Introduction
Secondary kidney damage is the most common
complication in children with Henoch-Schönlein purpura (HSP)
nephritis. Currently, HSP is often considered to be preceded by an
infection (1,2); however, the cause of immunity
function disorder remains unclear (3). Toll-like receptors (TLRs) are a class
of important pattern recognition receptors, which function as a
bridge, connecting inherent and adaptive immunities (4).
Allergic purpura is one of the most common types of
vasculitis in children and mainly affects the vessels of the skin,
gastrointestinal tract and kidneys. Approximately 20–60% of
children with allergic purpura are complicated with renal damage,
which is an important factor affecting the prognosis. Infection is
the most important risk factor of HSP nephritis, of which the
incidence is >70% (1,2). Group A Streptococcus is the most
common precipitant, demonstrable in up to one-third of cases, but
exposure to Bartonella, Haemophilus parainfluenza and
numerous vaccines and drugs may precede the development of HSP.
Pathogens may activate an abnormal immune response via a number of
methods. The role of microbial antigens in the pathogenesis of HSP
remains elusive.
Human TLRs are a class of transmembrane receptors
that induce signaling pathways. TLRs are the first line of defense
for the host to initiate an immune and inflammatory response.
Through recognition of pathogen-associated molecular patterns
(PAMPs) in pathogenic organisms, TLRs activate intracellular
signaling pathways, resulting in the release of a series of
inflammatory cytokines and the initiation of an adaptive immune
response. In addition, TLRs function as a bridge, connecting
inherent immunity and adaptive immunity (4). TLRs are expressed in immunocytes,
including monocytes, macrophages and dendritic cells, as well as in
renal cells. Abnormal expression of TLRs may cause numerous kidney
diseases, including interstitial nephritis, immune complex
nephritis, renal ischemia-reperfusion injury and rejection of renal
transplantation (5–8). However, whether expression of TLRs is
associated with the development of HSP nephritis remains
unclear.
The aim of the present study was to detect the
expression levels of TLRs in PBMCs from children with HSP nephritis
and analyze urinary protein excretion to explore the effects of
TLRs on the pathogenesis of HSP nephritis.
Subjects and methods
Subjects and groups
Between August 2011 and March 2013, 105 children
aged between 2 and 14 years-old were diagnosed with acute HSP, on
the basis of EULAR/PReS criteria for the classification of
childhood vasculitis in 2005 (9).
The subjects included 50 males and 55 females with an average age
of 6 years. The children initially presented with HSP and had not
been administered glucocorticoids, immunosuppressors or heparin in
the 4 weeks prior to disease occurrence. According to the 24-h
urinary protein measurements and the presence of renal damage, 105
cases were divided into groups A, B and C as follows: Group A, 57
children with HSP but without kidney damage; group B, 25 children
with HSP nephritis but no proteinuria; and group C, 23 children
with HSP nephritis and proteinuria. An additional 30 healthy
children in The Affiliated Hospital of Qingdao University Medical
College (Qingdao, China) were recruited for the normal control
group (group N), which included 16 males and 14 females with an
average age of 6.4 years-old (range, 3–12 years). These individuals
had no anaphylactic disease history prior to the study. The blood
samples were detected simultaneously. This study was conducted in
accordance with the Declaration of Helsinki and with approval from
the Ethics Committee of the Affiliated Hospital of Qingdao
University Medical College (Qingdao, China). Written informed
consent was obtained from patients or their families.
Isolation of PBMCs
Blood samples (2–3 ml) were obtained under sterile
conditions and PBMCs were isolated with lymphocyte separation
medium through density gradient centrifugation. Next, 1 ml RNAiso
Plus (Takara Biotechnology, Co., Ltd., Dalian, China) was added and
the samples were stored at −80°C.
cDNA synthesis
Total RNA was extracted from PBMCs with Takara
reagent, according to the manufacturer’s instructions (Takara
Biotechnology, Co., Ltd.). The concentration of total RNA was
detected using an ultraviolet spectrophotometer. Quantitative
polymerase chain reaction (qPCR) was performed with TLR3 and TLR4
primers [Table I; Sangon Biotech
(Shanghai) Co., Ltd., Shanghai, China]. Optimal reaction conditions
were 35–40 cycles of a two-stage PCR. The amplified PCR products of
TLR3 and TLR4 were resolved by 2% agarose gel electrophoresis, and
recycled. The products were sequenced by Sangon Biotech (Shanghai)
Co., Ltd. and the sequencing results were identical to those from
GenBank.
 | Table IPrimers of TLR3, TLR4 and GAPDH. |
Table I
Primers of TLR3, TLR4 and GAPDH.
| Gene | Sequences | Annealing temperature
(°C) | Fragment size
(bp) |
|---|
| TLR3 | Sense:
5′-GTCCAACGGCTTTGACGAGAT-3′
Antisense: 5′-CTGGCCCGAAAACCTTCTTCT-3′ | 56 | 188 |
| TLR4 | Sense:
5′-TGTCCTCCCACTCCAGGTAAGT-3′
Antisense: 5′-GATTGCTCAGACCTGGCAGTT-3′ | 55 | 144 |
| GAPDH | Sense:
5′-TCATGGGTGTGAACCATGAGAA-3′
Antisense: 5′-GGCATGGACTGTGGTCATGAG-3′ | 57 | 111 |
qPCR
First strand cDNA was amplified using a qPCR thermal
cycler (ABI7000; Applied Biosystems, Inc., Foster City, CA, USA).
For the relative comparison of each gene expression, the qPCR data
were analyzed using the 2−ΔΔCT method (10). The Ct value of the endogenous
control (GAPDH) was subtracted from the Ct value of each target
gene to normalize the quantity of sample cDNA added to each
reaction.
Flow cytometry (FCM)
Peripheral blood (400 μl) was added to four marked
tubes (100 μl/tube) and 5 μl anti-CD14+-fluorescein
isothiocyanate-conjugated antibody was added. The first tube
included 5 μl isotype control of anti-TLR4-phycoerythrin (PE)
antibody (mouse IgG2A marked by PE; K isotype), the second tube
contained 5 μl anti-TLR4-PE antibody, the third tube included 5 μl
isotype control of anti-TLR3 antibody (mouse IgG1; K isotype) and
the fourth tube contained 5 μl anti-TLR3-PE antibody. Samples were
then stored at room temperature in the dark for 15 min. The tubes
were centrifuged for 5 min (626 × g) and washed with
phosphate-buffered saline. Finally, the expression levels of TLR3
and TLR4 from CD14+ monocytes were measured by FCM
(Beckman Coulter, Miami, FL, USA). CellQuest software (BD
Biosciences, San Jose, CA, USA) was used to analyze the results.
All antibodies were purchased from eBioscience (San Diego, CA,
USA).
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA) and the results are
presented as the mean ± SEM. One-way analysis of variance was used
for multiple-group comparisons, after establishing that the data in
the groups were normally and equally distributed. The least
significant difference t-test was used for two-sample comparisons
from multiple groups, after establishing that the data in the
groups were not equally distributed. Pearson analysis was used to
analyze correlations between the groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
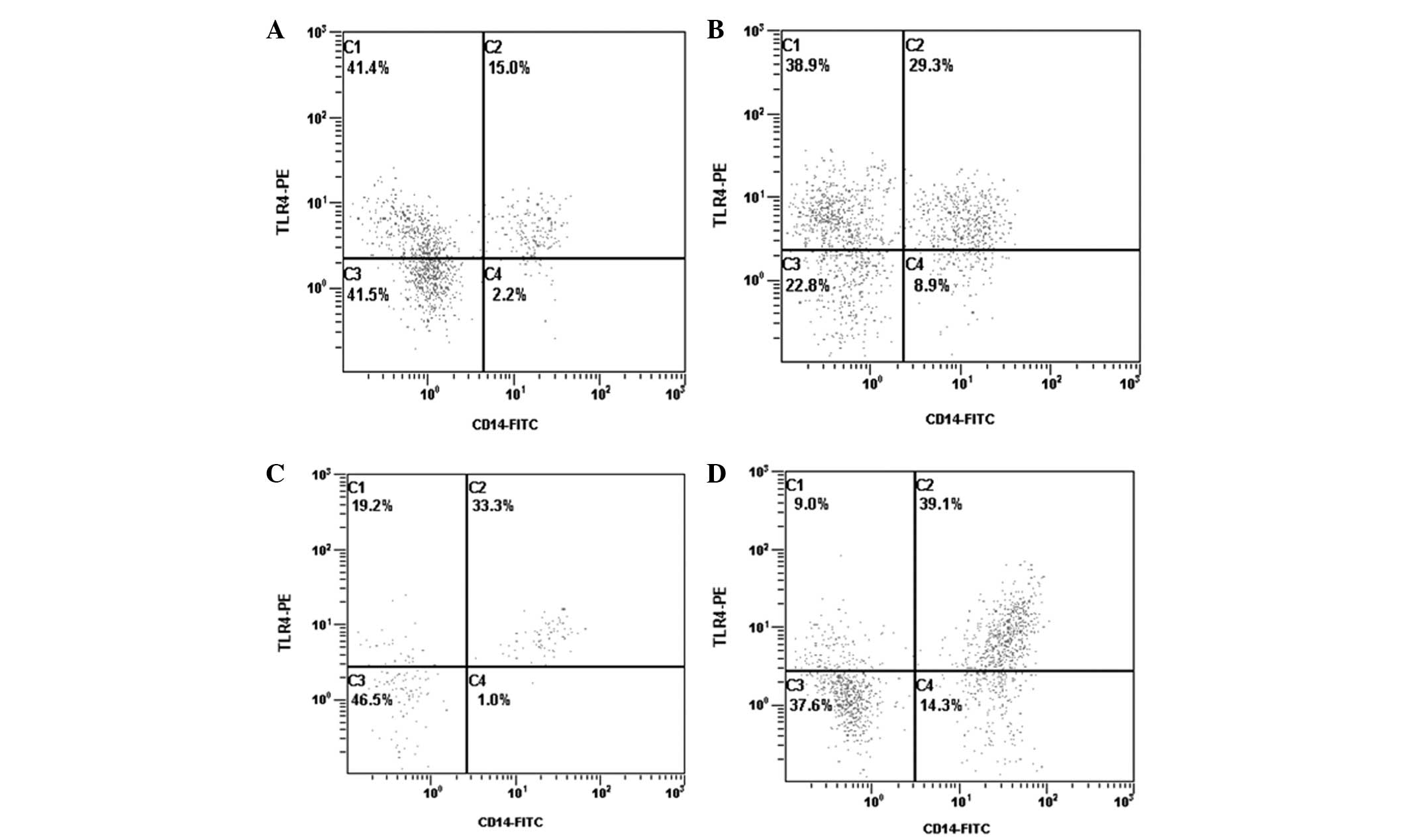
Expression of TLR3 and TLR4
mRNA expression levels of TLR4 were significantly
higher in groups A, B and C when compared with group N (P<0.05,
<0.01 and <0.01, respectively). Protein expression levels of
TLR4 were also significantly higher in groups A, B and C when
compared with group N (P<0.01, <0.01 and <0.01,
respectively). In addition, mRNA and protein expression levels of
TLR4 in group C were markedly higher when compared with group A
(P<0.01 and <0.05, respectively) and group B (P<0.05 and
<0.01, respectively). There were no significant differences in
TLR4 mRNA and protein expression levels between groups A and B
(P>0.05 and >0.05, respectively; Table II; Fig. 1). In addition, the expression
levels of TLR3 were not significantly different between each of the
4 groups (Table II).
 | Table IImRNA and protein expression levels of
TLR3 and TLR4 (mean ± SEM). |
Table II
mRNA and protein expression levels of
TLR3 and TLR4 (mean ± SEM).
| Group | Cases | TLR3 mRNA | TLR3 protein | TLR4 mRNA | TLR4 protein |
|---|
| A | 57 | 0.54±0.24 | 0.10±0.06 | 1.30±0.62ab | 0.23±0.11cd |
| B | 25 | 0.64±0.22 | 0.09±0.03 | 1.78±0.32cd | 0.32±0.12bc |
| C | 23 | 0.59±0.23 | 0.12±0.07 | 2.20±0.50c | 0.45±0.16c |
| N | 30 | 0.62±0.77 | 0.10±0.06 | 1.10±0.50 | 0.14±0.08 |
| F-value | | 1.78 | 0.86 | 37.33 | 24.01 |
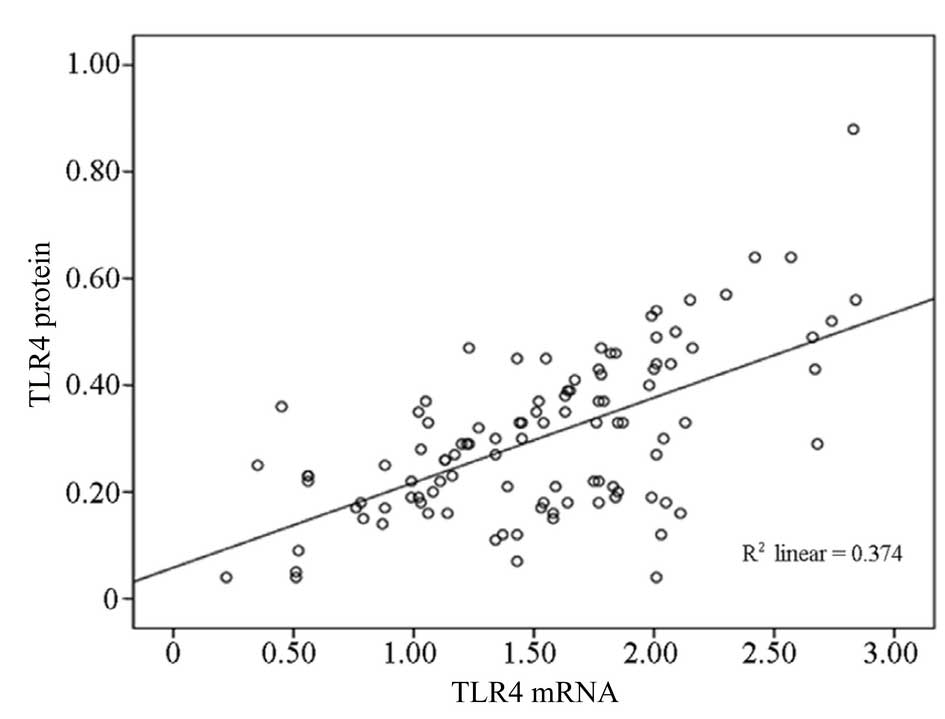
In addition, the mRNA expression levels of TLR4
(1.53±0.58, n=105) in all the patients with HSP (groups A, B, and
C) exhibited a positive correlation with the level of TLR4 protein
expression (0.30±0.17, n=105; r=0.61, P<0.01) (Fig. 2).
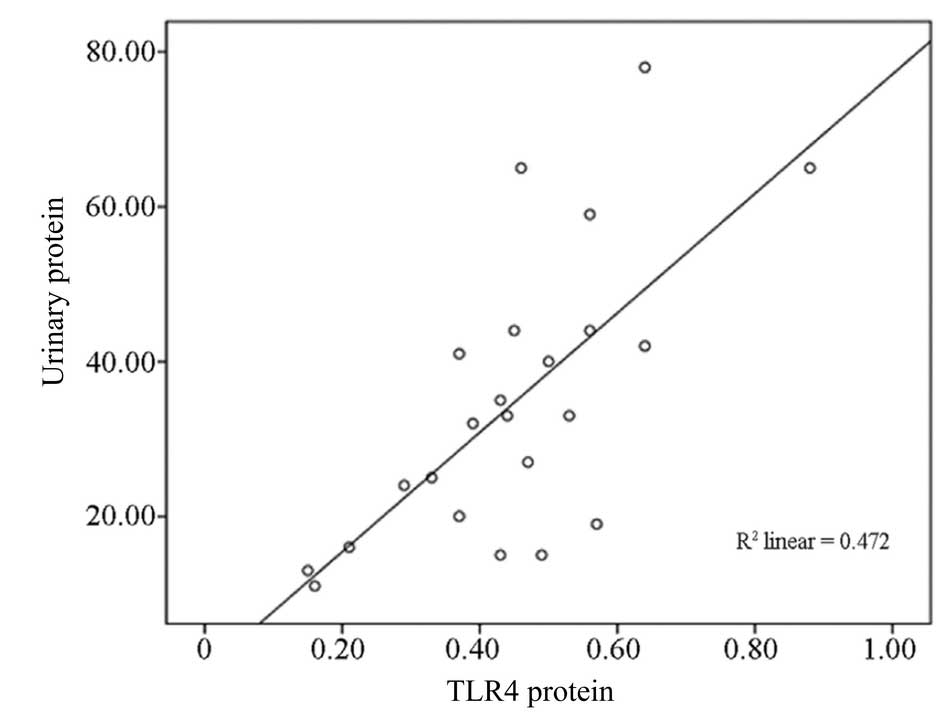
Correlation analysis between TLR4 protein
expression and 24-h proteinuria
TLR4 protein expression in group C (HSP with
proteinuria) and 24-h urinary protein (35±18 mg/kg) exhibited a
positive correlation (r=0.69, P<0.01; Fig. 3) in PBMCs from children with
HSP.
Discussion
Anaphylactoid purpura (also known as HSP) is
small-vessel vasculitis that involves the skin, connective tissues,
scrotum, joints, gastrointestinal tract and kidneys. Secondary
kidney damage is the most common complication in children with HSP
nephritis, which directly affects the course and prognosis of HSP
(1,2). However, the mechanism of action
remains unclear, although it may include the disorder of humoral
and cell immunities and cytokines or inflammatory mediators
(3). A number of studies have
indicated that HSP is often preceded by an infection. There is a
close correlation between the incidence of HSP and infection. In
particular, intractable HSP has a higher incidence of infection.
However, the cause of immunity function disorder currently remains
unclear.
TLRs are a class of important pattern recognition
receptors involved in the innate immune system that can recognize
PAMPs to activate cell signaling systems, generating a series of
proinflammatory cytokines to activate the adaptive immune response
(11–14). Activation of innate immunity is a
critical step in the development of antigen-specific acquired
immunity and individual receptors may be upregulated during
infection and inflammation. Thus, we hypothesized that the
incidence and development of HSP and HSP nephritis may be mediated
by TLRs when patients are infected by bacteria or viruses.
TLRs are single membrane-spanning, non-catalytic
receptors that are usually expressed in sentinel cells, including
macrophages and dendritic cells. Exogenous ligands of TLRs are from
PAMPs, including bacterial cell-surface lipopolysaccharides (LPSs),
teichoic acid and nucleic acids of bacteria and viruses. Endogenous
ligands include heat shock protein 60, fibrinogen and extracellular
matrix components. TLR3 is able to recognize viral RNA and
synthetic RNA, while TLR4 is able to recognize numerous ligands,
including LPS, HSPs, heparin, fibrinogen and taxol. At present, 13
types of TLRs have been identified (TLR1-TLR13).
Although TLR signals are the first defense against
infection from microbes, over-activated TLR signaling causes
inflammatory cell infiltration to generate cytokines and
autoantibodies, thus, inducing autoimmune diseases (15–18).
TLR upregulation may be involved in renal disease and there is
increasing data supporting a role for TLRs in infectious autoimmune
and inflammatory disorders of the kidney (3). In a crescentic glomerulonephritis
experiment, wild-type C57BL/6 mice and TLR-deficient mice were
treated with the same dose of LPS. The wild-type mice developed
severe glomerular injury, characterized by glomerular thrombosis,
crescent formation and glomerular macrophage infiltration. This
indicated that the TLR2 signaling pathway was involved in
crescentic glomerulonephritis (19). Additional studies have also
demonstrated that the expression of B7-1 was markedly increased
following LPS treatment via the TLR4 signaling pathway, which
causes proteinuria (20). Pawar
et al reported that LPS may activate the TLR2 and TLR4
signaling pathway in a mouse model of lupus nephrosis, and promote
the inflammatory factor secretion (21). This process leads to continuous
kidney damage. Coppo et al (20) found that protein and gene
expression levels of TLR4 increased in circulating mononuclear
cells of patients with IgA nephropathy. This indicated that TLR4
signaling mediated the abnormal immunity of IgA nephropathy.
Therefore, it was hypothesized that TLRs participate in the
pathogenesis of HSP nephritis, however, the correlation between
TLRs and HSP nephritis is yet to be studied.
The results of the present study demonstrated that
mRNA and protein expression levels of TLR3 did not significantly
differ between the normal control group and the other groups.
However, the mRNA and protein expression levels of TLR4 in PBMCs
were shown to be higher in groups A, B and C when compared with the
control group. Furthermore, protein and mRNA expression levels of
TLR4 were higher in group C than in groups A and B, and protein
expression levels of TLR4 in group C exhibited a positive
correlation with 24-h urinary protein excretions.
In conclusion, the mRNA and protein expression
levels of TLR4 in PBMCs from HSP nephritis children were
significantly increased, which positively correlated with the level
of 24-h urinary protein. This was consistent with our previous
hypothesis. The results indicated that activation of TLR4 signaling
was associated with HSP and aberrant activation of TLR4 may cause
kidney damage. Thus, downregulating TLR4 expression levels or
blockading the TLR4 signaling pathway are candidate targets for
future HSP nephritis treatments (22), although the exact mechanism of
action requires further study. In the present study, the mRNA and
protein expression levels of TLR3 and TLR4 in children with HSP
nephritis were investigated only in PBMCs. Other TLRs and
associated signaling factors have not yet been studied or TLR
expression in renal tissue. Therefore, further study to elucidate
the role of the innate immune system in renal injury is
required.
References
|
1
|
Jauhola O, Ronkainen J, Koskimies O, et
al: Clinical course of extrarenal symptoms in Henoch-Schonlein
purpura: a 6-month prospective study. Arch Dis Child. 95:871–876.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Punnoose AR, Lynm C and Golub RM: JAMA
patient page. Henoch-Schönlein purpura. JAMA. 307:7422012.
|
|
3
|
Lau KK, Suzuki H, Novak J and Wyatt RJ:
Pathogenesis of Henoch-Schönlein purpura nephritis. Pediatr
Nephrol. 25:19–26. 2010.
|
|
4
|
Brown J, Wang H, Hajishengallis GN and
Martin M: TLR-signaling networks: an integration of adaptor
molecules, kinases, and cross-talk. J Dent Res. 90:417–427. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gluba A, Banach M, Hannam S, Mikhailidis
DP, Sakowicz A and Rysz J: The role of Toll-like receptors in renal
diseases. Nat Rev Nephrol. 6:224–235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Akil I, Ozkinay F, Onay H, Canda E,
Gumuser G and Kavukcu S: Assessment of Toll-like receptor-4 gene
polymorphism on pyelonephritis and renal scar. Int J Immunogenet.
39:303–307. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eleftheriadis T, Pissas G, Liakopoulos V,
Stefanidis I and Lawson BR: Toll-like receptors and their role in
renal pathologies. Inflamm Allergy Drug Targets. 11:464–477. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Amura CR, Renner B, Lyubchenko T, Faubel
S, Simonian PL and Thurman JM: Complement activation and toll-like
receptor-2 signaling contribute to cytokine production after renal
ischemia/reperfusion. Mol Immuno. 52:249–257. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ozen S, Ruperto N, Dillon MJ, et al:
EULAR/PReS endorsed consensus criteria for the classification of
childhood vasculitides. Ann Rheum Dis. 65:936–941. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Method. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brown J, Wang H, Hajishengallis GN and
Martin M: TLR-signaling networks: an integration of adaptor
molecules, kinases, and cross-talk. J Dent Res. 90:417–427. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin B, Sun T, Yu XH and Yeo AE: The
effects of TLR activation on T-cell development and
differentiation. Clin Dev Immunol. 2012:8364852012.PubMed/NCBI
|
|
13
|
Qian C and Cao X: Regulation of Toll-like
receptor signaling pathways in innate immune responses. Ann NY Acad
Sci. 1283:67–74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Negishi H, Yanai H, Nakajima A, et al:
Cross-interference of RLR and TLR signaling pathways modulates
antibacterial T cell responses. Nat Immunol. 13:659–666. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mills KH: TLR-dependent T cell activation
in autoimmunity. Nat Rev Immunol. 11:807–822. 2011.PubMed/NCBI
|
|
16
|
Urbonaviciute V, Starke C, Pirschel W, et
al: Toll-like receptor 2 is required for autoantibody production
and development of renal disease in pristane-induced lupus.
Arthritis Rheum. 65:1612–1623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miranda-Hernandez S and Baxter AG: Role of
toll-like receptors in multiple sclerosis. Am J Clin Exp Immunol.
2:75–93. 2013.PubMed/NCBI
|
|
18
|
Kirchner M, Sonnenschein A, Schoofs S,
Schmidtke P, Umlauf VN and Mannhardt-Laakmann W: Surface expression
and genotypes of Toll-like receptors 2 and 4 in patients with
juvenile idiopathic arthritis and systemic lupus erythematosus.
Pediatr Rheumatol Online J. 11:92013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brown HJ, Sacks SH and Robson MG:
Toll-like receptor 2 agonists exacerbate accelerated nephrotoxic
nephritis. J Am Soc Nephrol. 17:1931–1939. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Coppo R, Camilla R, Amore A, et al:
Toll-like receptor 4 expression is increased in circulating
mononuclear cells of patients with immunoglobulin A nephropathy.
Clin Exp Immunol. 159:73–81. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pawar RD, Castrezana Lopez L, Allam R, et
al: Bacterial lipopeptide triggers massive albuminuria in murine
lupus nephritis by activating Toll-like receptor 2 at the
glomerular filtration barrier. Immunology. 128(1 Suppl): e206–e221.
2009. View Article : Google Scholar
|
|
22
|
Lucas K and Maes M: Role of the Toll like
receptor (TLR) radical cycle in chronic inflammation: possible
treatments targeting the TLR4 pathway. Mol Neurobiol. 48:190–204.
2013. View Article : Google Scholar : PubMed/NCBI
|