilm
thickness at different time points (μm, mean ± standard
deviation). |
Table VI
C. tropicalis biofilm
thickness at different time points (μm, mean ± standard
deviation).
| Time (h) |
|---|
|
|
|---|
| Group | 4 | 8 | 12 | 24 | 48 |
|---|
| Intracellular
proteins | 35.33±5.74 | 33.91±4.58a | 27.72±4.00a | 37.69±3.57a | 76.69±5.17 |
| Exocrine
proteins | 33.28±2.15 | 47.36±2.79 | 62.36±4.46 | 75.18±4.60 | 103.04±6.55 |
| Chlorhexidine | 32.23±2.83 | 33.13±3.63 | 35.91±3.60 | 42.55±5.05 | 58.18±4.45 |
| Deionized
water | 33.91±2.06 | 44.08±4.68 | 58.21±3.38 | 71.17±4.40 | 112.26±5.27 |
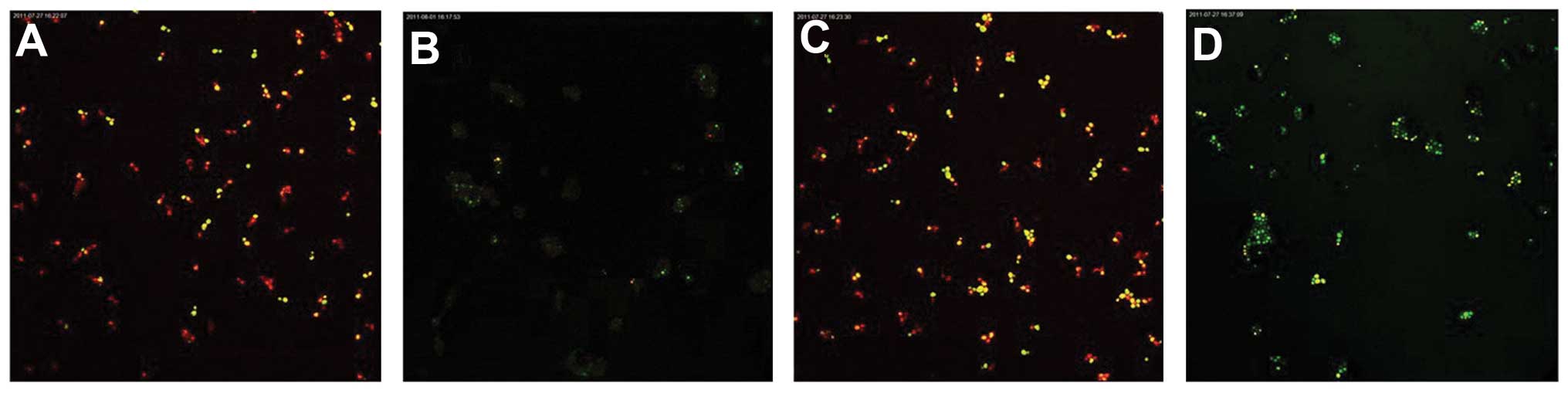
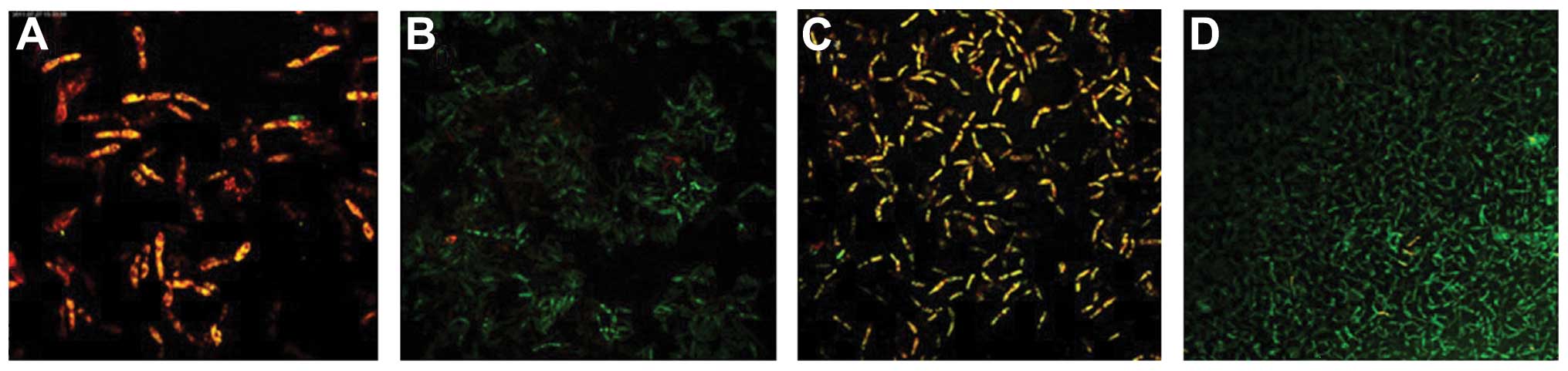
Following fluorescent staining, it was identified
that there were a number of dead fungi present at the bottom of the
biofilm formed by the C. albicans and C. tropicalis
co-culture. The intermediate layer was a mixture of live and dead
fungi, while there were few fungi in the surface layer. The
quantity of live C. albicans and C. tropicalis
following treatment with the intracellular proteins was less than
that of the control group for 4–12 h, while no significant
difference was observed after 24–48 h. The quantity of live C.
albicans and C. tropicalis following treatment with the
exocrine proteins was fundamentally the same as that of the control
group (Figs. 2 and 3).
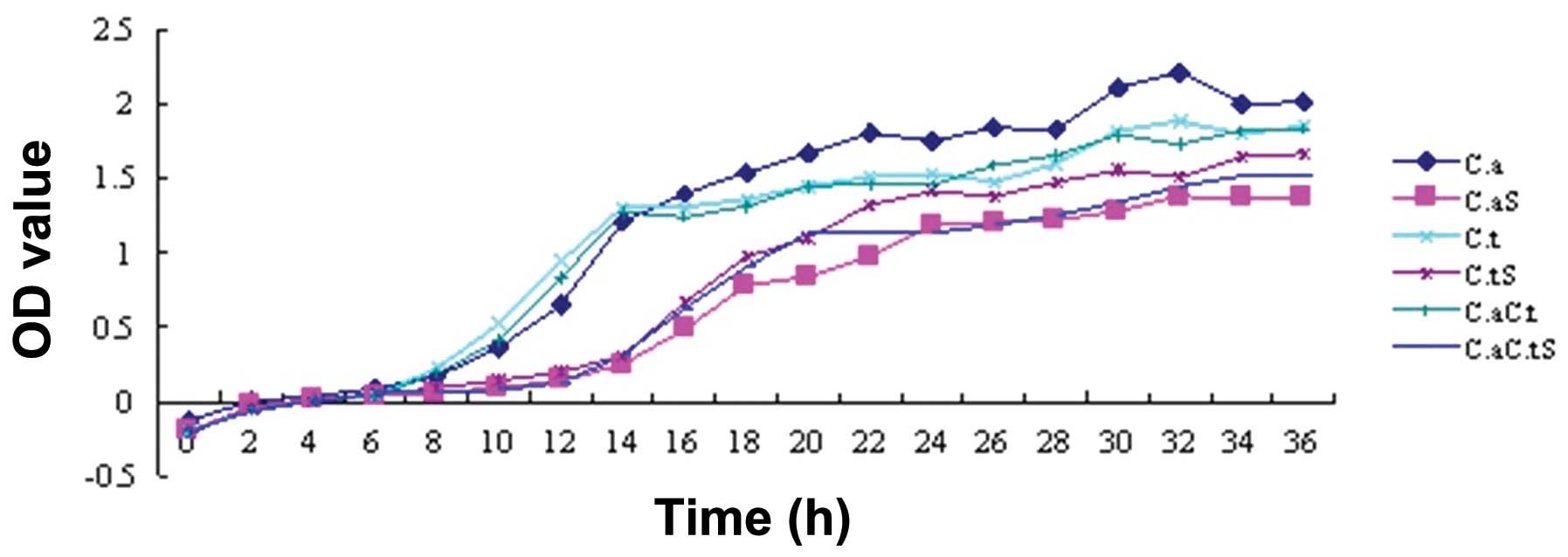
Growth curve
The OD values represent the radiation absorbed by a
detected object. The turbidity of a bacterial suspension is
proportional to the OD, which is precisely measured by an
ultraviolet spectrophotometer; therefore, OD values represent the
relative quantity of bacteria under a certain experimental
condition, thus reflecting the relative amount of growth. The
results showed a lag phase growth of C. albicans, C.
tropicalis and their co-culture following treatment with the
intracellular proteins for 14 h after seeding, after which the
bacteria proliferated rapidly and entered a logarithmic phase,
while the control groups entered this phase at 6 h. It was
indicated that the growth of C. albicans, C.
tropicalis and their co-culture was inhibited by the
intracellular proteins of S. sanguinis; the inhibitory
effect began after 8 h of incubation and was no longer observed at
14 h (Fig. 4).
Morphological observation
Using OM, it was observed that there was a large
number of spores and hyphae in the control group culture of C.
albicans, C. tropicalis or their co-culture. The hyphae
were different in length, and segmentally and irregularly
distributed among round or oval spores. When treated with the
intracellular proteins, the number of spores was markedly reduced,
and there was no hypae growth among round or oval spores (Fig. 5).
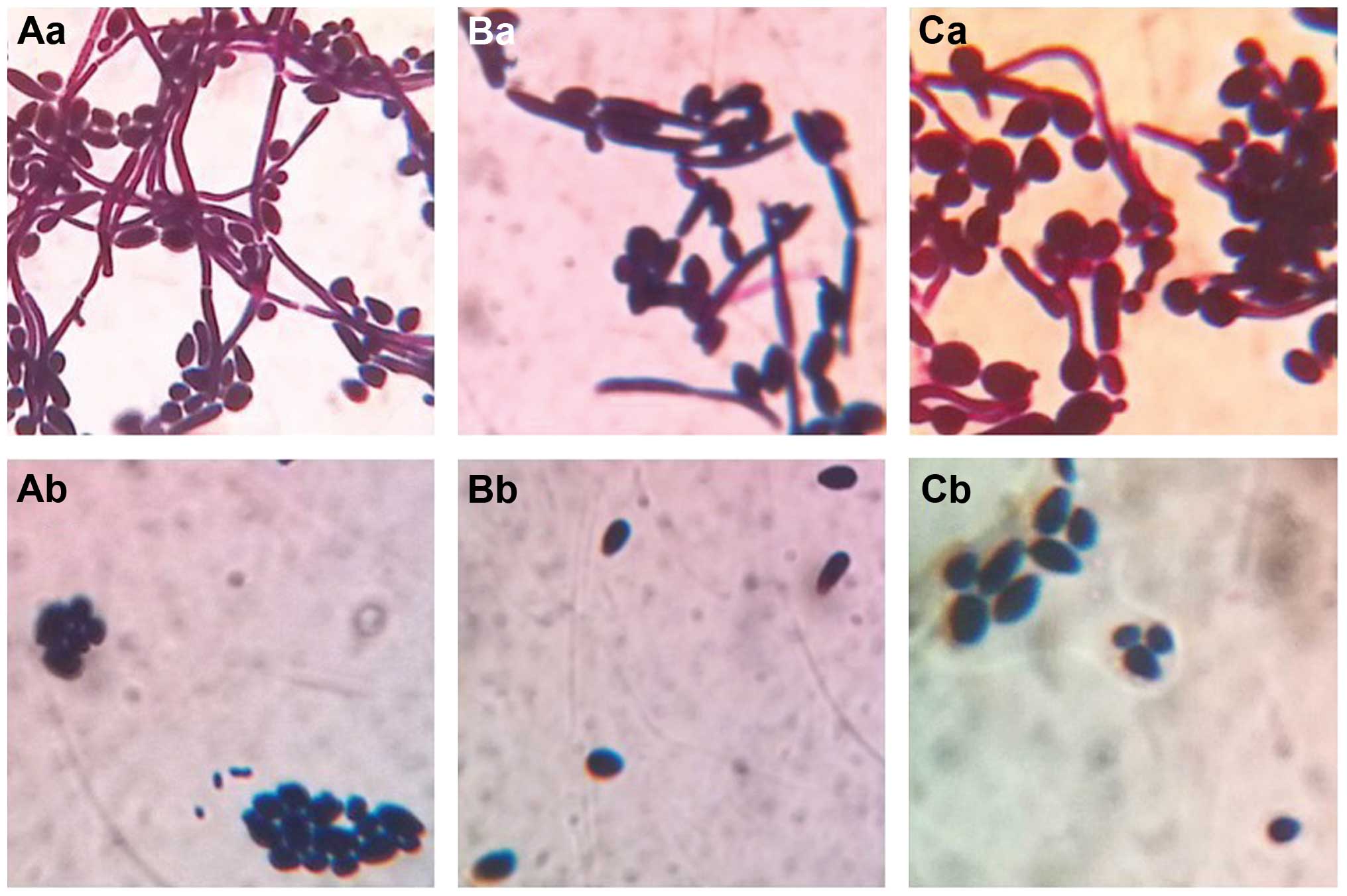 | Figure 5Morphology of C. albicans and
C. tropicalis in the control and the test groups under OM
observation. (Aa) C. albicans, 12 h; (Ab) intracellular
protein-treated C. albicans, 12 h. (Ba) C.
tropicalis, 12 h; (Bb) intracellular protein-treated C.
tropicalis, 12 h. (Ca) C. albicans and C.
tropicalis co-culture, 12 h; (Cb) intracellular protein-treated
co-culture, 12 h. Magnification, ×1,000. C. albicans, Candida
albicans; C. tropicalis, Candida tropicalis; OM, optical
microscopy. |
Using SEM, it was identified that there were round
or oval spores in the control group cultures of C. albicans
and C. tropicalis; the spores were 2–5 μm in diameter, had a
full shape and smooth surface, and there were adhesions among a
number of the spores. Round or oval spores and segmental hyphae
were observed in the C. albicans and C. tropicalis
co-culture control group; the spores had a full shape and smooth
surface, the spore diameter was 2–5 μm, and the hyphae took up the
entire photograph with a diameter of 1–3 μm. When treated with the
intracellular proteins, certain oblate or irregular spores were
identified in the C. albicans and C. tropicalis
monoculture groups. Disc-like depressions of different depths were
present in the surfaces of the spores, and the diameter of the
depressions was 0.2–0.8 μm. The majority of the spores had only one
depression, while their surfaces were smooth and free of wrinkles.
In the intracellular protein-treated C. albicans and C.
tropicalis co-culture group, disc-like depressions were
observed in the surfaces of a number of spores, and the diameter of
the depressions was 0.2–0.8 μm. The majority of the spores had only
one depression, although two depressions were occasionally
observed. Depressions were also identified in the surface of the
segmental hyphae. Each segment had a depression, the diameter of
the hyphae was 2 μm and that of the depression was 0.6 μm. The
surfaces of the hyphae and the majority of the spores were smooth;
however, wrinkles were present in the surfaces of certain spores
(Fig. 6).
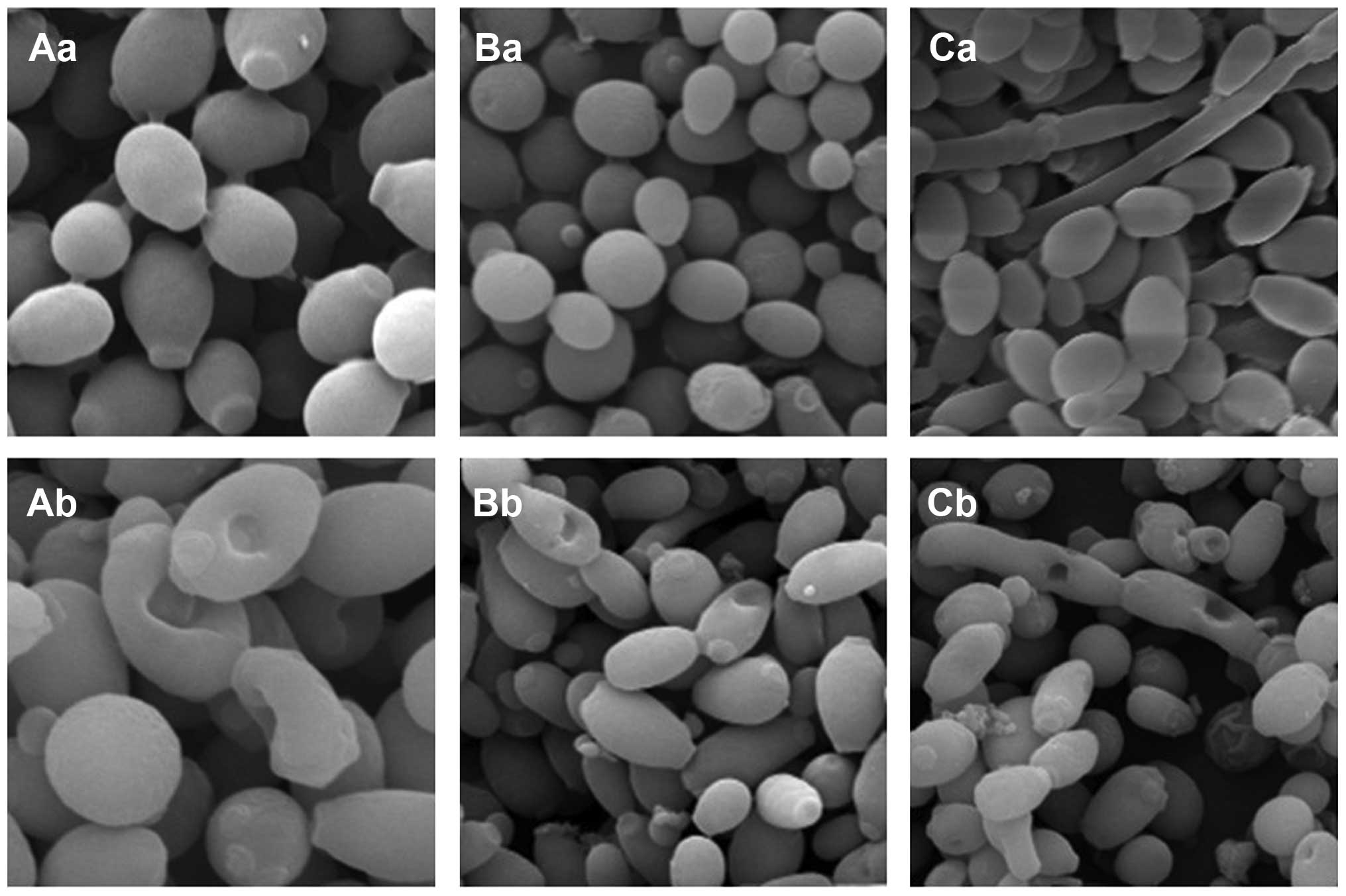 | Figure 6Morphology of C. albicans and
C. tropicalis in the control and the test groups under SEM
observation. (Aa) C. albicans, 12 h; (Ab) intracellular
protein-C. albicans, 12 h (magnification, ×4,000). (Ba)
C. tropicalis, 12 h; (Bb) intracellular protein-C.
tropicalis, 12 h (magnification, ×2,000). (Ca) C.
albicans and C. tropicalis co-cultures, 12 h; (Cb)
intracellular protein-co-cultures, 12 h (magnification, ×2,000).
C. albicans, Candida albicans; C. tropicalis, Candida
tropicalis; SEM, scanning electron microscopy. |
Discussion
It is controversial whether the antibacterial
substance generated by S. sanguinis under anaerobic
conditions exists in the cell or is released into the extracellular
environment. In this study, intracellular and exocrine proteins
were extracted from S. sanguinis, and their antagonistic
effects on P. intermedia, P. gingivalis, C.
albicans and C. tropicalis were detected. The results
showed that the antibacterial substance of S. sanguinis was
obtainable through a series of methods, namely centrifugation,
ultrasonication, salting out, Sephadex G-50 filtration and
dialysis. The extracts are also known as bacteriocins. The putative
periodontal pathogens P. intermedia and P. gingivalis
are bacteria, while C. albicans and C. tropicalis are
fungi, so they are discussed separately in this study.
In the present study, a number of effects of the
protein extracts on P. intermedia and P. gingivalis
were observed. P. intermedia is a Gram-negative anaerobic
bacteria that produces melanin, and is a potentially pathogenic
bacteria that is frequently detected in the subgingival plaques of
patients with periodontitis, pregnancy gingivitis, acute
necrotizing gingivitis and human immunodeficiency virus-associated
gingivitis (14). P.
gingivalis is considered as another main periodontal pathogen,
and is associated with adult periodontitis, juvenile periodontitis,
periodontal abscesses, alveolar abscesses, pulp infection and
refractory periodontitis (15).
P. intermedia and P. gingivalis are two important
putative periodontal pathogens, and their virulence factors
penetrate and destroy the host tissue, allowing them to escape the
host defense system. The detection rate is high in deep periodontal
pockets and the attachment loss sites of periodontitis. It has been
reported that the development of periodontitis is severely
associated with P. gingivalis and moderately associated with
P. intermedia (16).
Biofilms are the main form of survival for pathogens; they adapt
themselves to the circumstances and resist attack from phagocytes,
and inflammatory factors released by them cause the local
inflammatory response. Complicated biofilms are the main pattern of
existence of putative periodontal pathogens, and adhesive
interactions between the bacteria form barriers that develop
resistance to antibiotics. The results of the present study showed
a marked effect of the intracellular proteins on the growth of
P. intermedia, P. gingivalis and their co-cultures.
Also, the biofilm viability of the P. intermedia and P.
gingivalis co-culture was significantly reduced following
treatment with the intracellular proteins, compared with that of
the control group. It may speculated that: i) Secretory
immunoglobulin A (SIgA) in the saliva plays a pivotal role in the
local anti-infective immune response of the oral mucosa. SIgA may
bind specifically to antigens and play an inhibitory role in
sterilization through a biological effect on bacteria. As the
intracellular proteins of S. sanguinis interacted with the
biofilm formed by the P. intermedia and P. gingivalis
co-culture and the viability of the biofilm was reduced, it was
considered that the mechanisms of action of SIgA and S.
sanguinis may be similar. ii) Certain small molecules of the
intracellular proteins of S. sanguinis may act as ligands
that specifically bind to cell-membrane or intracellular receptors
of P. intermedia and P. gingivalis, and then trigger
a cascade of cellular events.
In the present study, a significant inhibitory
effect of the intracellular proteins on the growth of C.
albicans, C. tropicalis and their co-culture was
observed compared with that of the control groups. In clinical
practice, the commonly used antifungal drugs mainly function
through the inhibition of ergosterol biosynthesis, and as
ergosterol is the major component of the fungal cell membrane,
fungal growth is inhibited (17,18).
To investigate whether the inhibitory mechanism of the
intracellular proteins of S. sanguinis on C. albicans
and C. tropicalis was consistent with the mechanism of
typical antifungal drugs, in the present study, the effects of the
proteins of S. sanguinis on the morphology of C.
albicans and C. tropicalis were analyzed. It was
demonstrated that the number of spores and hyphae of C.
albicans and C. tropicalis was markedly reduced
following treatment with the intracellular proteins, and that
disc-like depressions were present in the surfaces of spores and
hyphae. Combined with the MIC values, the conclusion that the
intracellular proteins of S. sanguinis not only caused the
morphological changes of C. albicans and C.
tropicalis, but also the inhibition of their growth was
reached. There may be a number of associations between them, so it
is considered that the intracellular proteins of S.
sanguinis function through the following mechanisms: i)
Numerous intracellular proteins assemble on the cell surface by
electrostatic forces, which leads to a change in the distribution
of certain molecules in the outer layer of the cell and eventually
to a change in the morphology of the cell. ii) Intracellular
proteins may specifically bind to certain substances of the cell
membrane or walls of C. albicans and C. tropicalis,
to cause the breakdown of the outer layer. Wiedemann et al
(19) and Christ et al
(20) showed that the primary
force of the antibacterial effect of the bacteriocin of lactic acid
bacteria is the formation of pores of diameter 2–2.5 nm on the
surface of target cells, leading to an increase in cell membrane
permeability, which allows the release of ATP, amino acids and ions
from the cell. This results in bacterial metabolic disturbances and
ultimately causes the death of the cell. These morphological
changes are accordant with the results generated by the
intracellular proteins of S. sanguinis in the present study,
so it may be speculated that the antifungal effect was the result
of a loss of integrity of the cell surface and changes in cell
membrane permeability. Whether the intracellular proteins of S.
sanguinis are able to enter cells and influence signal
transduction and gene expression requires further study. iii) A
certain type of enzyme may be released from S. sanguinis,
which interacts with the cell walls through electrostatic
attraction, and then the cell is depressed and lysosomal enzyme is
released, which induces autocytolysis. iv) The intracellular
proteins may enter the cell and inhibit the synthesis of
chromosomal DNA, releasing the cytoskeleton and thereby inducing
autocytolysis. v) The intracellular proteins may enter the cell and
inhibit the synthesis of chromosomal DNA; this is likely to damage
the cytoskeleton to a certain extent, and ultimately result in
cytoskeleton collapse and the formation of disc-like depressions on
the bacterial surface.
The results of the growth curve analysis in the
present study indicated that the growth of C. albicans,
C. tropicalis and their co-culture was inhibited by the
intracellular proteins of S. sanguinis at a concentration of
1 g/l; the inhibition began at 8 h and ceased at 14 h. The reasons
for this observation may be that: i) There may be a
‘half-life period’ of the intracellular proteins of S.
sanguinis; that is, they are degraded by fungi or fungal
metabolites as the treatment time is extended. ii) The inhibition
process of the intracellular proteins of S. sanguinis may be
a form of active transport; the intracellular proteins are
transported by an active transcellular route against the
concentration gradient, and as the process requires energy
expenditure, the inhibitory effect reduces gradually.
The thickness of the biofilms formed by C.
albicans and C. tropicalis was reduced following
treatment with the intracellular proteins within 24 h and minimized
in 12 h, suggesting the intracellular proteins of S.
sanguinis have significant antifungal effects. It may be
speculated that: i) The antifungal substance of S. sanguinis
is located within the cell. ii) The intracellular proteins are
gradually degraded by fungi or fungal metabolites, their
‘half-life period’ is minimized and biological activity is
lost. iii) The intracellular proteins are characterized by
amphoteric dissociation; when they become positively charged, the
negative charges of C. albicans or C. tropicalis are
neutralized, which triggers a cascade of cellular potential
disorders and finally causes the death of the bacteria. iv) The
intracellular proteins may cause changes in the osmotic balance of
C. tropicalis in vitro and in vivo; as the proteins
are gradually degraded through facilitated diffusion within 24 h,
the antifungal effect is then reduced. v) The van der Waals force
between the bacteria increases along with the maturation of the
biofilm, which is tightly packed, so the antifungal substance is
not able to enter the biofilm and the inhibitory effect is
invalidated, with little effect on the bacterial surface.
No inhibitory effect of the exocrine proteins on
the growth of P. intermedia, P. gingivalis, C.
albicans and C. tropicalis was observed in the present
study, which is not accordant with the findings of previous studies
(21). The standard strain of
S. sanguinis or the culture conditions in the present study
may have been different from those of the other studies, resulting
in the antimicrobial substance of S. sanguinis not being
exported out of the cell in the previous studies.
In the present study, significant inhibitory
effects of the intracellular proteins of S. sanguinis on the
growth of P. intermedia, P. gingivalis, C.
albicans, C. tropicalis and their biofilms were
observed, and the morphology of C. albicans and C.
tropicalis was also affected. However the inhibitory mechanism
of S. sanguinis was not identified. Whether the inhibition
caused the morphological changes or the morphological changes
caused the inhibition, or they were essential prerequisites of each
other was not fully elucidated. Furthermore, identification of
where the antimicrobial substance of S. sanguinis is located
requires further study.
Acknowledgements
This study was funded by Natural Science Foundation
of Heilongjiang Province (D2007-03).
References
|
1
|
Qi F and Kreth J: Characterization of
anti-competitor activities produced by oral bacteria. Methods Mol
Biol. 666:151–166. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhu L and Kreth J: Role of
Streptococcus mutans eukaryotic-type serine/threonine
protein kinase in interspecies interactions with Streptococcus
sanguinis. Arch Oral Biol. 55:385–390. 2010.
|
|
3
|
Fujimura S and Nakamura T: Sanguicin, a
bacteriocin of oral Streptococcus sanguis. Antimicrob Agents
Chemother. 16:262–265. 1979. View Article : Google Scholar
|
|
4
|
Hillman JD, Socransky SS and Shivers M:
The relationships between streptococcal species and
periodontopathic bacteria in human dental plaque. Arch Oral Biol.
30:791–795. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hammond BF, Lillard SE and Stevens RH: A
bacteriocin of Actinobacillus actinomycetemcomitans. Infect
Immun. 55:686–691. 1987.
|
|
6
|
Byrne DP, Potempa J, Olczak T and Smalley
JW: Evidence of mutualism between two periodontal pathogens:
co-operative haem acquisition by the HmuY haemophore of
Porphyromonas gingivalis and the cysteine protease interpain
A (InpA) of Prevotella intermedia. Mol Oral Microbiol.
28:219–229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sbordone L, Di Genio M and Bortolaia C:
Bacterial virulence in the etiology of periodontal diseases.
Minerva Stomatol. 49:485–500. 2000.(In Italian).
|
|
8
|
Gomes BP, Endo MS and Martinho FC:
Comparison of endotoxin levels found in primary and secondary
endodontic infections. J Endod. 38:1082–1086. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
López NJ: Occurrence of Actinobacillus
actinomycetemcomitans, Porphyromonas gingivalis, and
Prevotella intermedia in progressive adult periodontitis. J
Periodontol. 71:948–954. 2000.
|
|
10
|
de Lillo A, Teanpaisan R, Fierro JF and
Douglas CW: Binding and degradation of lactoferrin by
Porphyromonas gingivalis, Prevotella intermedia and
Prevotella nigrescens. FEMS Immunol Med Microbiol.
14:135–143. 1996.
|
|
11
|
Bruder-Nascimento A, Camargo CH, Sugizaki
MF, Sadatsune T, Montelli AC, Mondelli AL and Bagagli E: Species
distribution and susceptibility profile of Candida species
in a Brazilian public tertiary hospital. BMC Res Notes. 3:12010.
View Article : Google Scholar
|
|
12
|
Azenha MR, Caliento R, Brentegani LG and
de Lacerda SA: A retrospective study of oral manifestations in
patients with paracoccidioidomycosis. Braz Dent J. 23:753–757.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vazquez JA: Therapeutic options for the
management of oropharyngeal and esophageal candidiasis in HIV/AIDS
patients. HIV Clin Trials. 1:47–59. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Okamoto M, Maeda N, Kondo K and Leung KP:
Hemolytic and hemagglutinating activities of Prevotella
intermedia and Prevotella nigrescens. FEMS Microbiol
Lett. 178:299–304. 1999. View Article : Google Scholar
|
|
15
|
Lakhdar L, Hmamouchi M, Rida S and Ennibi
O: Antibacterial activity of essential oils against periodontal
pathogens: a qualitative systematic review. Odontostomatol Trop.
35:38–46. 2012.PubMed/NCBI
|
|
16
|
Consensus report. Periodontal diseases:
pathogenesis and microbial factors. Ann Periodontol. 1:926–932.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Joseph-Horne T and Hollomon DW: Molecular
mechanisms of azole resistance in fungi. FEMS Microbiol Lett.
149:141–149. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Urbina JM, Cortés JC, Palma A, López SN,
Zacchino SA, Enriz RD, Ribas JC and Kouznetzov VV: Inhibitors of
the fungal cell wall. Synthesis of 4-aryl-4-N-arylamine-1-butenes
and related compounds with inhibitory activities on beta(1–3)
glucan and chitin synthases. Bioorg Med Chem. 8:691–698.
2000.PubMed/NCBI
|
|
19
|
Wiedemann I, Benz R and Sahl HG: Lipid
II-mediated pore formation by the peptide antibiotic nisin: a black
lipid membrane study. J Bacteriol. 186:3259–3261. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Christ K, Wiedemann I, Bakowsky U, Sahl HG
and Bendas G: The role of lipid II in membrane binding of and pore
formation by nisin analyzed by two combined biosensor techniques.
Biochim Biophys Acta. 1768:694–704. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kou YR and Pan YP: Bacteriostatic activity
of bacteriocin from Streptococcus sanguis. J Microbiol.
2:100–102. 2005.(In Chinese).
|