Introduction
The proliferation and migration of vascular smooth
muscle cells (VSMCs) may play a key role in the development of
intimal thickening following arterial-wall injury or in
atherosclerosis (1). Matrix
metalloproteinases (MMPs) are a broad family of zinc-dependent
proteinases, which are implicated in extracellular matrix turnover,
vascular wall remodeling, angiogenesis and atherosclerosis
(2). Among the MMP family, several
lines of evidence have hypothesized that MMP-2 plays a key role in
promoting VSMC proliferation, migration and the weakening of
atherosclerotic plaque stability (3). In addition, MMP-2 expression in VSMCs
has been associated with a variety of pathological situations,
particularly in atherosclerotic plaques, which show significantly
increased MMP-2 expression and activation levels, most prominently
in vulnerable regions, indicating a pathogenic role for MMP-2 in
the progression of atherosclerosis (4,5).
Curcumin (diferuloylmethane), a bioactive constituent from
Curcuma longa, possesses marked anti-inflammatory,
antioxidant and anticarcinogenic properties (6,7). A
previous study has shown that curcumin possesses anti-inflammatory,
antioxidant, anticancer, antibacterial and antiviral activities,
and exhibits a strong potency in inhibiting transcription factors,
protein kinases, cytokines, adhesion molecules and oxidative stress
(8). In the present study, the
inhibitory effect of curcumin on tumor necrosis factor
(TNF)-α-induced VSMC migration and MMP-2 expression and activity
was investigated, with the aim of identifying the pathways
involved.
Materials and methods
Cell culture and treatment
Rat aortic smooth muscle cells were isolated from
male Sprague-Dawley rats (purchased from the laboratory animal
center of Southern Medical University, Guangzhou, China), as
described previously (9). The
cells were cultured in Dulbecco’s modified Eagle’s medium
(Gibco-BRL, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum (Gibco-BRL), 25 mM HEPES, 100 U/ml penicillin and 100 μg/ml
streptomycin at 37°C. Cells that had grown to 80–90% confluence
were used for all the experiments. Cells were placed in serum-free
medium for 24 h prior to treatment with TNF-α (Sigma, St. Louis,
MO, USA). The study was conducted in strict accordance with the
recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health. Prior to the addition
of TNF-α to the medium, the cells were pretreated with curcumin
(Solon, OH, USA). The study was approved by the Ethics Committee of
Southern Medical University.
Cell viability assay
Cells were plated with a variety of concentrations
of curcumin (0–40 μM) in 96-well microtiter plates, and were then
cultured for 24 h at 37°C in a 5% CO2 incubator. Cell
viability was determined using the conventional methylthiazolyl
tetrazolium (MTT) reduction assay. Following the treatment of the
cells with curcumin, MTT solution was added (final concentration, 5
mg/ml) and incubation was continued for 4 h at 37°C. The dark blue
formazan crystals formed in the intact cells were solubilized with
dimethyl sulfoxide and the absorbance of the blue color was
measured at 490 nm using a microplate reader.
Preparations of nuclear proteins
Cells (1×107 cells/ml) were harvested,
washed with ice-cold phosphate-buffered saline (PBS), centrifuged
and resuspended in ice-cold isotonic buffer A [10 mM HEPES (pH
7.9), 10 mM KCl, 1.5 mM MgCl2, 0.5 mM dithiothreitol
(DTT) and 0.5 mM phenylmethanesulfonyl fluoride (PMSF)]. Following
incubation in an ice bath for 15 min, the cells were centrifuged at
16,000 × g for 5 min at 4°C. The cells were then resuspended in
ice-cold buffer C [20 mM HEPES (pH 7.9), 20% glycerol, 0.4 M NaCl,
1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT and 0.5 mM PMSF],
which was followed by incubation at 4°C for 40 min. Following
vortex-mixing, the resulting suspension was centrifuged at 16,000 ×
g for 10 min at 4°C, and the supernatant was stored at −80°C. The
protein content was determined by bicinchoninic protein assay
reagent.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was isolated from cells using
TRIzol-reagent (Invitrogen Life Technologies, Carlsbad, CA, USA)
and quantified by ultraviolet absorption at 260 and 280 nm. RT-PCR
was performed according to the manufacturer’s instructions.
According to GenBank, the RT-PCR primers were designed as follows:
MMP-2 sense, 5′-ACCTGTCACTCCGGAGATCTGCAA-3′ and antisense,
5′-TCACGCTCTTGAGACTTTGGTTCT-3′. The PCR conditions were as follows:
30 cycles of 94°C for 30 sec; 55°C for 30 sec; and 72°C for 45 sec.
The amplified products were visualized by 1.5% agarose gel
electrophoresis, stained with ethidium bromide and images were then
captured under ultraviolet light. Densitometric analysis of the
different observations was performed using Quantity One Software
(Bio-Rad, Hercules, CA, USA). The quantity of each transcript was
normalized against GAPDH.
Western blot analysis
VSMCs were harvested at the indicated time points
and lysed into lysis buffer. Total proteins (50 μg per well) were
separated by 10% SDS-PAGE and electrophoretically transferred to
nitrocellulose membranes. Following blocking for 1 h with 5%
skimmed milk in Tris-buffered saline (TBS; 10 mM Tris and 150 mM
NaCl), the membrane was washed three times for 15 min each with TBS
Tween-20 buffer (10 mM Tris, 150 mM NaCl and 0.1% Tween-20).
Immunoreactive bands were visualized using horseradish
peroxidase-conjugated secondary antibodies and an enhanced
chemiluminescence (ECL) western blotting detection kit (GE
Healthcare, Little Chalfont, UK). The bands were visualized using
the ECL system, and the band density was determined using Image J
software. All the antibodies were purchased from the Beyotime
Institute of Biotechnology (Shanghai, China).
Gelatin zymography
Enzymatic activity levels of MMP-2 were assessed by
gel zymographic analysis (10).
The protein content of the samples was measured by the colorimetric
method using serum albumin as the standard. In total, 50-μg samples
of proteins from the VSMC lysate were loaded on an 11% SDS-PAGE gel
containing 0.1% gelatin for electrophoresis in a 4°C cold room.
Subsequently, the gels were incubated with collagenase buffer for
16 h at 37°C, stained with 0.25% Coomassie Brilliant Blue,
destained with 30% isopropanol in 10% acetic acid and
visualized.
Cell migration assay
The invasion of VSMCs through the extracellular
matrix was determined using a commercial cell invasion assay kit
(Chemicon International, Temecula, CA, USA), as described in a
previous study (11). VSMCs were
resuspended in conditioned medium that had been collected following
pretreatment with curcumin and TNF-α-treated cells for 23 h, and
were added to the upper components of the migration chamber. Next,
a 500-μl sample of the same conditioned medium was added to the
lower compartment of the migration chamber. Cells without
TNF-α-treated conditioned medium served as the control. The
migration chambers were incubated at 37°C for 24 h in an atmosphere
of 5% CO2. Following incubation, the inserts were
removed from the wells and the cells on the upper side of the
filter were removed using cotton swabs. The filters were fixed and
stained according to the manufacturer’s instructions. Next, 100 μl
dye mixture was transferred to a 96-well plate and the optical
density was measured at 560 nm.
Measurement of reactive oxygen species
(ROS)
Prior to chemical treatment, the cells were
incubated in culture medium containing 30 μM
2′,7-dichlorofluorescein (DCF; Beyotime Institute of Biotechnology,
Shanghai, China), a fluorescent dye, for 30 min to establish a
stable intracellular level of the probe. Subsequently, the cells
were washed with PBS, removed from the Petri dishes by scraping and
evaluated for DCF fluorescence intensity. This was used as an index
of the intracellular levels of ROS. The fluorescent DCF was
detected using a laser scanning confocal microscope (TCS-NT; Leica
Microsystems, Heidelberg, Germany) with excitation and emission
wavelengths of 488 and 520 nm, respectively. The cell number in
each sample was counted and utilized to normalize the fluorescence
intensity of DCF.
Statistical analysis
Data are expressed as the mean ± standard deviation
of three assays. Statistical analysis was conducted using one-way
analysis of variance and P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using SPSS 13.0 software (SPSS, Inc., Chicago, IL,
USA).
Results
Assessment of cell toxicity of
curcumin
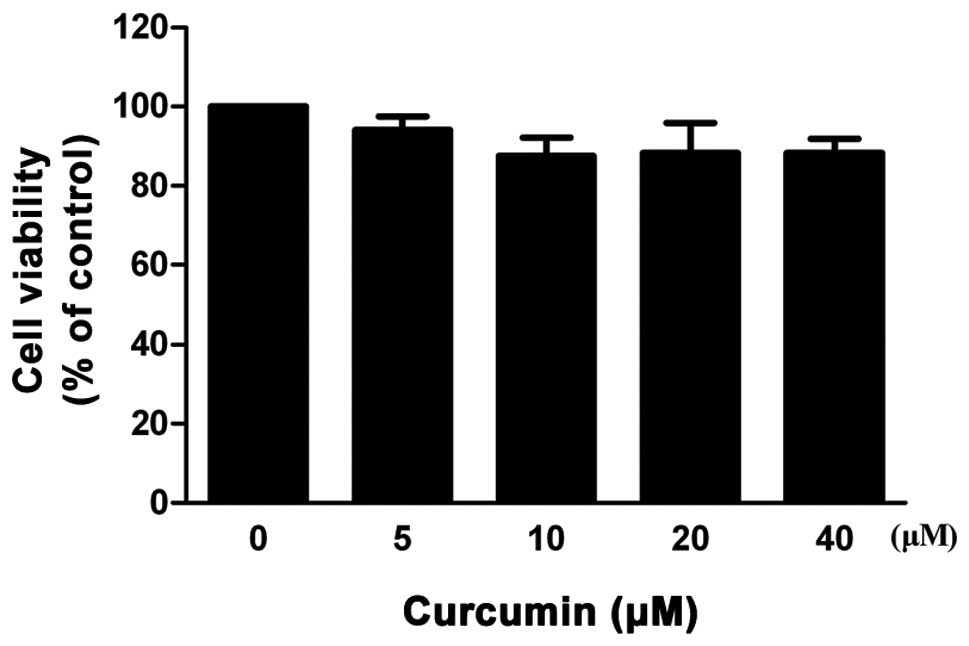
The MTT assay was performed to evaluate the
cytotoxicity of curcumin on VSMCs. As shown in Fig. 1, curcumin did not exhibit a
dose-dependent (0–40 μM) cytotoxic effect in VSMCs. According to
the results of the MTT assay, curcumin concentrations of 20 and 40
μM were selected for all the following experiments.
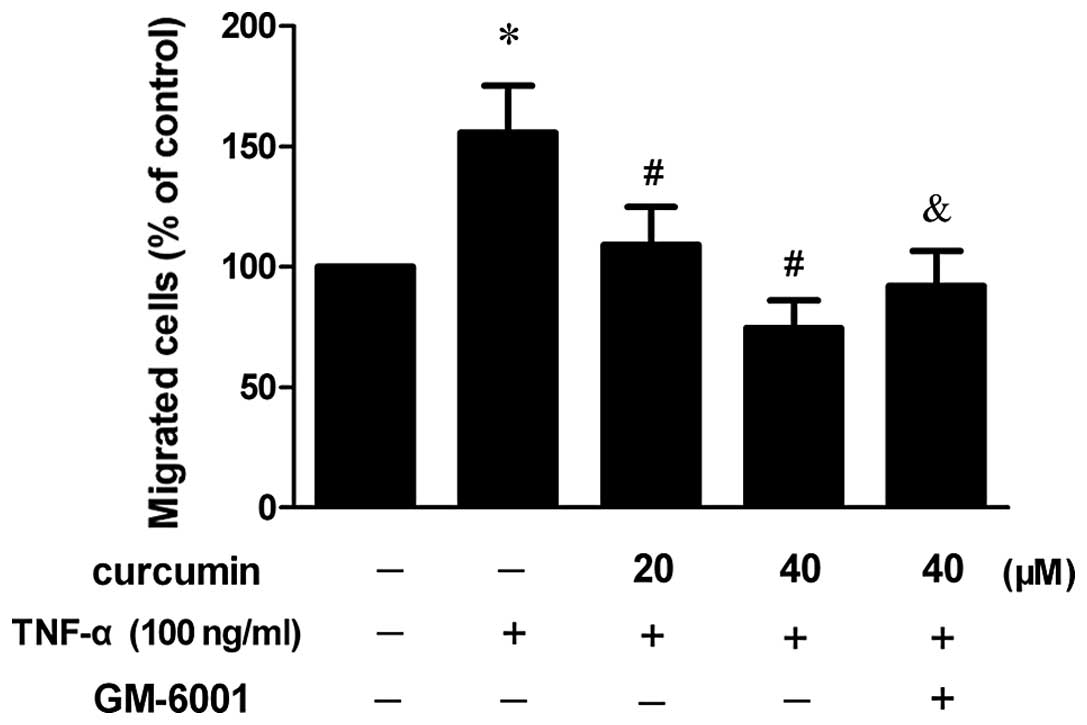
Curcumin inhibits TNF-α-stimulated
migration in VSMCs
To investigate the effect of curcumin on the
migration ability of VSMCs, a cell migration assay was performed.
As shown in Fig. 2, the migration
of VSMCs increased following treatment with 100 ng/ml TNF-α,
whereas 20 and 40 μM curcumin reduced cell migration in
TNF-α-induced VSMCs and a statistically significant difference was
observed (P<0.05). When VSMCs were pretreated with an MMP
inhibitor (GM-6001), the inhibitory effect was partly
diminished.
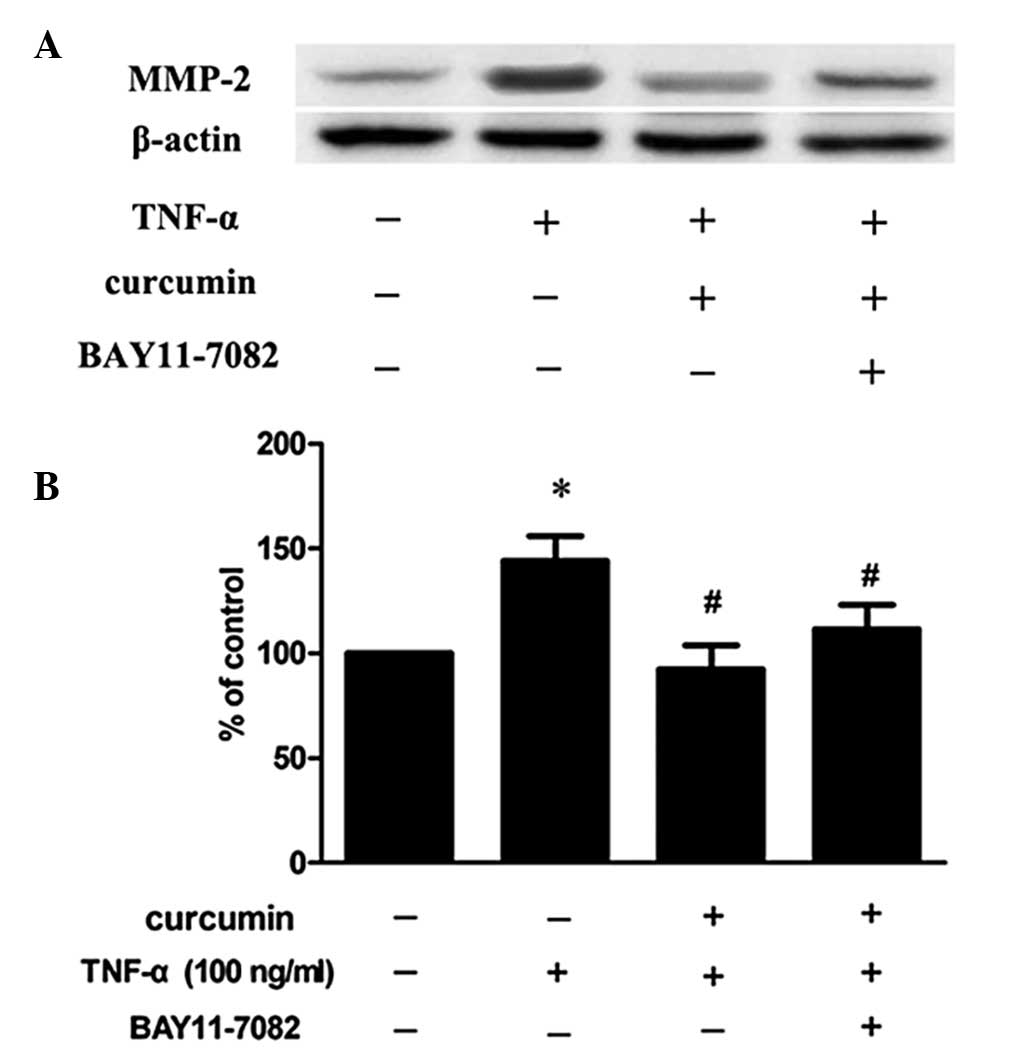
Curcumin inhibits TNF-α-induced MMP-2
expression and activity in VSMCs
VSMCs were treated with 100 ng/ml TNF-α in the
presence or absence of various concentrations of curcumin. Compared
with TNF-α alone, 20 and 40 μM curcumin significantly reduced MMP-2
expression and activity levels (P<0.05; Fig. 3). Treatment with 40 μM curcumin was
more effective at decreasing the levels of protein expression and
activity of MMP-2 when compared with 20 μM curcumin treatment.
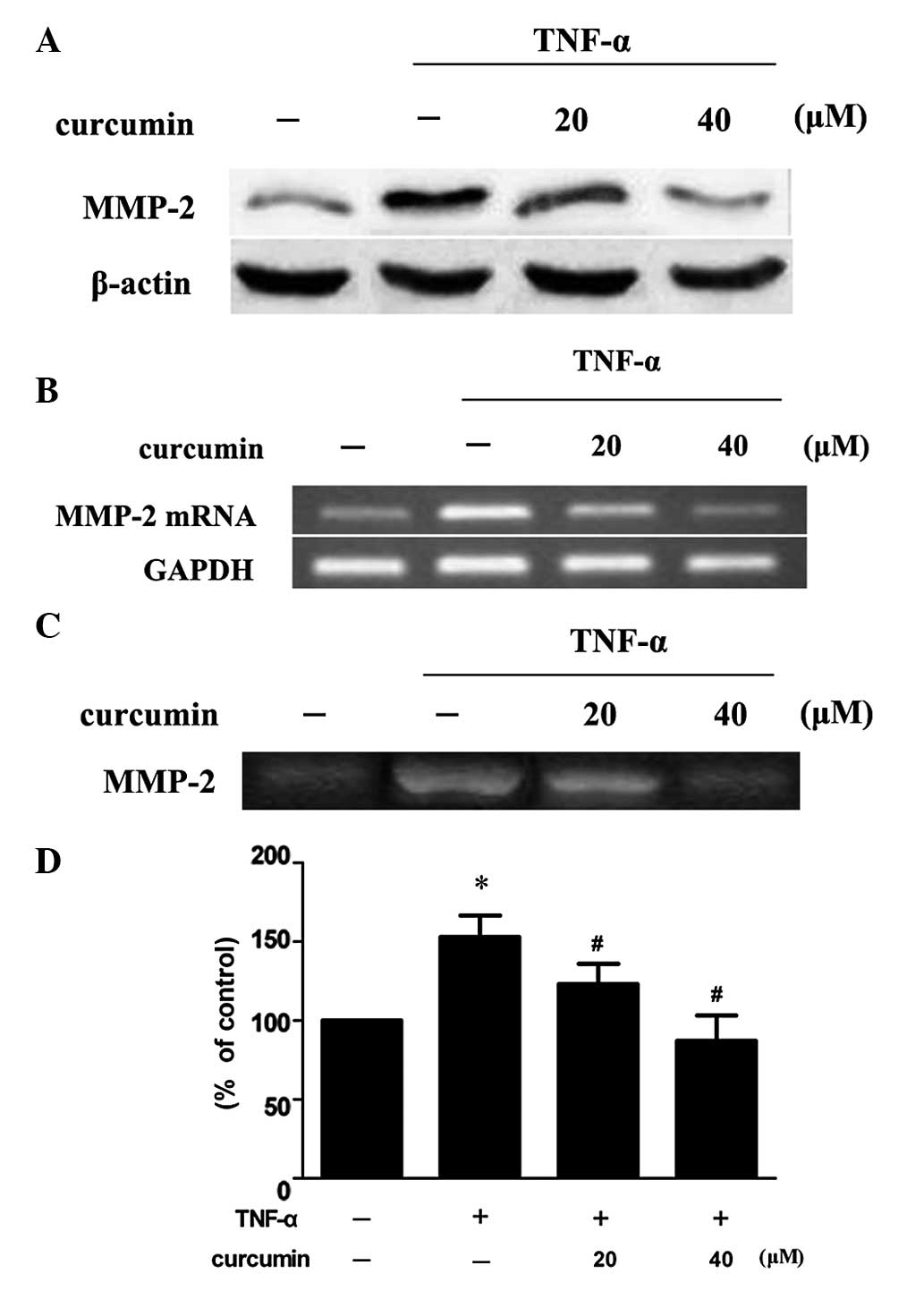 | Figure 3Curcumin inhibits TNF-α-induced MMP-2
expression and activity in VSMCs. VSMCs were pretreated with
curcumin (20 or 40 μM) for 1 h and exposed to 100 ng/ml TNF-α for
an additional 23 h. Following treatment, the (A) protein
expression, (B) mRNA expression and (C) activity levels of MMP-2
were assessed by western blot analysis, RT-PCR and gelatin
zymography, respectively. (D) Densitometric analysis was conducted
with Image J software to quantify the gelatin zymography data.
*P<0.05, vs. control group; #P<0.05,
vs. TNF-α + curcumin group. Data are expressed as the mean ±
standard deviation of three independent experiments. VSMCs,
vascular smooth muscle cells; TNF, tumor necrosis factor; MMP,
matrix metalloproteinase; RT-PCR, reverse transcription polymerase
chain reaction. |
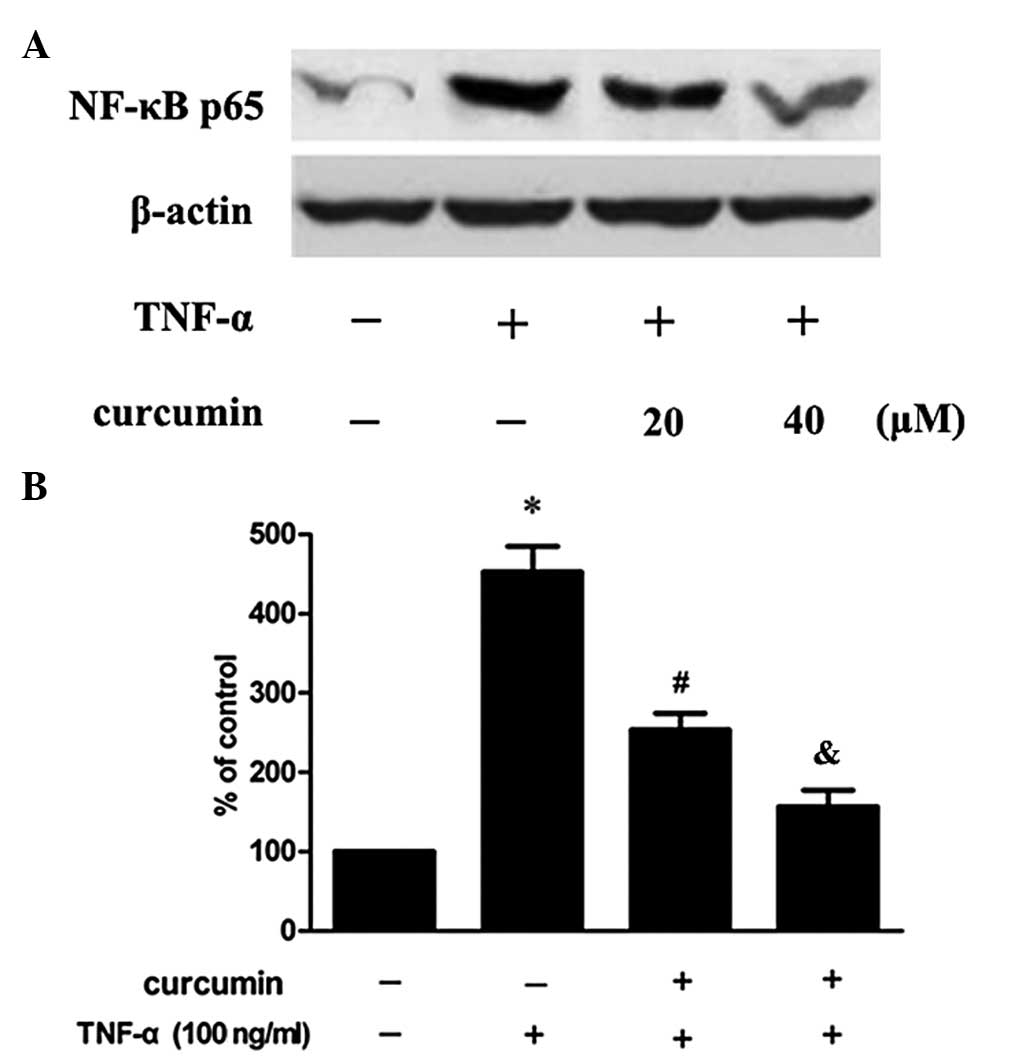
Curcumin suppresses TNF-α-induced MMP-2
expression in VSMCs via the nuclear factor (NF)-κB pathway
In order to further investigate whether the NF-κB
signaling pathway is involved in the inhibitory effect of curcumin
on the TNF-α-induced expression of MMP-2, cells were pretreated
with an inhibitor of NF-κB (BAY11–7082). The results revealed that
the inhibitory effect was partly eliminated (Fig. 4). In addition, the effect of
curcumin on the p65 subunit, induced by TNF-α, was also determined.
As a result, TNF-α was found to increase the expression of the p65
subunit in the nucleus, but this increase was inhibited when the
cells had been preincubated with curcumin (Fig. 5).
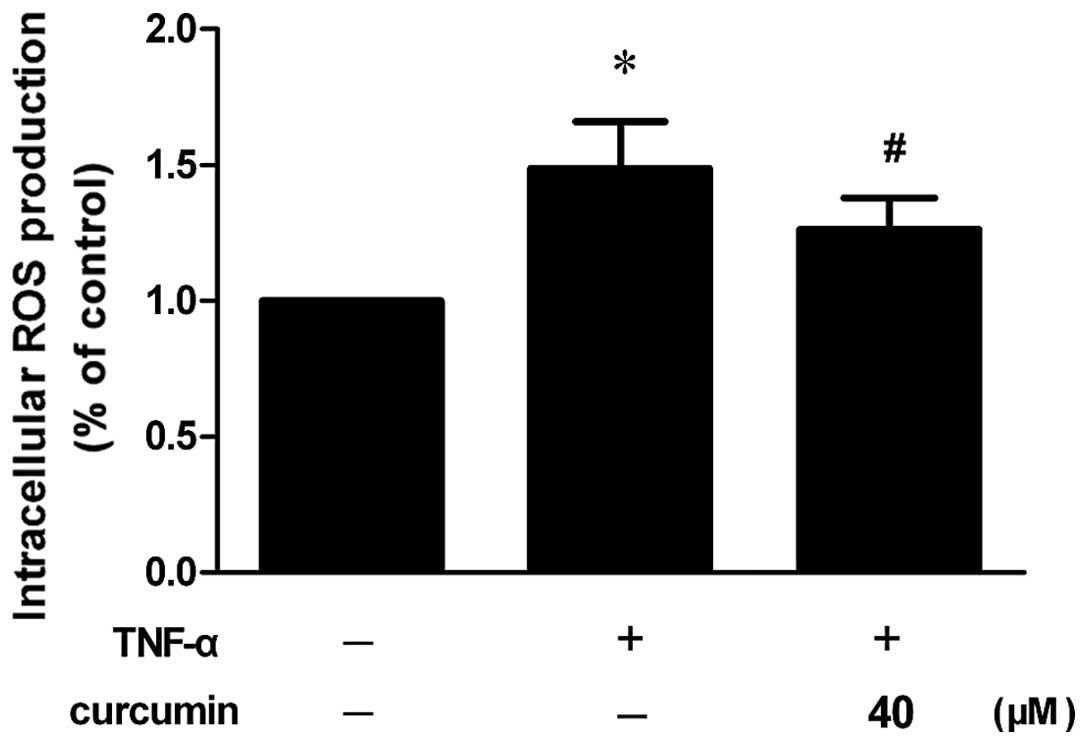
Curcumin prevents TNF-α-induced ROS
generation
Increased ROS generation was observed in VSMCs that
had been stimulated with TNF-α, whereas the inhibitory effect was
significantly blocked by pretreatment with curcumin (Fig. 6; P<0.05).
Discussion
VSMC migration evidently plays a critical role in
the pathophysiology of several prominent cardiovascular disease
states, including atherosclerosis and restenosis (12,13).
Previous studies have shown that the MMP system may be a potential
therapeutic target for the treatment of restenosis or
atherosclerosis since the MMP system plays a role in VSMC migration
and neointima formation following vascular injury (14). In the present study, the inhibitory
effect of curcumin on TNF-α-induced VSMC migration was
investigated, as well as the possible mechanisms involved. Curcumin
was found to inhibit the TNF-α-stimulated migration of VSMCs, which
is consistent with a previous study (15). In addition, administration of an
MMP inhibitor (GM-6001) was shown to partly diminish the inhibitory
effect, indicating that MMP-2 may play an important role in this
process.
Accumulating evidence has indicated that gelatinase
MMP-2 plays a pivotal role in the initiation and progression of
atherosclerotic lesions. MMP-2 is constitutively expressed in VSMCs
in normal arteries (9), and MMP-2
expression and activity levels may contribute to the pathogenesis
of atherosclerosis by facilitating the migration of VSMCs (16). Therefore, the inhibitory effect of
curcumin on the TNF-α-induced expression and activity of MMP-2 was
further investigated. Subsequently, curcumin was demonstrated to
significantly inhibit TNF-α-induced MMP-2 expression and activity,
which indicated that MMP-2 is possibly involved in the inhibitory
effect of curcumin on TNF-α-induced cell migration.
TNF-α is one of the major inflammatory cytokines
that mediates a wide range of biological responses, including
inflammation, infection, injury and apoptosis (17). The effects of TNF-α are initiated
by binding to its receptors, which causes the activation of two
major transcription factors, AP-1 and NF-κB. This in turn induces
the expression of genes involved in inflammatory responses and
apoptosis (18). A previous study
also demonstrated that inflammatory cytokines, including TNF-α, may
induce the expression of the genes that encode MMPs (19). Thus, the present study provides new
evidence that TNF-α enhances MMP-2 expression and activity in
cultured VSMCs, and to the best of our knowledge, the present study
shows for the first time that curcumin significantly inhibits
TNF-α-induced MMP-2 expression and activity. Previous studies have
indicated that transcriptional regulation involving NF-κB
activation has been implicated in the TNF-α-induced activation of
VSMCs (20). A key component of
MMP expression is the redox-sensitive transcription factor NF-κB
(21).
Therefore, to further investigate whether NF-κB
contributes to the regulatory effect of curcumin on TNF-α-induced
MMP-2 expression and activity, the cells were pretreated with
BAY11-7082 (an NF-κB inhibitor) and curcumin prior to the addition
of TNF-α. The inhibitory effect was shown to be blocked by
BAY11-7082, which indicated that NF-κB is involved in this
process.
In unstimulated cells, inactive NF-κB exists as a
heterodimeric complex of the subunits, p50 and p65, that are
complexed with the inhibitory protein, IκB. Upon activation,
phosphorylation of IκB results in its degradation, which is
followed by the translocation of the liberated NF-κB to the nucleus
where the dimer interacts with regulatory κB elements in promoters
and enhancers, thereby controlling gene transcription (22). Consistent with previous
observations (19,23,24),
the present study also demonstrated that TNF-α activates NF-κB in
VSMCs. In addition, a key component of MMP expression is the
redox-sensitive transcription factor NF-κB (21). These results indicate that MMP
expression, in response to TNF-α, may be mediated by this
transcription factor.
In the present study, curcumin was found to reduce
TNF-α-induced nuclear translocation of NF-κB p65 in VSMCs. In
addition, it was demonstrated that ROS play an essential role in
NF-κB activation via proinflammatory cytokines (TNF-α and
interleukin-1β) and lipopolysaccharide, two major components of
innate immunity (25). The vast
majority of studies concerning oxidant-induced NF-κB activation
have used H2O2 as a direct source of ROS.
Schreck et al (26) were
the first to demonstrate that the direct addition of
H2O2 to a culture medium containing a
subclone of Jurkat cells (Jurkat JR) resulted in the activation of
NF-κB. Excess ROS activate the redox-sensitive transcription
factor, NF-κB, resulting in an increase in its activity and
expression (27). The activation
of NF-κB can be inhibited by antioxidants (28). Thus, the effect of curcumin on
TNF-α-induced ROS generation was investigated. Subsequently,
TNF-α-induced ROS generation and increased ROS generation was shown
to be significantly blocked by pretreatment with curcumin.
Therefore, the present study demonstrated that curcumin suppresses
TNF-α-stimulated MMP-2 expression and activity in VSMCs via the
NF-κB pathway.
In conclusion, curcumin effectively inhibited the
TNF-α-induced migration of VSMCs. Levels of ROS production, MMP-2
activation and expression and nuclear translocation of NF-κB p65
were also all reduced by curcumin pretreatment. These results
demonstrate that curcumin suppresses TNF-α-induced MMP-2 expression
and activity in rat VSMCs via the NF-κB signaling pathway, thereby
suppressing cell migration. Therefore, these observations support
an emerging role of curcumin as a candidate for the treatment of
atherosclerosis. In addition, the ROS/NF-κB pathway may be an
additional potential therapeutic target for
atherosclerosis-associated diseases.
Acknowledgements
The study was supported by a grant from the
Affiliated Hospital of Luzhou Medical College (no. 12298).
References
|
1
|
Ross R: The pathogenesis of
atherosclerosis: a perspective for the 1990s. Nature. 362:801–809.
1993. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Augé N, Maupas-Schwalm F, Elbaz M, Thiers
JC, Waysbort A, Itohara S, Krell HW, Salvayre R and Nègre-Salvayre
A: Role for matrix metalloproteinase-2 in oxidized low-density
lipoprotein-induced activation of the sphingomyelin/ceramide
pathway and smooth muscle cell proliferation. Circulation.
110:571–578. 2004.
|
|
3
|
Aoyagi M, Yamamoto M, Azuma H, Nagashima
G, Niimi Y, Tamaki M, Hirakawa K and Yamamoto K: Immunolocalization
of matrix metalloproteinases in rabbit carotid arteries after
balloon denudation. Histochem Cell Biol. 109:97–102. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Newby AC and Zaltsman AB: Fibrous cap
formation or destruction - the critical importance of vascular
smooth muscle cell proliferation, migration and matrix formation.
Cardiovasc Res. 41:345–360. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Caird J, Napoli C, Taggart C, Farrell M
and Bouchier-Hayes D: Matrix metalloproteinases 2 and 9 in human
atherosclerotic and non-atherosclerotic cerebral aneurysms. Eur J
Neurol. 13:1098–1105. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruby AJ, Kuttan G, Babu KD, Rajasekharan
KN and Kuttan R: Anti-tumour and antioxidant activity of natural
curcuminoids. Cancer Lett. 94:79–83. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Surh YJ: Anti-tumor promoting potential of
selected spice ingredients with antioxidative and anti-inflammatory
activities: a short review. Food Chem Toxicol. 40:1091–1097. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goel A, Kunnumakkara AB and Aggarwal BB:
Curcumin as ‘Curecumin’: from kitchen to clinic. Biochem Pharmacol.
75:787–809. 2008.
|
|
9
|
Kamimura M, Bea F, Akizawa T, Katus HA,
Kreuzer J and Viedt C: Platelet-derived growth factor induces
tissue factor expression in vascular smooth muscle cells via
activation of Egr-1. Hypertension. 44:944–951. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang W, Lim S, Song H, Song BW, Kim HJ,
Cha MJ, Sung JM, Kim TW and Hwang KC: Cordycepin inhibits vascular
smooth muscle cell proliferation. Eur J Pharmacol. 597:64–69. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu YM and Lin HC: Curcumin prevents human
aortic smooth muscle cells migration by inhibiting of MMP-9
expression. Nutr Metab Cardiovasc Dis. 20:125–132. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schwartz SM, Heimark RL and Majesky MW:
Developmental mechanisms underlying pathology of arteries. Physiol
Rev. 70:1177–1209. 1990.PubMed/NCBI
|
|
13
|
Owens GK: Regulation of differentiation of
vascular smooth muscle cells. Physiol Rev. 75:487–517.
1995.PubMed/NCBI
|
|
14
|
Lijnen HR: Plasmin and matrix
metalloproteinases in vascular remodeling. Thromb Haemost.
86:324–333. 2001.PubMed/NCBI
|
|
15
|
Sheu MJ, Lin HY, Yang YH, Chou CJ, Chien
YC, Wu TS and Wu CH: Demethoxycurcumin, a major active curcuminoid
from Curcuma longa, suppresses balloon injury induced
vascular smooth muscle cell migration and neointima formation: An
in vitro and in vivo study. Mol Nutr Food Res. 57:1586–1597.
2013.PubMed/NCBI
|
|
16
|
Baeuerle PA and Baltimore D: I kappa B: a
specific inhibitor of the NF-kappa B transcription factor. Science.
242:540–546. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baud V and Karin M: Signal transduction by
tumor necrosis factor and its relatives. Trends Cell Biol.
11:372–377. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Barnes PJ and Karin M: Nuclear
factor-kappaB: a pivotal transcription factor in chronic
inflammatory diseases. N Engl J Med. 336:1066–1071. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee B and Moon SK: Resveratrol inhibits
TNF-alpha-induced proliferation and matrix metalloproteinase
expression in human vascular smooth muscle cells. J Nutr.
135:2767–2773. 2005.
|
|
20
|
McKellar GE, McCarey DW, Sattar N and
McInnes IB: Role for TNF in atherosclerosis? Lessons from
autoimmune disease. Nat Rev Cardiol. 6:410–417. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Newby AC: Metalloproteinase expression in
monocytes and macrophages and its relationship to atherosclerotic
plaque instability. Arterioscler Thromb Vasc Biol. 28:2108–2114.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hayden MS and Ghosh S: Signaling to
NF-kappaB. Genes Dev. 18:2195–2224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim HS, Kim HJ, Park KG, Kim YN, Kwon TK,
Park JY, Lee KU, Kim JG and Lee IK: Alpha-lipoic acid inhibits
matrix metalloproteinase-9 expression by inhibiting NF-kappaB
transcriptional activity. Exp Mol Med. 39:106–113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim CH and Moon SK:
Epigallocatechin-3-gallate causes the p21/WAF1-mediated G(1)-phase
arrest of cell cycle and inhibits matrix metalloproteinase-9
expression in TNF-alpha-induced vascular smooth muscle cells. Arch
Biochem Biophys. 435:264–272. 2005. View Article : Google Scholar
|
|
25
|
Gloire G, Legrand-Poels S and Piette J:
NF-kappaB activation by reactive oxygen species: fifteen years
later. Biochem Pharmacol. 72:1493–1505. 2006.PubMed/NCBI
|
|
26
|
Schreck R, Rieber P and Baeuerle PA:
Reactive oxygen intermediates as apparently widely used messengers
in the activation of the NF-kappa B transcription factor and HIV-1.
EMBO J. 10:2247–2258. 1991.PubMed/NCBI
|
|
27
|
Janssen-Heininger YM, Poynter ME and
Baeuerle PA: Recent advances towards understanding redox mechanisms
in the activation of nuclear factor kappaB. Free Radic Biol Med.
28:1317–1327. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bar-Shai M, Carmeli E, Ljubuncic P and
Reznick AZ: Exercise and immobilization in aging animals: the
involvement of oxidative stress and NF-kappaB activation. Free
Radic Biol Med. 44:202–214. 2008. View Article : Google Scholar : PubMed/NCBI
|