Introduction
Nasopharyngeal carcinoma (NPC) is a common
malignancy with a high metastatic and invasive rate that originates
in the epithelial lining of the nasopharynx. NPC is most prevalent
in southeast Asia, parts of Africa and southern China (1,2) and
there were an estimated 84,400 cases of NPC and 51,600 mortalities
in 2008 worldwide (3). Since NPC
is radiosensitive, radiotherapy serves as the primary treatment,
achieving 5-year overall survival rates of 90 and 84% for early
stage I and IIA NPC, respectively (4). Although NPC is sensitive to
radiotherapy and chemotherapy, the majority of patients are
diagnosed during the middle-late stage and despite local lesions
being well controlled, treatments for lymph node metastasis and
local recurrence are limited. A number of clinical studies
(5,6) have found that the nodal status is an
independent prognostic factor that affects the overall survival
rate of NPC patients without distant metastasis. However, for
advanced NPC, treatment outcomes have been unsatisfactory due to
distant metastases and local recurrence following radiotherapy
(7). The clinical use of
chemoradiotherapy is often limited by unacceptable levels of normal
tissue toxicity (8). Thus, more
effective treatment methods and drugs are required to effectively
control lymph node metastasis and improve the overall survival rate
(9).
Crucifers, including broccoli, cauliflower and
carrots, are plants with natural antitumor activity.
3,3′-Diindolylmethane (DIM) is a specific compound extracted from
crucifers that has been identified to provide the main antitumor
function. A number of studies (10–13)
have reported that DIM inhibits proliferation and induces apoptosis
in a variety of tumor cells in vitro and in vivo. In
a previous study (14), DIM was
found to effectively inhibit the proliferation of NPC cells and
induce them to undergo apoptosis. Multiple signaling pathways,
including the phosphoinositide-3-kinase, protein kinase B, mitogen
activated protein kinase and nuclear factor-κB pathways, were shown
to be regulated by DIM and the expression of key proteins
associated with these pathways was also shown to be inhibited. In
addition, no signs of toxicity were observed in the normal tissues
and organs in the animal model, indicating that DIM is safe to use
as an antitumor drug. The study concluded that DIM effectively
induced apoptosis of NPC cells and had a preventive and curative
role in the development and progression of NPC.
However, whether DIM inhibits lymph node metastasis
of NPC cells remains to be investigated. Thus, the aim of the
present study was to explore whether DIM has the ability to inhibit
the lymph node metastasis of NPC cells. The molecular mechanism was
also investigated.
Materials and methods
Cell culture and grouping
The 5–8F Human NPC cell line (China Center for Type
Culture Collection, Wuhan University, Wuhan, China) was cultured in
RMPI-1640 (Invitrogen Life Technologies, Carlsbad, CA, USA) with
10% fetal bovine serum (FBS; HyClone Laboratories, Inc., South
Logan, UT, USA) in an environment of 5% CO2 and 37°C.
Cells in a logarithmic growth phase were used in the experiment.
DIM, at final concentrations of 0, 25, 50 and 100 μM, was added to
the 5–8F cell cultures.
Transwell assay
Transwell assays were performed using polycarbonate
membrane Transwell plates coated with Matrigel (Corning Inc.,
Tewksbury, MA, USA). The bottom chamber included 0.5 ml medium
containing 5% FBS. Cells were seeded into the upper chamber at a
density of 3.0×105 cells/ml and incubated for 24 h at
37°C and 5% CO2. The remaining cells on the upper
surface were mechanically removed. Membranes were then washed,
fixed and stained with methyl violet. Using a microscope, the
migration ability of the cells was determined by counting the cells
that had migrated to the lower side of the filter. Experiments were
performed in triplicate and three fields were counted in each
experiment.
Animal feeding and grouping
Female BALB/c nude mice (age, 4–6 weeks-old) were
purchased from Beijing Huafukang Biological Technology Co. Ltd.
(Beijing, China). The animals were fed the control diet for one
week prior to the experiment in order to adapt. A conventional diet
and 0.5% DIM-supplemented diet were manufactured by Beijing
Huafukang Biological Technology Co. Ltd. Animal welfare and
transplanted tumor inoculation procedures were performed as
previously described (14).
Animals were divided into three groups. The preventive treatment
group received a 0.5% DIM-supplemented diet prior to the
inoculation of NPC cells. The drug treatment group received the
0.5% DIM-supplemented diet following the inoculation of NPC cells
and the tumor diameter reaching 5 mm, while the control group was
inoculated with NPC cells and fed a control diet. The duration of
DIM treatment was 8 weeks and the time of lymph node metastasis
occurrence was observed and recorded. Eight weeks after
inoculation, the animals were sacrificed and the xenografts,
metastatic lymph nodes, hearts, livers and kidneys were preserved
for further testing. The study was conducted in strict accordance
with the recommendations in the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health (eighth
edition). The animal use protocol was reviewed and approved by the
Institutional Animal Care and Use Committee of Wuhan University
(Wuhan, China).
Western blot analysis
Cells were stimulated with various concentrations of
DIM, harvested and then lysed in buffer containing 1% Nonidet-P40
supplemented with complete protease inhibitor cocktail (Roche
Diagnostics, Mannheim, Germany) and 2 mM dithiothreitol. Lysates
were resolved by 12% SDS-PAGE, transferred to nitrocellulose
membranes and immunoblotted with primary antibodies against
E-cadherin, matrix metalloproteinase (MMP)-9, vimentin, slug, snail
and GAPDH (Cell Signaling Technology, Inc., Danvers, MA, USA).
Following immunoblotting with secondary antibodies (Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China),
proteins were detected with enhanced chemiluminescence reagent
(Thermo Fisher Scientific, Waltham, MA, USA). All experiments were
performed in triplicate.
Statistical analysis
All values are expressed as mean ± SD. Statistical
analysis was conducted using one-way analysis of variance with SPSS
statistical software version 15.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
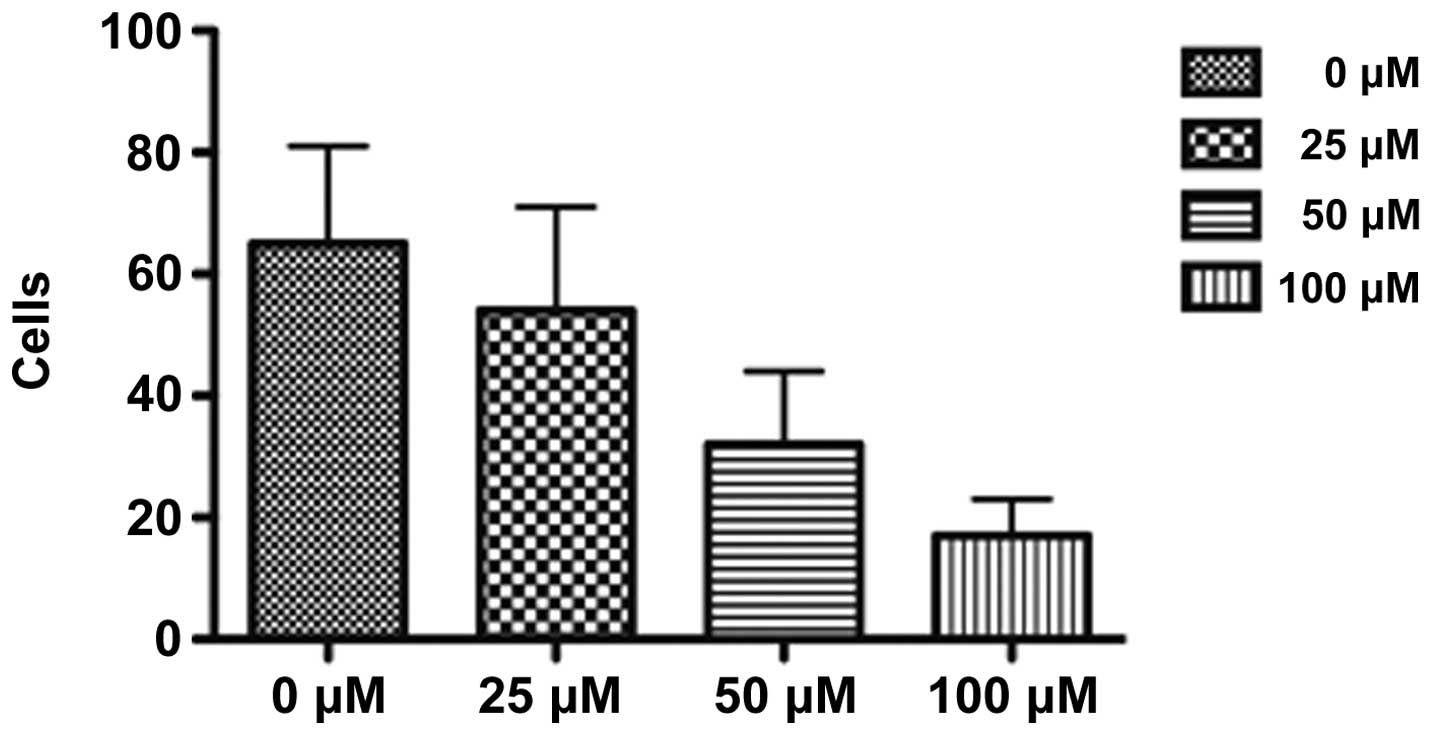
Invasive ability of NPC cells in
vitro
The migration and invasive abilities of NPC cells
were detected using the Transwell assay. The results demonstrated
that NPC cells treated with DIM had a decreased ability to migrate
and invade. In addition, the weakening effect was increased as the
concentration of DIM increased (Fig.
1).
Invasive ability of NPC cells in
vivo
In the control group, lymph node metastasis occurred
4 weeks following the implantation of NPC cells, and lymph node
metastasis was observed in 10/12 rats at week 8 following
implantation. In the drug treatment group, lymph node metastasis
occurred 6 weeks following implantation and lymph node metastasis
was observed in 6/12 rats at week 8 following implantation. In the
preventive treatment group, lymph node metastasis occurred 7 weeks
following implantation and lymph node metastasis was observed in
2/12 rats at week 8 following implantation. The differences between
the three groups were statistically significant (Table I).
 | Table IIncidence of xenograft tumor and lymph
node metastasis (n=12 per group). |
Table I
Incidence of xenograft tumor and lymph
node metastasis (n=12 per group).
| Groups | Xenograft tumor, n
(%) | Lymph node
metastasis, n (%) |
|---|
| Control | 12/12 (100) | 10/12 (83.3) |
| Preventive
treatment | 9/12 (75) | 2/9 (22.2) |
| Drug treatment | 10/12 (83.3) | 6/10 (60) |
Expression of key proteins associated
with epithelial mesenchymal transition (EMT)
Expression levels of EMT-associated key proteins in
NPC cells treated with DIM were detected to investigate the
molecular mechanisms underlying the inhibition of invasion and
metastasis in vitro and in vivo. The results
demonstrated that the protein expression level of E-cadherin
increased gradually with increasing concentrations of DIM. By
contrast, vimentin, slug and snail expression levels gradually
decreased (Fig. 2).
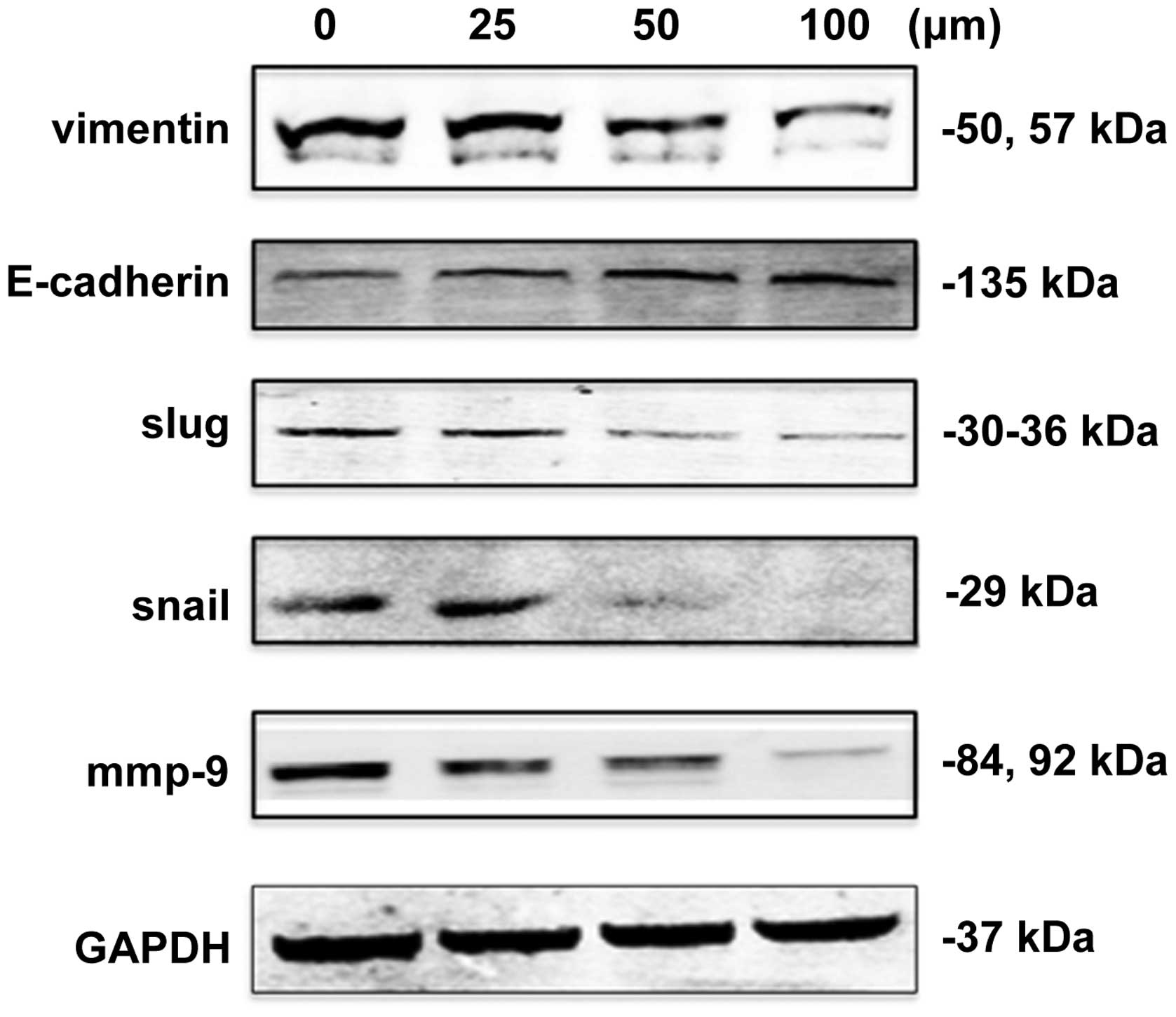 | Figure 2Expression of EMT-associated proteins
in 5–8F NPC cells treated with various concentrations of DIM.
Expression levels of the EMT-associated proteins vimentin,
E-cadherin, slug, snail and MMP-9 were detected by western blot
analysis. The expression levels of vimentin, slug, snail and MMP-9
decreased with increasing concentrations of DIM, while the
expression levels of E-cadherin increased with increasing
concentrations of DIM. NPC, nasopharyngeal carcinoma; DIM,
3,3′-diindolylmethane; EMT, epithelial mesenchymal transition. |
Discussion
The majority of patients with NPC are found to have
a palpable mass in their neck following physical examination. Once
neck lymph node metastasis has occurred, the treatment outcomes of
NPC are poor (15). This is mainly
due to the limitations of conventional chemoradiotherapy treatment
in controlling lymph node metastasis (16). In previous years, although
radiation technology has developed and a variety of new
chemotherapy drugs have been applied in clinical practice, the
5-year overall survival rate of NPC patients has not significantly
improved (17–23).
A number of studies (24–27)
have demonstrated that EMT plays a key role in tumor development.
The migration capacity of tumor cells is associated with cell
phenotype. EMT is typically characterized by spindle-shaped cells
that are unable to form homophilic cell-cell interactions due to
the addition of a vimentin cytoskeleton and the loss of E-cadherin
(28,29). Tumor cells with a mesenchymal
phenotype, in contrast to epithelial tumor cells, have higher
expression levels of cell movement-associated proteins and exhibit
improved migration and invasion (30). The results of the present study
indicate that lymph node metastasis of NPC was significantly
inhibited by DIM. To the best of our knowledge, the function of DIM
in inhibiting lymph node metastasis has not been previously
reported. The molecular mechanism of DIM was considered to involve
inhibition of the EMT phenotype of NPC cells.
E-cadherin and vimentin are important markers in EMT
(31–33). E-cadherin is an epithelial cell
marker, while vimentin is a mesenchymal cells marker. Wong et
al (34) reported that
E-cadherin expression levels decreased in NPC cells undergoing
migration. In addition, the migration potential of NPC cells
significantly decreased when exogenous E-cadherin was expressed,
according to gene technology. Xu et al (35) found that E-cadherin expression
exhibited a significant positive correlation with the long-term
outcome of NPC patients; that is, the higher the E-cadherin
expression levels, the better the long-term outcome of patients.
Among the NPC patients who received the antitumor therapy, the
reoccurrence and lymph node metastasis rates of the patients with
higher E-cadherin expression were lower than those with lower
E-cadherin expression. The study concluded that EMT is one of the
factors that affects the long-term outcomes of patients with NPC.
In the present study, the NPC cell line 5–8F had a marked
capability for invasion and were identified to have an EMT
phenotype with low E-cadherin expression levels and high vimentin
expression levels. However, the invasion ability of 5–8F cells
decreased markedly following treatment with DIM and the E-cadherin
expression levels increased with increasing concentrations of DIM.
In addition, the expression levels of vimentin decreased with
increasing concentrations of DIM. In the animal model, lymph node
metastasis occurrence was reduced and delayed in rats fed the 0.5%
DIM-supplemented diet compared with that in the control group.
These results indicate that DIM significantly inhibits the invasion
ability and lymph node metastasis of NPC cells.
Slug and snail are transcription factors that play
an important role in tumor development as they inhibit E-cadherin
expression and promote EMT (36,37).
It has been reported that by inhibiting slug and snail, which in
turn inhibit EMT, the invasion and metastasis of tumor cells is
inhibited, causing the expression levels of E-cadherin to increase.
In the present study, the expression levels of slug and snail in
the NPC cells treated with DIM were detected and found to decrease
with increasing concentrations of DIM. Therefore, it may be
hypothesized that the reductions in the slug and snail expression
levels caused by DIM were associated with the inhibition of
invasion and metastasis of the NPC cells, and that the inhibition
of invasion and metastasis was directly caused by reductions in the
E-cadherin and vimentin expression levels.
The control of lymph node metastasis is crucial in
the treatment of NPC. The expression levels of key markers of the
EMT phenotype have been shown to directly correlate with the
prognosis of patients with NPC (38). In the present study, a natural and
non-toxic compound extracted from plants was shown to effectively
inhibit the invasion and metastasis of NPC cells by inhibiting EMT
in vivo and in vitro. However, the treatment effects
of DIM combined with conventional chemotherapy and radiotherapy, as
well as the function of inhibiting EMT by DIM for use in clinical
practice, require further study.
In conclusion, DIM affects the expression levels of
a number of EMT-associated key proteins and effectively induces the
inhibition of invasion and metastasis of NPC cells in vitro
and in vivo.
Acknowledgements
The study was supported by grants from the National
Natural Science Foundation of China (no. 30901662), the Doctoral
Discipline Foundation in the Higher Education Institutions of
Ministry of Education (no. 20110141110062), the Science and
Technology Program of Hubei Province of China (no. 2007AA302B08)
and the Science and Technology Program of Wuhan City (nos.
200951199455 and 200950431168).
References
|
1
|
Lo KW, To KF and Huang DP: Focus on
nasopharyngeal carcinoma. Cancer Cell. 5:423–428. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lo KW, Chung GT and To KF: Deciphering the
molecular genetic basis of NPC through molecular, cytogenetic, and
epigenetic approaches. Semin Cancer Biol. 22:79–86. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
4
|
Lee AW, Sze WM, Au JS, et al: Treatment
results for nasopharyngeal carcinoma in the modern era: the Hong
Kong experience. Int J Radiat Oncol Biol Phys. 61:1107–1116. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He JR, Shen GP, Ren ZF, et al:
Pretreatment levels of peripheral neutrophils and lymphocytes as
independent prognostic factors in patients with nasopharyngeal
carcinoma. Head Neck. 34:1769–1776. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang PY, Wang CT, Cao KJ, et al:
Pretreatment body mass index as an independent prognostic factor in
patients with locoregionally advanced nasopharyngeal carcinoma
treated with chemoradiotherapy: findings from a randomised trial.
Eur J Cancer. 49:1923–1931. 2013. View Article : Google Scholar
|
|
7
|
Lee AW, Poon YF, Foo W, et al:
Retrospective analysis of 5037 patients with nasopharyngeal
carcinoma treated during 1976–1985: overall survival and patterns
of failure. Int J Radiat Oncol Biol Phys. 23:261–270.
1992.PubMed/NCBI
|
|
8
|
Tofilon PJ and Camphausen K: Molecular
targets for tumor radiosensitization. Chem Rev. 109:2974–2988.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ho FC, Tham IW, Earnest A, Lee KM and Lu
JJ: Patterns of regional lymph node metastasis of nasopharyngeal
carcinoma: a meta-analysis of clinical evidence. BMC Cancer.
12:982012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim SJ, Lee JS and Kim SM:
3,3′-Diindolylmethane suppresses growth of human esophageal
squamous cancer cells by G1 cell cycle arrest. Oncol Rep.
27:1669–1673. 2012.
|
|
11
|
Shorey LE, Hagman AM, Williams DE, Ho E,
Dashwood RH and Benninghoff AD: 3,3′-Diindolylmethane induces G1
arrest and apoptosis in human acute T-cell lymphoblastic leukemia
cells. PLoS One. 7:e349752012.
|
|
12
|
Yin XF, Chen J, Mao W, Wang YH and Chen
MH: A selective aryl hydrocarbon receptor modulator
3,3′-Diindolylmethane inhibits gastric cancer cell growth. J Exp
Clin Cancer Res. 31:462012.
|
|
13
|
Zhu J, Li Y, Guan C and Chen Z:
Anti-proliferative and pro-apoptotic effects of
3,3′-diindolylmethane in human cervical cancer cells. Oncol Rep.
28:1063–1068. 2012.
|
|
14
|
Chen C, Chen SM, Xu B, et al: In vivo and
in vitro study on the role of 3,3′-diindolylmethane in treatment
and prevention of nasopharyngeal carcinoma. Carcinogenesis.
34:1815–1821. 2013.
|
|
15
|
Pan Y and Claret FX: Targeting Jab1/CSN5
in nasopharyngeal carcinoma. Cancer Lett. 326:155–160. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng SH, Jian JJ, Tsai SY, et al:
Long-term survival of nasopharyngeal carcinoma following
concomitant radiotherapy and chemotherapy. Int J Radiat Oncol Biol
Phys. 48:1323–1330. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee AW, Tung SY, Ngan RK, et al: Factors
contributing to the efficacy of concurrent-adjuvant chemotherapy
for locoregionally advanced nasopharyngeal carcinoma: combined
analyses of NPC-9901 and NPC-9902 Trials. Eur J Cancer. 47:656–666.
2011. View Article : Google Scholar
|
|
18
|
Xiao WW, Huang SM, Han F, et al: Local
control, survival, and late toxicities of locally advanced
nasopharyngeal carcinoma treated by simultaneous modulated
accelerated radiotherapy combined with cisplatin concurrent
chemotherapy: long-term results of a phase 2 study. Cancer.
117:1874–1883. 2011. View Article : Google Scholar
|
|
19
|
Pan ZQ, He XY, Guo XM, et al: A phase III
study of late course accelerated hyperfractionated radiotherapy
versus conventionally fractionated radiotherapy in patients with
nasopharyngeal carcinoma. Am J Clin Oncol. 35:600–605. 2012.
View Article : Google Scholar
|
|
20
|
Peng G, Wang T, Yang KY, et al: A
prospective, randomized study comparing outcomes and toxicities of
intensity-modulated radiotherapy vs. conventional two-dimensional
radiotherapy for the treatment of nasopharyngeal carcinoma.
Radiother Oncol. 104:286–293. 2012. View Article : Google Scholar
|
|
21
|
Cao CN, Luo JW, Gao L, et al: Clinical
outcomes and patterns of failure after intensity-modulated
radiotherapy for T4 nasopharyngeal carcinoma. Oral Oncol.
49:175–181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Y, Sun Y, Liang SB, et al: Progress
report of a randomized trial comparing long-term survival and late
toxicity of concurrent chemoradiotherapy with adjuvant chemotherapy
versus radiotherapy alone in patients with stage III to IVB
nasopharyngeal carcinoma from endemic regions of China. Cancer.
119:2230–2238. 2013.
|
|
23
|
Zhang W, Dou H, Lam C, et al: Concurrent
chemoradiotherapy with or without adjuvant chemotherapy in
intermediate and locoregionally advanced nasopharyngeal carcinoma.
Tumour Biol. 34:1729–1736. 2013. View Article : Google Scholar
|
|
24
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: new insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: at the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsai JH, Donaher JL, Murphy DA, Chau S and
Yang J: Spatiotemporal regulation of epithelial-mesenchymal
transition is essential for squamous cell carcinoma metastasis.
Cancer Cell. 22:725–736. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Grünert S, Jechlinger M and Beug H:
Diverse cellular and molecular mechanisms contribute to epithelial
plasticity and metastasis. Nat Rev Mol Cell Biol. 4:657–665.
2003.PubMed/NCBI
|
|
29
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Onder TT, Gupta PB, Mani SA, Yang J,
Lander ES and Weinberg RA: Loss of E-cadherin promotes metastasis
via multiple downstream transcriptional pathways. Cancer Res.
68:3645–3654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013.PubMed/NCBI
|
|
32
|
Scanlon CS, Van Tubergen EA, Inglehart RC
and D’Silva NJ: Biomarkers of epithelial-mesenchymal transition in
squamous cell carcinoma. J Dent Res. 92:114–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shirkoohi R: Epithelial mesenchymal
transition from a natural gestational orchestration to a bizarre
cancer disturbance. Cancer Sci. 104:28–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wong TS, Chan WS, Li CH, et al: Curcumin
alters the migratory phenotype of nasopharyngeal carcinoma cells
through up-regulation of E-cadherin. Anticancer Res. 30:2851–2856.
2010.PubMed/NCBI
|
|
35
|
Xu L, Jiang Y, Zheng J, et al: Aberrant
expression of β-catenin and E-cadherin is correlated with poor
prognosis of nasopharyngeal cancer. Hum Pathol. 44:1357–1364.
2013.
|
|
36
|
Nieto MA: The snail superfamily of
zinc-finger transcription factors. Nat Rev Mol Cell Biol.
3:155–166. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cano A, Pérez-Moreno MA, Rodrigo I, et al:
The transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Luo W, Fang W, Li S and Yao K: Aberrant
expression of nuclear vimentin and related epithelial-mesenchymal
transition markers in nasopharyngeal carcinoma. Int J Cancer.
131:1863–1873. 2012. View Article : Google Scholar : PubMed/NCBI
|