Introduction
Osteonecrosis of the femoral head (ONFH) has a high
incidence, however, the underlying pathogenesis of ONFH remains
unclear. To date, theories of metabolic disturbance, osteoporosis,
intraosseous hypertension, intracellular coagulation and
cytotoxicity have been hypothesized to explain its pathogenesis,
but the underlying pathological change in ONFH is microcirculation
disturbance (1,2). Furthermore, the treatment of ONFH
remains a controversial challenge as no safe or effective
prevention and treatment methods have been identified (3–5).
As molecular biology has developed, bone marrow
derived mesenchymal stem cells (BMSCs) have been applied in the
treatment of ONFH; this has initiated a novel strategy for the
treatment of ONFH and has been identified as effective in
preliminary investigations (6,7).
However, the application of cajan leaf in combination with BMSCs in
the treatment of ONFH has not yet been investigated.
In the present study, the effect of combining
traditional Chinese cajan leaf with BMSCs, for the treatment of
ONFH, was observed and the underlying mechanisms were analyzed.
Materials and methods
Animals
A total of 40 healthy Sprague Dawley male rats
(weight, 200±20 g) were purchased from the Laboratory Animal Center
of Zhejiang University (Zhejiang, China). This study was conducted
in strict accordance with the recommendations in the Guide for the
Care and Use of Laboratory Animals of the National Institutes of
Health (2011). The animal use protocol was reviewed and approved by
the Institutional Animal Care and Use Committee of Xi’an Jiaotong
University College of Medicine (Xi’an, China). The study was
approved by the ethics committee of the medical ethics committee of
Xi’an Jiaotong University, Xi’an, China.
Grouping
Following three days of adaptive feeding, the rats
were randomized into groups of ten: A, control with no treatment;
B, treated with cajan leaf; C, treated with BMSCs and D, treated
with cajan leaf and BMSCs. The groups were subject to left-sided
ONFH modeling.
Modeling and handling
Modeling was performed via liquid nitrogen freezing.
Following administration of an intraperitoneal anesthesia with 1%
pentobarbital sodium (45 mg/kg), each rat was fixed supinely to the
surgical table. The skin was prepared and disinfected three times.
An anterior longitudinal incision was performed, to cut open the
skin, and the muscles were detached to expose the joint capsule and
femur head, ensuring that the femoral artery was not damaged. Half
of the femur head was dislocated out of the acetabulum, the femur
head was drilled using a rat puncture needle and the surrounding
tissues were protected using sterile gauze. Subsequently, the front
of the femoral head was punctured 10 times (for 20 secs each time)
using the metal bar in the nitrogen canister. Subsequent to heating
with warm physiological saline, groups C and D were injected with
BMSCs. BMSCs were obtained from the femur and the shinbone of
Sprague Dawley rats, which were cultured in Dulbecco’s Modified
Eagle Medium (DMEM) containing 10% fetal bovine serum, 100 μ/ml
penicillin and 100 μ/ml streptomycin. Next, ~5 μl BMSCs (containing
~105 cells) were injected using a microinjector. The
incision was sealed using gelatin and sutured layer by layer. The
muscular layer was sutured at first, and then the skin was sutured.
The rats were injected with penicillin for three consecutive days
to prevent infection following the modeling. No other treatment was
administered and the rats were caged separately. Administration of
cajan leaf commenced one day following the cell injection. An
aqueous solution of the cajan leaf was prepared (Beijing Normal
University, Beijing, China) and injected locally into the hip joint
on the side that was used for preparing the model. A dose of 40
mg/kg cajan leaf was administered in an injection volume of 0.4 ml
per rat; groups A and C received an equal volume of physiological
saline. Each rat was injected once per day for 30 consecutive
days.
Sample collection
Ten rats from each group were sacrificed 30 days
following treatment. The integral femoral heads were obtained under
aseptic conditions, wrapped with wet gauze and stored in a low
temperature refrigerator.
Decalcified bone sample preparation
The whole femoral head was fixed in 4% formaldehyde
solution for 72 h, which was subsequently decalcified in 10% edetic
acid solution for ~14 days (the decalcifying fluid was changed
every 7 days). Histomorphological observation and
immunohistochemical staining were performed until the cancellous
bone was pierceable with a pin.
Histomorphological observation
Segments of the decalcified bone samples were
dehydrated using a gradient of ethanol, wax-dipped, embedded in
paraffin and cut into 4- μm sections. One section was subject to
hematoxylin and eosin staining and the other section was used for
immunohistochemical staining of vascular endothelial growth factor
(VEGF) and image analysis.
Immunohistochemical staining and image
analysis
The sections were loaded on to polylysine pretreated
microscopic slides and placed in a 60°C oven for 30 min to increase
adhesion. They were routinely dewaxed and placed in a 3%
H2O2 solution for 10 min at room temperature,
to block endogenous peroxidase activity. Subsequently, the sections
were rinsed three times in distilled water, soaked in 0.01 M
citrate buffer and heated to boiling point, at which point the
power was immediately cut off. After 10 min, the heating process
was repeated and the sections were reacted with antigen retrieval
buffer for 5–10 min to expose additional antigens. The sections
were rinsed three times and incubated with normal goat serum
confining liquid for 20 min. Redundant liquid was removed and the
sections were incubated overnight at 4°C with a 1:150 dilution of
rabbit anti-mouse VEGF antibody (Sigma, St. Louis, MO, USA).
Phosphate-buffered saline (PBS) was used to wash the sections,
which were then incubated at 37°C, with biotinylated goat
anti-rabbit IgG. Subsequent to this, sections were incubated with
streptavidin-biotin complex for 20 min. The sections were washed
four times with PBS and stained with 3,3′-diaminobenzidine solution
at room temperature, rinsed with distilled water, counterstained
with hematoxylin, dehydrated, cleared, mounted and observed under a
microscope (Olympus, Tokyo, Japan). The cells exhibiting
homogeneously buffy-stained cytoplasm and membranes were considered
to exhibit positive expression.
Following staining, the sections were observed under
a light microscope (magnification, ×200). Visual fields from each
section were randomly selected to determine positive expression.
Positive expression of VEGF at the broken ends of the fractured
bones was determined using mean gray scale values and compared
using an image analysis system (Nikon ACT-1U, Nikon Corporation,
Tokyo, Japan). The gray scales represented the signal intensity
within the cells, whereby a reduced gray scale indicated a higher
intensity. Five visual fields were randomly selected, total cells
and positive cells were counted from which the positive cell
percentage was calculated.
Statistical analysis
All data were analyzed using SPSS software (SPSS
Inc., Chicago, IL, USA). Enumeration data are presented as mean ±
standard deviation.
Results
Histomorphological changes
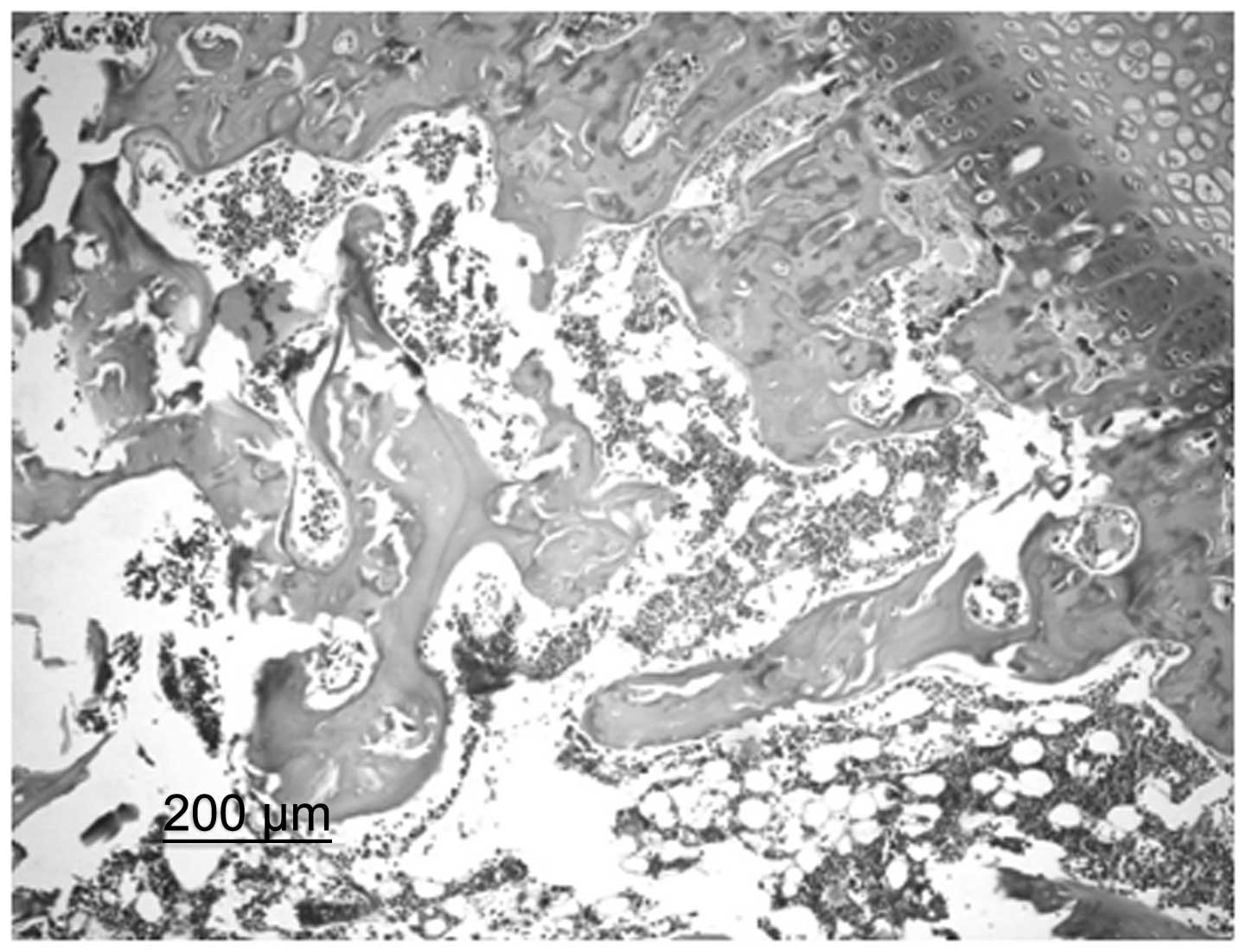
In the control group, bone trabeculae were sparse,
thin and exhibited ruptures. Structural disorder and bone fragments
were apparent. Osteocytes in the bone trabeculae displayed pyknosis
or margination and in specific cases, osteocytes were not present.
In addition, the number of empty bone lacunae markedly increased,
the volume of the lipocytes in the pulp chamber increased and
specific lipocytes fused into a bubble shape. Spindle-shaped
osteoblasts were observed along the margins of the bone trabeculae
in small numbers (Fig. 1). In the
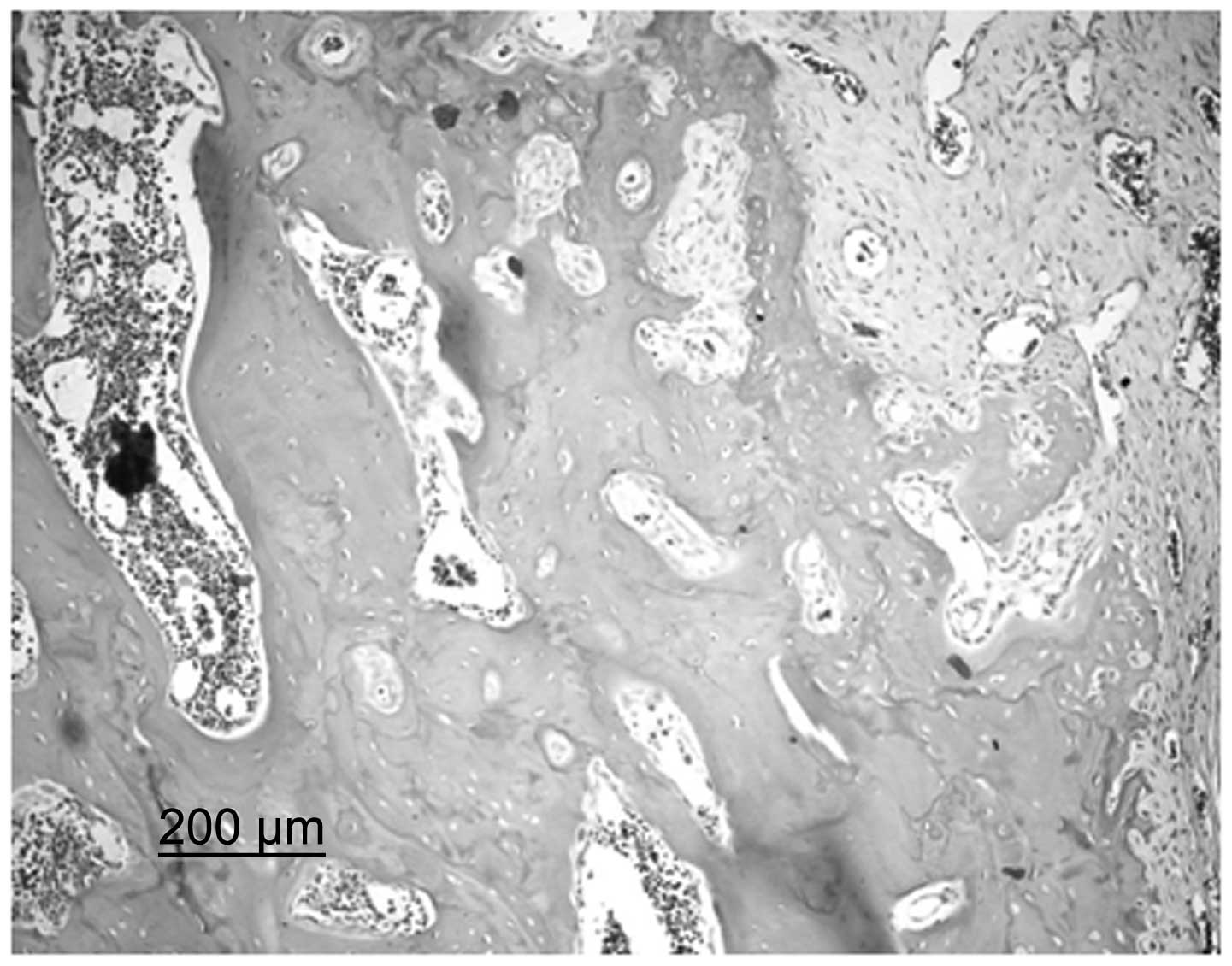
BMSC group, bone trabeculae were incomplete and, although their
alignment was ordered, a small number were broken. It was possible
to observe the nuclei of osteocytes on the bone trabeculae and a
number of empty bone lacunae were present. A large quantity of
spindle-shaped osteoblasts were observed along the margins of the
bone trabeculae. In the cajan leaf and cajan leaf + BMSC groups,
bone trabeculae exhibited a regular alignment. Trabeculae were
dense and full without ruptures and the nuclei of osteocytes were
clearly observed. In addition, the presence of empty bone lacunae
was rare, hematopoietic cells were abundant in the pulp chamber and
no large fat drops were observed. Furthermore, the quantity of
spindle-shaped osteoblasts in a dense alignment along the margins
of the bone trabeculae increased (Fig.
2).
Immunohistochemical staining
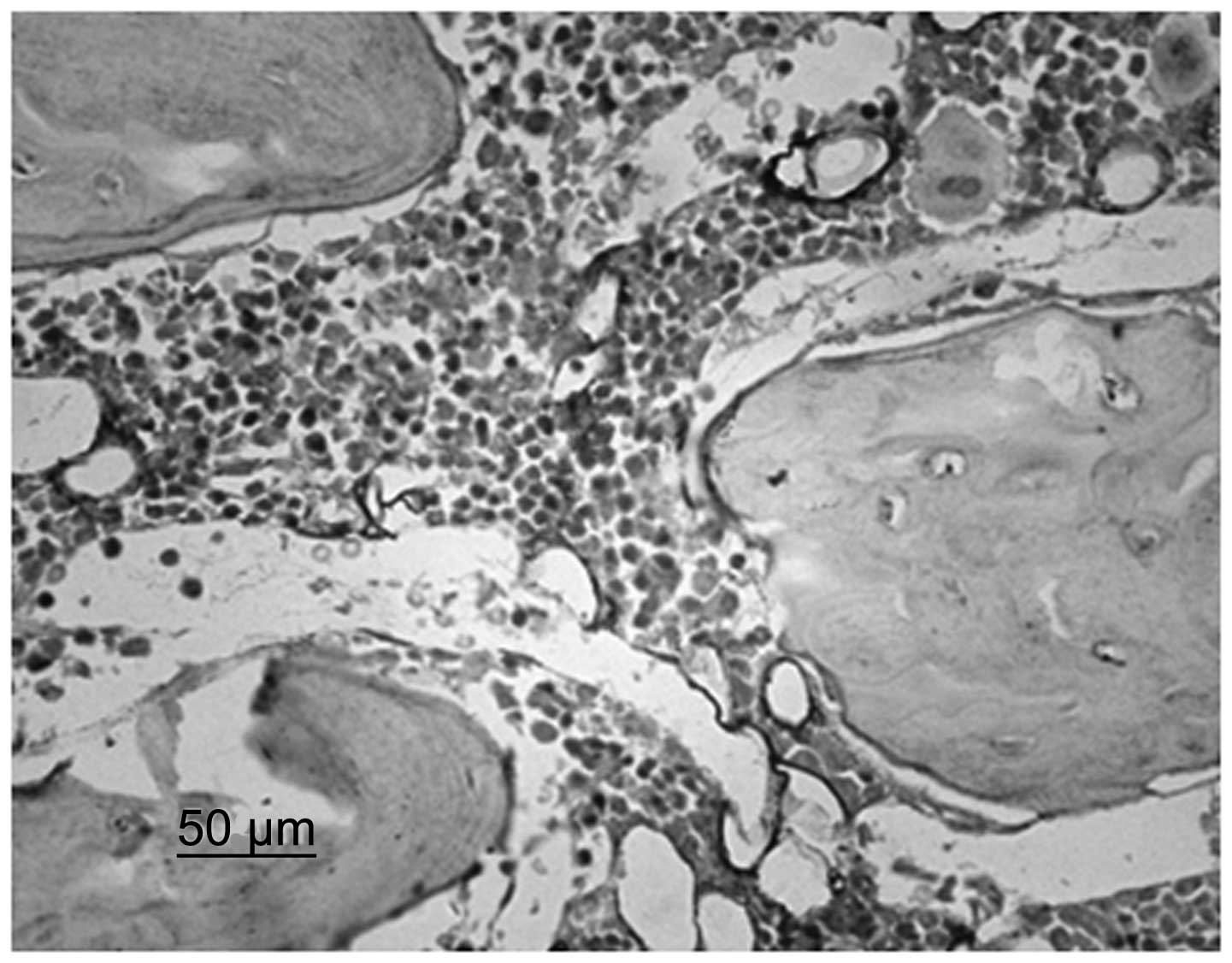
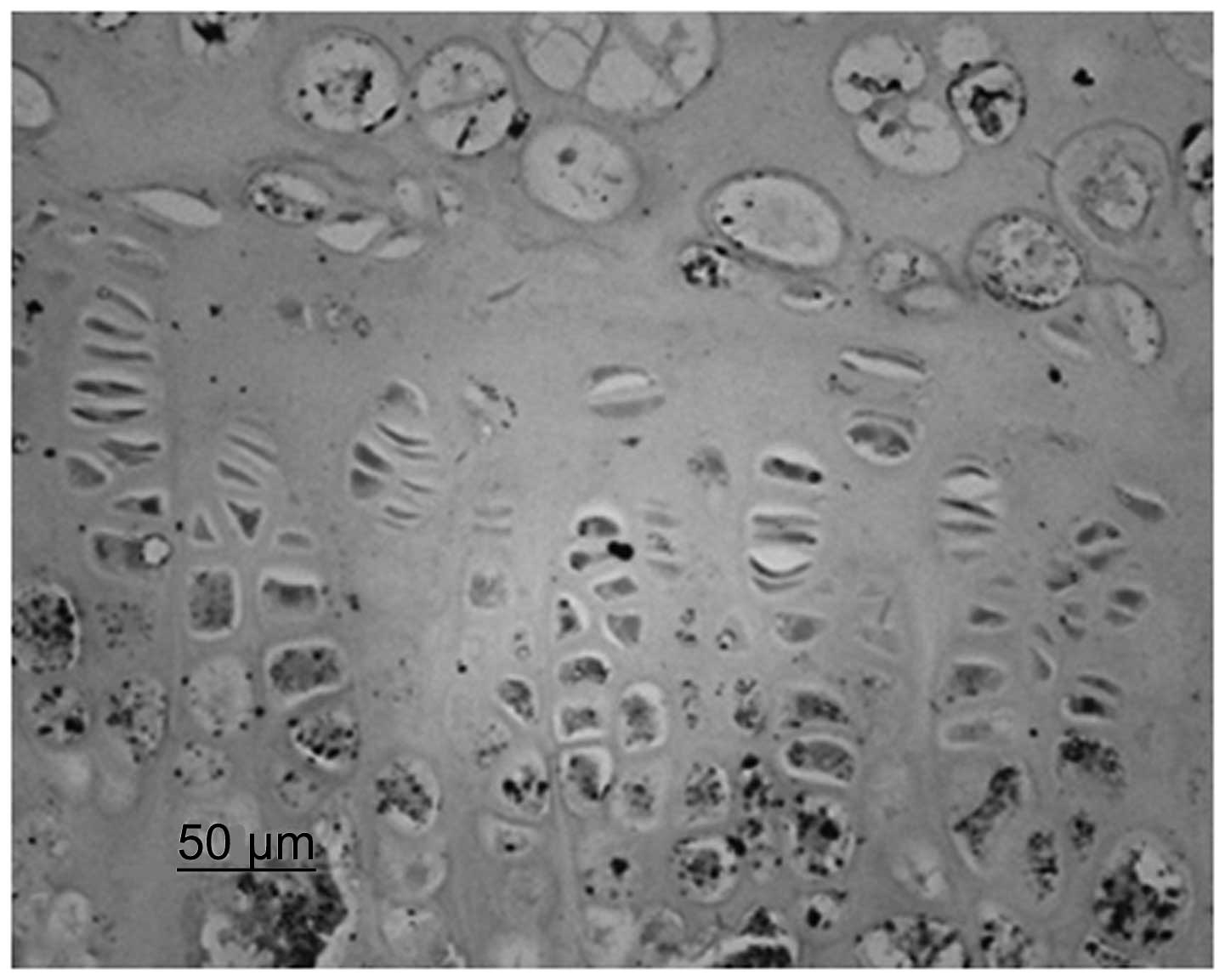
Immunohistochemistry identified that chondrocytes
and VEGF were stained. Although immature and mature chondrocytes in
the chondrogenic zone were stained, the staining of the mature
chondrocytes was deeper. Compared with the control group, the cajan
leaf and cajan leaf + BMSC groups exhibited deeper staining of
chondrocytes and VEGF, and a greater quantity of positively
expressed cells (Figs. 3 and
4).
Image analysis
The gray scale assays identified that the positive
expression intensity of groups B–D significantly increased compared
with that of group A (P<0.01). Although no significant
difference was observed between groups B and D (P>0.05), group D
exhibited a greater tendency towards a positive expression
intensity. The results showed that the positive expression rates of
groups B–D were markedly higher than the rates of group A
(P<0.01). Furthermore, groups A–C showed significant differences
in positive expression compared with group D (P<0.01). The
results are summarized in Table
I.
 | Table IPositive expression rate and gray
scale of each group. |
Table I
Positive expression rate and gray
scale of each group.
| Group | Positive expression
rate, % | Gray scale |
|---|
| Control |
22.1323±2.6852b |
140.7500±2.5495b |
| Cajan leaf |
40.0572±5.2344a |
125.1250±3.6815ab |
| BMSC alone |
30.8064±2.2825ab |
135.125±3.1820ab |
| Cajan leaf +
BMSC |
47.0581±4.8905b |
124.0000±4.7509a |
Discussion
The primary pathological change in ONFH is the
obstruction of intraosseous blood supply, which leads to a
disturbance in the microcirculation of the femoral head. Although
the exact incidence rate of ONFH remains unknown, 1,000–2,000
patients are diagnosed with this condition annually in the United
States (8). The treatment of ONFH
is complex and generally classified into surgical and non-surgical
treatment methods; however, the curative effects of the two methods
vary (9–13). Li and Wang (14) observed the effect of Epimedium
brevicornum on hormonal ONFH and identified that its effect may
be correlated with the improvement of local blood circulation in
the femoral head and the promotion of the proliferation,
differentiation and maturation of osteoblasts in vitro.
These observations highlighted the possibility of administering
cajan leaf for the treatment of ONFH.
Bone tissue engineering is a novel branch of
scientific research, which aims to design, construct, culture and
maintain living cells, to study biological substitutes, repair and
reconstruct the structure of human tissues and organs, and to
maintain or improve their functions based on the principles and
techniques of biology and engineering. The key components of tissue
engineering may be summarized as stent materials, seed cells and
signal factors. BMSCs are non-hematopoietic stem cells in the bone
marrow, which support and regulate hematogenesis in vivo, as
well as in vitro. BMSCs are distributed in a variety of
tissues and organs in vivo and have a multi-directional
differentiation potential, which enables them to differentiate into
osteoblasts, fibroblasts, reticulocytes, lipocytes and endothelial
cells; for these reasons, BMSCs are currently being extensively
studied. In addition, the gradual development of the technique of
inducing the differentiation of BMSCs towards osteoblasts,
indicates a general trend towards the utilization of BMSCs for
treatment of ONFH. Although BMSCs have a certain curative effect on
ONFH (7,15,16),
the underlying mechanisms have not been elucidated. Therefore, the
present study aimed to identify the possible mechanisms underlying
the curative effect of BMSCs on ONFH, based on the hypothesis that
BMSCs improve ONFH repair by promoting revascularization.
The regeneration and repair of ONFH is a complex
physiological and biochemical process, which is accompanied by
vascularization. VEGF specifically acts on endothelial cells to
promote their proliferation and vascularization, thereby
participating in bone regeneration and repair. When using bone
grafts in clinical practice to repair defects, a sufficient blood
supply to the bone graft bed is required. In autogenous bone
implantation, a vascular pedicle bone graft or a graft with
muscular flaps is frequently used to increase blood supply in order
to promote the early survival of the bone graft. Therefore, for
bone formation, an optimal vascular net is necessary to provide
nutrition and oxygen, transport osteogenic precursor cells and
secrete growth factors, which are required by the osteoblasts
(17,18). In the present study, the results
showed that treatment with cajan leaf, BMSCs alone and cajan leaf +
BMSCs were all capable of locally stimulating a high expression of
VEGF, especially in the cajan leaf and cajan leaf + BMSCs groups.
The mechanisms underlying the repair-promoting effect of cajan leaf
+ BMSCs on ONFH may, therefore, be as follows. Although local
hypoxia following ONFH stimulated the expression of VEGF, cajan
leaf combined with BMSCs increased VEGF expression. Such an
increase strengthened the vascular proliferation in the necrotic
area and formed a complete vascular net. Accordingly, the formation
of this net provided increased nutrition and oxygen for the
necrotic area, which conveyed increased osteogenic precursor cells
and secreted related growth factors, thereby accelerating ONFH
repair.
As investigation of ONFH continues, the analysis of
genes associated with this condition has gained increasing
attention worldwide (19–23). Although further progress in the
investigation of BMSCs, as well as the application of BMSCs in the
treatment of ONFH has been made, the study of cajan leaf combined
with BMSCs is in its infancy. Furthermore, the underlying
mechanisms remain unknown; however, previous studies have laid the
foundations and provided a direction for further investigation.
In conclusion, the application of traditional
Chinese medicine in the treatment of orthopedic disorders has a
long history and has achieved a marked curative effect. Therefore,
analyzing traditional Chinese medicine using modern technology
whilst continuing to link traditional Chinese medicine with gene
research, may provide a direction for future investigation.
References
|
1
|
Assouline-Dayan Y, Chang C, Greenspan A,
Shoenfeld Y and Gershwin ME: Pathogenesis and natural history of
osteonecrosis. Semin Arthritis Rheum. 32:94–124. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aaron RK: Concepts of the pathogenesis of
osteonecrosis. Tech Orthop. 16:101–104. 2001. View Article : Google Scholar
|
|
3
|
Ivankovich DA, Rosenberg AG, Malamis A and
Aaron RK: Reconstructive options for osteonecrosis of the femoral
head. Tech Orthop. 16:66–79. 2001. View Article : Google Scholar
|
|
4
|
Jones LC and Hungerford DS: Overview of
osteonecrosis of the hip and current treatment options. Curr Opin
Orthop. 14:12–16. 2003. View Article : Google Scholar
|
|
5
|
Zhao DW and Hu YC: Chinese experts’
consensus on the diagnosis and treatment of osteonecrosis of the
femoral head in adults. Orthop Surg. 4:125–130. 2012.
|
|
6
|
Zhao D, Cui D, Wang B, et al: Treatment of
early stage osteonecrosis of the femoral head with autologous
implantation of bone marrow-derived and cultured mesenchymal stem
cells. Bone. 50:325–330. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gangji V, De Maertelaer V and Hauzeur JP:
Autologous bone marrow cell implantation in the treatment of
non-traumatic osteonecrosis of the femoral head: Five year
follow-up of a prospective controlled study. Bone. 49:1005–1009.
2011.PubMed/NCBI
|
|
8
|
Babis GC, Sakellariou V, Parvizi J and
Soucacos P: Osteonecrosis of the femoral head. Orthopedics.
34:392011. View Article : Google Scholar
|
|
9
|
Baksi DP, Pal AK and Baksi DD: Long-term
results of decompression and muscle-pedicle bone grafting for
osteonecrosis of the femoral head. Int Orthop. 33:41–47. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marker DR, Seyler TM, McGrath MS, Delanois
RE, Ulrich SD and Mont MA: Treatment of early stage osteonecrosis
of the femoral head. J Bone Joint Surg Am. 90(Suppl 4): 175–187.
2008. View Article : Google Scholar
|
|
11
|
Nozawa M, Maezawa K, Matsuda K, et al:
Rotational acetabular osteotomy for osteonecrosis of the femoral
head after intracapsular fracture of the neck of the femur. J
Orthop Trauma. 22:658–662. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei BF and Ge XH: Treatment of
osteonecrosis of the femoral head with core decompression and bone
grafting. Hip Int. 21:206–210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Malizos KN, Karantanas AH, Varitimidis SE,
Dailiana ZH, Bargiotas K and Maris T: Osteonecrosis of the femoral
head: etiology, imaging and treatment. Eur J Radiol. 63:16–28.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li HY and Wang YS: Effect of epimedium on
blood rheology and bone density of rats with steroid-induced
femoral head necrosis. Chin J Exp Surg. 29:1226–1228. 2012.(In
Chinese).
|
|
15
|
Yamasaki T, Yasunaga Y, Ishikawa M, Hamaki
T and Ochi M: Bone-marrow-derived mononuclear cells with a porous
hydroxyapatite scaffold for the treatment of osteonecrosis of the
femoral head: a preliminary study. J Bone Joint Surg Br.
92:337–341. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshioka T, Mishima H, Akaogi H, Sakai S,
Li M and Ochiai N: Concentrated autologous bone marrow aspirate
transplantation treatment for corticosteroid-induced osteonecrosis
of the femoral head in systemic lupus erythematosus. Int Orthop.
35:823–829. 2011. View Article : Google Scholar
|
|
17
|
Liu B, Cao Y, Wang D, Yao G and Bi Z:
Vascular endothelial growth factor -634G/C polymorphism associated
with osteonecrosis of the femoral head in a Chinese population.
Genet Test Mol Biomarkers. 16:739–743. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hang D, Wang Q, Guo C, Chen Z and Yan Z:
Treatment of osteonecrosis of the femoral head with VEGF165
transgenic bone marrow mesenchymal stem cells in mongrel dogs.
Cells Tissues Organs. 195:495–506. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hong JM, Kim TH, Kim HJ, Park EK, Yang EK
and Kim SY: Genetic association of angiogenesis- and
hypoxia-related gene polymorphisms with osteonecrosis of the
femoral head. Exp Mol Med. 42:376–385. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim TH, Hong JM, Kim HJ, Park EK and Kim
SY: Lack of association of MTHFR gene polymorphisms with the risk
of osteonecrosis of the femoral head in a Korean population. Mol
Cells. 29:343–348. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee HJ, Choi SJ, Hong JM, et al:
Association of a polymorphism in the intron 7 of the SREBF1 gene
with osteonecrosis of the femoral head in Koreans. Ann Hum Genet.
73:34–41. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tateda K, Okazaki S, Nagoya S, et al: The
suppression of TRIM21 and the accumulation of IFN-α play crucial
roles in the pathogenesis of osteonecrosis of the femoral head. Lab
Invest. 92:1318–1329. 2012.PubMed/NCBI
|
|
23
|
Tang TT, Lu B, Yue B, et al: Treatment of
osteonecrosis of the femoral head with hBMP-2-gene-modified
tissue-engineered bone in goats. J Bone Joint Surg Br. 89:127–129.
2007. View Article : Google Scholar : PubMed/NCBI
|