Introduction
Malignant ventricular arrhythmias (VAs) are the most
common cause of sudden cardiac death (1). Arrhythmia monitoring is not available
in the majority of patients with VAs, and patients with malignant
VAs often are not able to benefit from effective and timely
hospital treatment as the majority of VAs occur outside hospital
(1,2). Ideally, patients at a high risk of
VAs should be continuously monitored to benefit from rapid
diagnosis and timely intervention to save lives. Devices that
monitor electrocardiograms (ECGs) and provide timely intervention
are required to save the lives of patients with malignant VAs
(1–4).
Currently available non-invasive techniques,
including Holter and ECG telemetry systems, as well as invasive
implantable subcutaneous cardiac monitors, are valuable in the
detection of arrhythmias, including VAs (1,4–6).
Implantable cardioverter-defibrillators (ICDs) detect and terminate
VAs and save the lives of patients (7,8).
However, false alarms may occur and patients may thus suffer
inappropriate shocks (7–10). An ICD with a remote monitoring
function has been successfully used to effectively terminate VAs by
anti-tachycardia pacing (ATP) and shocks, and manages alarm
messages from patients (9–11). The PainFREE Rx and PainFREE Rx II
trials showed that ATP terminates the majority of malignant
ventricular tachycardias (VTs) (11,12).
Animal models are helpful for testing the efficacy of novel drugs
and the feasibility of using novel implantable electronic
cardiovascular devices (IECDs) with telecommunications technologies
for monitoring and terminating VAs.
In the present study, the feasibility of using a
novel IECD system with remote wireless cardiac arrhythmia
monitoring and ATP functions was explored in rabbits with
experimental myocardial infarction (MI).
Materials and methods
IECD system
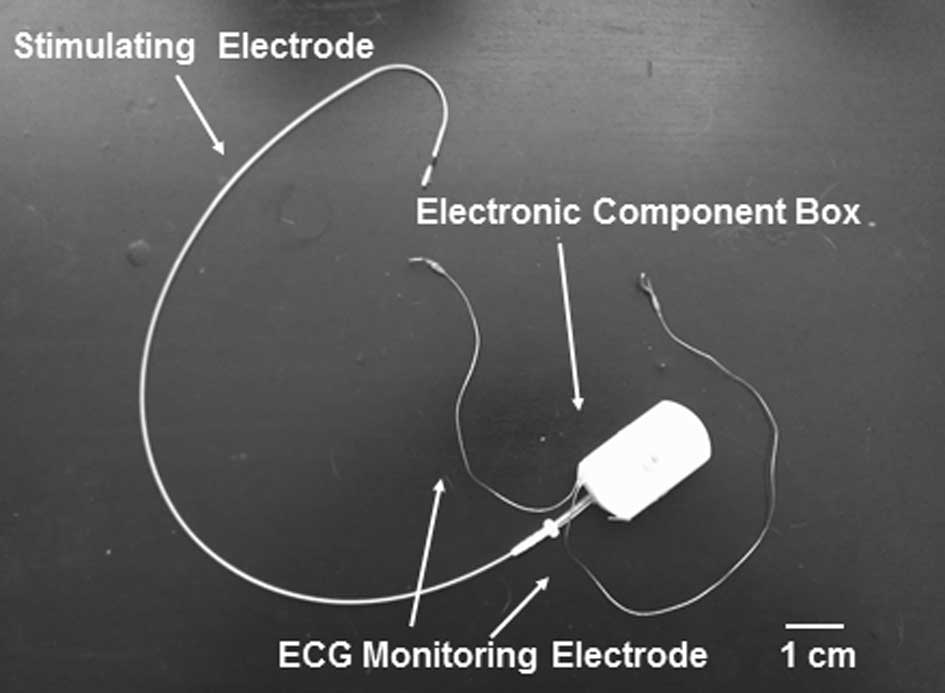
The IECD system was provided by Genix Biotek Science
Technology (Shanghai) Co., Ltd. (Shanghai, China). The IECD
consists of one electronic component box, <5 ml in volume and 15
g in weight, two ECG monitoring electrodes and one bipolar
stimulation electrode (5076 lead; Medtronic, Inc., Minneapolis, MN,
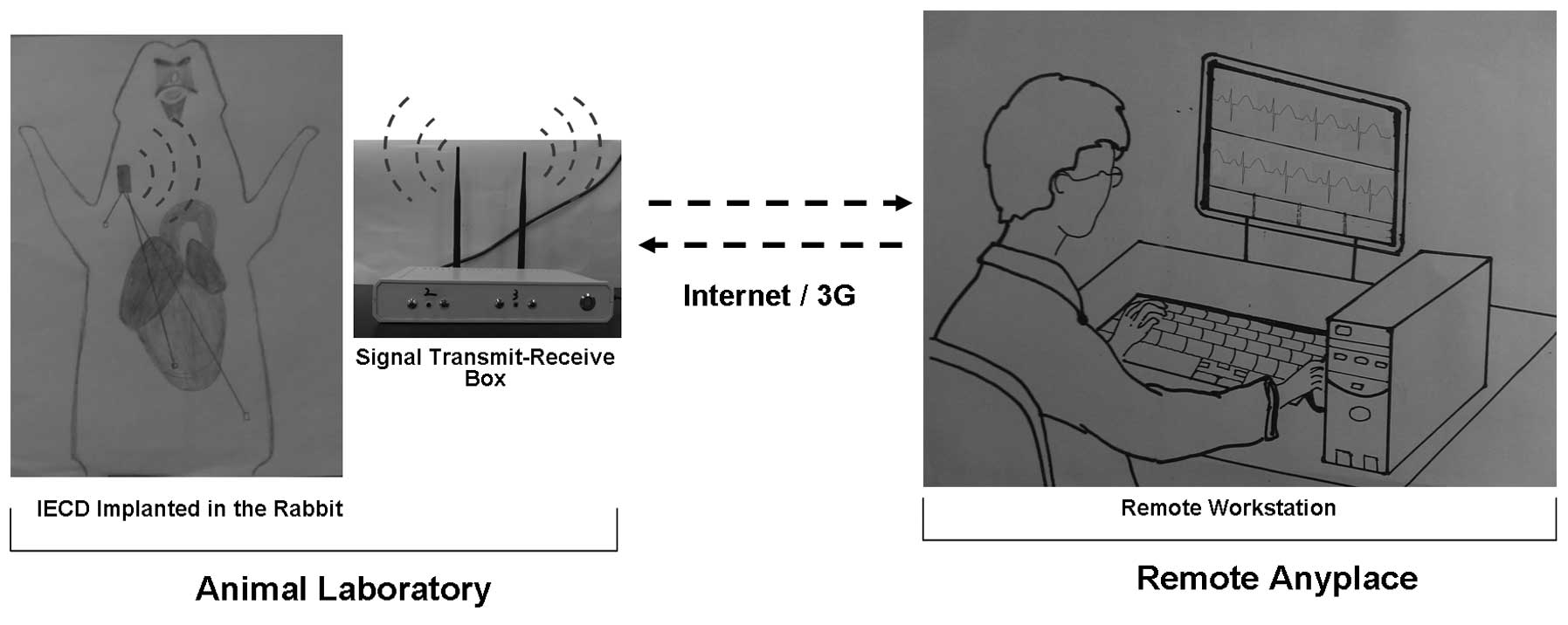
USA; Fig. 1). The extracorporeal
system consisted of a signal transmit-receive box which connected
with the internet or the third-generation communication system
(virtual private network system), and a personal computer with ECG
monitoring and electrical stimulation software (Fig. 2). The personal computer was also
used as a central workstation in the system. The computer received
remote digital signals and converted them into real-time ECG data.
The real-time ECG was saved as a compressed ECG signal file with
precise time stamps for off-line analysis. When the IECD was
implanted, the extracorporeal system also remotely sent out a
stimulation signal wirelessly to the IECD to stimulate the heart
via a stimulation electrode. Two stimulation modes were designed in
this IECD system: The regular stimuli (S1S1), and the regular
stimuli with an added extra stimulus (S1S2). The stimulation
parameters of this IECD system were as follows: Stimulation current
between 0.1 and 5 mA, stimulation time length between 0.5 and 5
msec, and stimulation rate between 1 and 50 Hz. In the S1S2
program, every seventh S1 was followed by one S2, and the
stimulation interval was 1–10 sec.
Ethics statement and animal model
preparation
A total of 20 adult New Zealand white rabbits
(weight, 2.5–3.5 kg; Shanghai Jiagan biological technology Co.,
Ltd., Shanghai, China) were used. Animal care and handling
procedures were approved by the Animal Care and Use Committee,
Research Institute of Medicine, Shanghai Jiao Tong University, in
accordance with the Guide for the Care and Use of Laboratory
Animals published by the National Institute of Health (1996). All
animals involved received humane care in compliance with Chinese
Association for Accreditation of Laboratory Animal Care. Each
surgery was performed under general anesthesia, and all efforts
were made to minimize suffering.
The rabbits were anesthetized with sodium
pentobarbital (30 mg/kg induction, and 2.0–5.0 mg/kg/h with
intermittent boluses as required). The skin was excised at the
location of the jugular vein, and then the jugular vein was
separated. The monitoring electrodes of the IECD were fixed
subcutaneously, with one electrode at the right upper limb joint as
the cathode and another at the left upper quadrant of the abdomen
as the anode. Following the incision of the jugular vein, the
stimulation electrode of the IECD was implanted in the right
ventricle through the jugular vein aided by laboratory digital
subtraction angiography (Fig. 2).
Following confirmation of the lowest stimulation current threshold
(0.2–1.0 mA) of ventricle pacing, the stimulation electrode was
fixed and the IECD was implanted subcutaneously into the back of
each rabbit, close to the cervicum. After the IECD was implanted, a
left thoracotomy was performed through the fourth intercostal space
to expose the heart. The major branch of the left anterior
descending branch was occluded to induce MI. The rabbits with the
implanted IECD were placed within 10 m of the signal
transmit-receive box. To prevent infections, 400,000 U penicillium
sodium were injected intramuscularly daily for 3–5 days as
required.
Remote ECG monitoring and ventricular
stimulation protocol
The cardiac electrical signal filtration used for
the ECG was between 0.5 and 100 Hz. The rabbits with the implanted
IECD were placed at different distances from the signal
transmit-receive box to test the quality of the wireless ECG signal
and the intensity of the stimulating signals. The wireless signal
intensity of the IECD implanted in the rabbits was also checked
with a home-made electronic detection device, based on low power
consumption and wireless transceiver technologies (CC1101; Texas
Instruments Inc., Dallas, TX, USA) and upper monitor control
software. The signal-to-noise ratio (SNR) was used to analyze the
IECD wireless signal intensity, which was deemed reliable if the
SNR was >80 decibels (dB), according to the CC1101
manufacturer’s instructions (13).
Prior to and on the second day following the MI
surgery, the surface ECG leads were connected with the cathode and
anode electrodes, which were placed near the monitoring electrodes
of the IECD in the animal laboratory. In a remote location with
internet access, another investigator from the group manipulated
the internet-based remote system using a personal computer for
remote ECG monitoring and ventricular stimulation. Surface and
remote ECG data were recorded synchronously, and the voltage
amplitudes of the stimulation signal with different stimulating
currents were compared subsequently. Simultaneously, the time delay
between the beginning of the stimulation command at the remote
personal computer and the appearance of stimulation signals on the
ECG was tested. Sustained VT was defined as VT continuing for
>30 sec. The remote S1S1 and S1S2 stimulation programs were
repeated to induce VT, and then to terminate the sustained VT.
Briefly, the initial S1S1 stimulation rate, which was >20% of
the sinus rhythm with 10 msec descending steps, was used until a
refractory period appeared. The initial S1S2 stimulation rate,
which was >20% of the sinus rhythm with 10 msec S1 descending
steps and 5 msec descending S2 steps, was also used until a
refractory period appeared. The S1S1 and S1S2 stimulation programs
were repeated 7–10 times for each electrophysiological study, or
until sustained VT and/or ventricular fibrillation (VF) appeared.
If sustained VT was induced, ventricular pacing with a rate >20%
of the induced VT rate was performed routinely to terminate it. If
VF was induced, S1S1 and S1S2 ventricular stimulation was attempted
to terminate the VF. We checked the function of
monitoring/stimulation everyday to observe the IECD battery
capacities by using the 2 rabbits until the battery was
exhausted.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Linear regression analysis was used to determine the
correlation among the stimulation current and the amplitude of the
stimulation voltage signal shown by the surface and remote ECGs.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Animals
Two out of the 20 rabbits died from hemorrhages
during the MI surgery. The IECD implantation and MI induction were
successful in the remaining 18 rabbits. Two rabbits died of
spontaneous VT/VF and cardiac arrest within 24 h after the surgery,
and another rabbit died of induced VT/VF during S1S1 and S1S2
stimulation on the second day after the surgery. A total of 13
rabbits were sacrificed with deep anesthesia within one week after
the induction of MI. Remote wireless ECG monitoring and stimulation
function were conducted in the remaining two rabbits for three
months to observe the battery capacities of the IECD.
Remote wireless ECG monitoring and
ventricular stimulation
The SNR was between −40±5.2 dB at 1 m and −61±3.8 dB
at 10 m, demonstrating that the wireless signal intensity of the
implanted IECD was stable and reliable within a 10-m distance. The
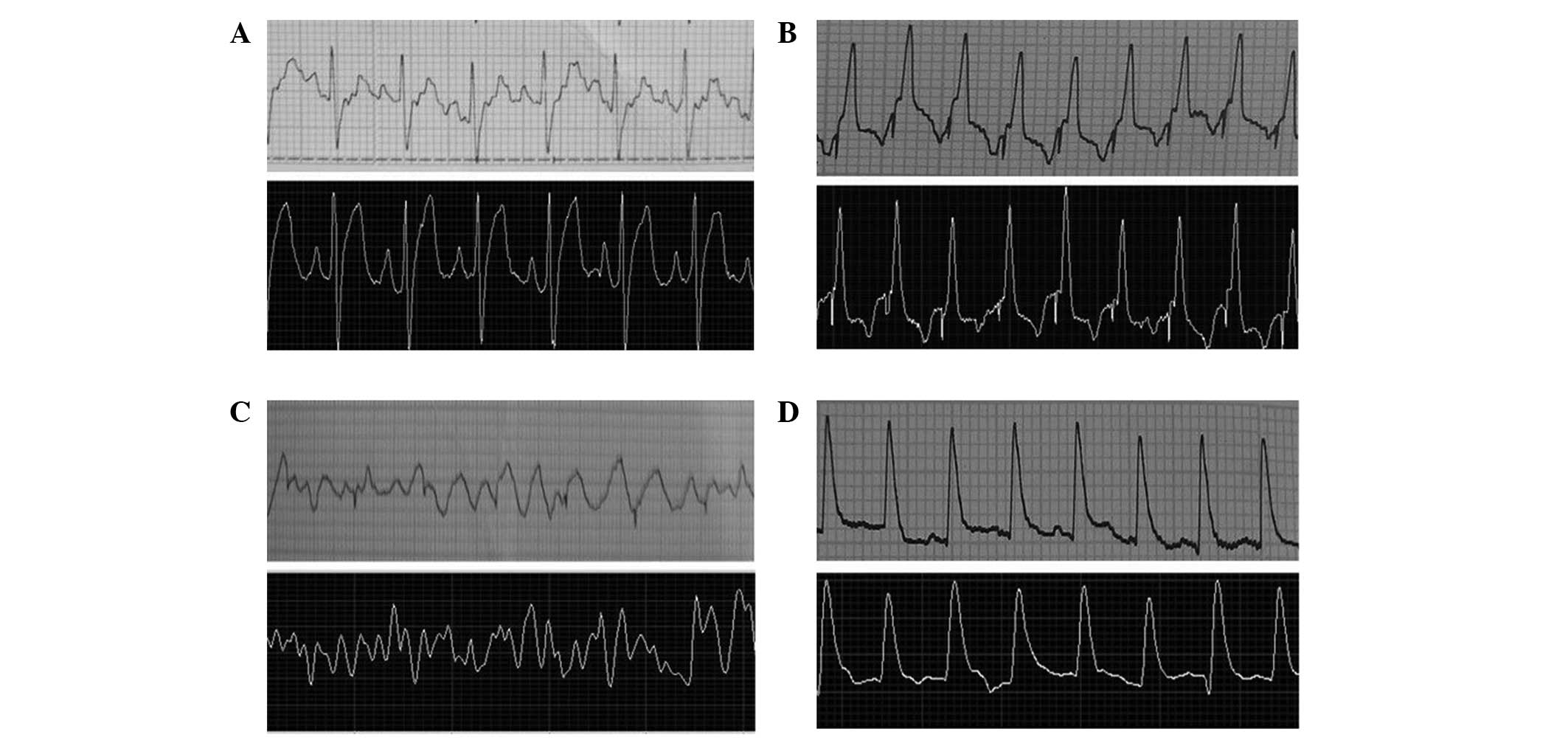
ECG monitoring and ventricular stimulation were successfully
performed within 10-m distances. The system achieved real-time ECG
monitoring with a 2–3-sec time delay depending on network speed,
and the ECG signal was saved in a compressed file with precise time
stamps. The morphology of the remote ECG signal was similar to that
of the surface ECG signal (Fig.
3).
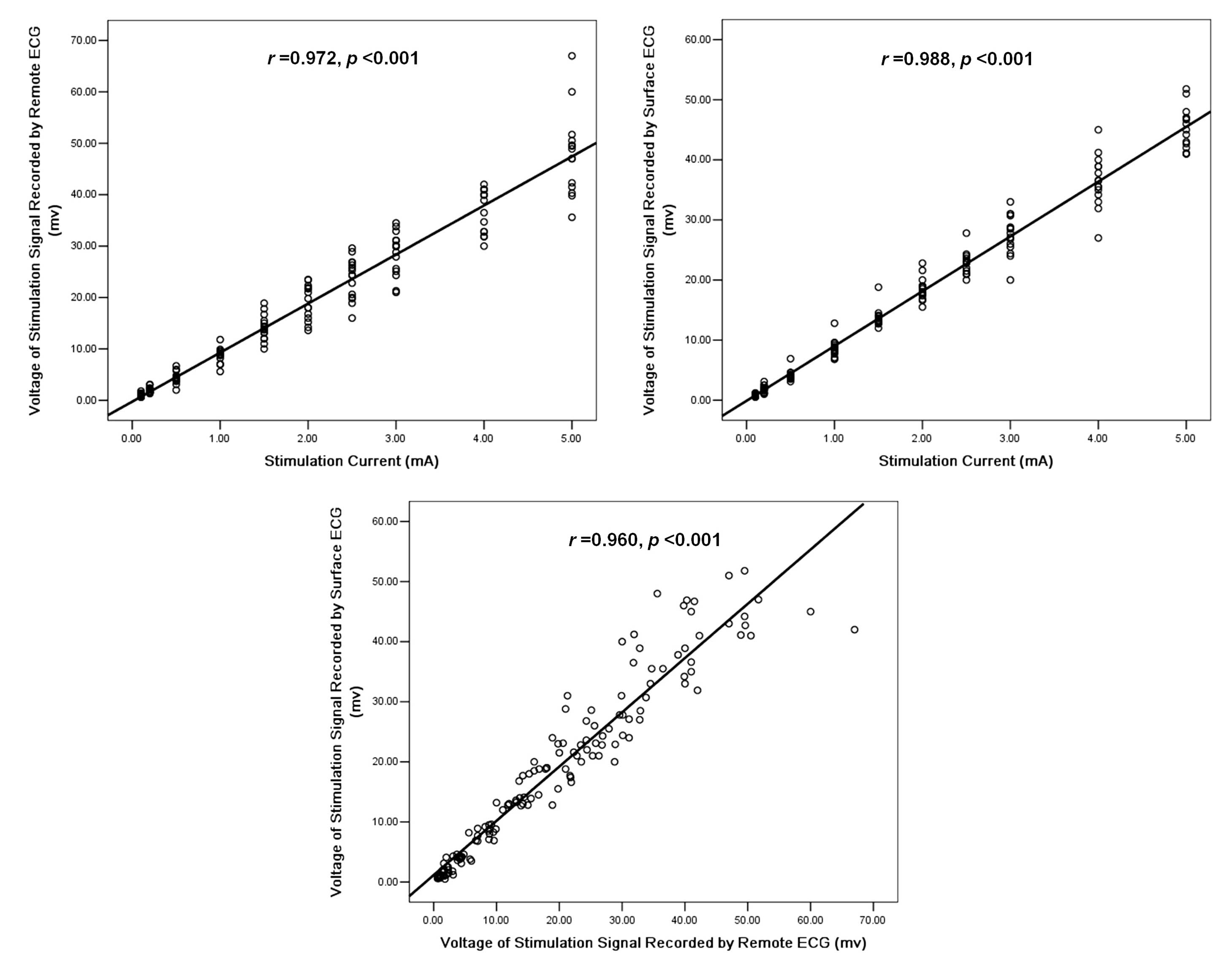
S1S1 and S1S2 stimulations were performed
successfully in 16 rabbits on the second day following the MI
surgery (Fig. 4). The stimulation
signal voltage recorded by the surface and remote ECGs increased in
proportion with the increasing stimulation current (remote ECG,
r=0.972 and surface ECG, r=0.988; P<0.001), and the voltage of
the stimulation signals recorded by the remote ECG also correlated
well with those of the surface ECG (r=0.960; P<0.001; Fig. 5).
The compressed ECG signal file of those 16 rabbits
was analyzed offline. Within one week after the MI induction,
spontaneous VTs were identified in six rabbits, including three
with sustained VTs. Two of the six rabbits died due to spontaneous
VT/VF and cardiac arrest within 24 h after the MI induction and the
other spontaneous VT was self-terminated.
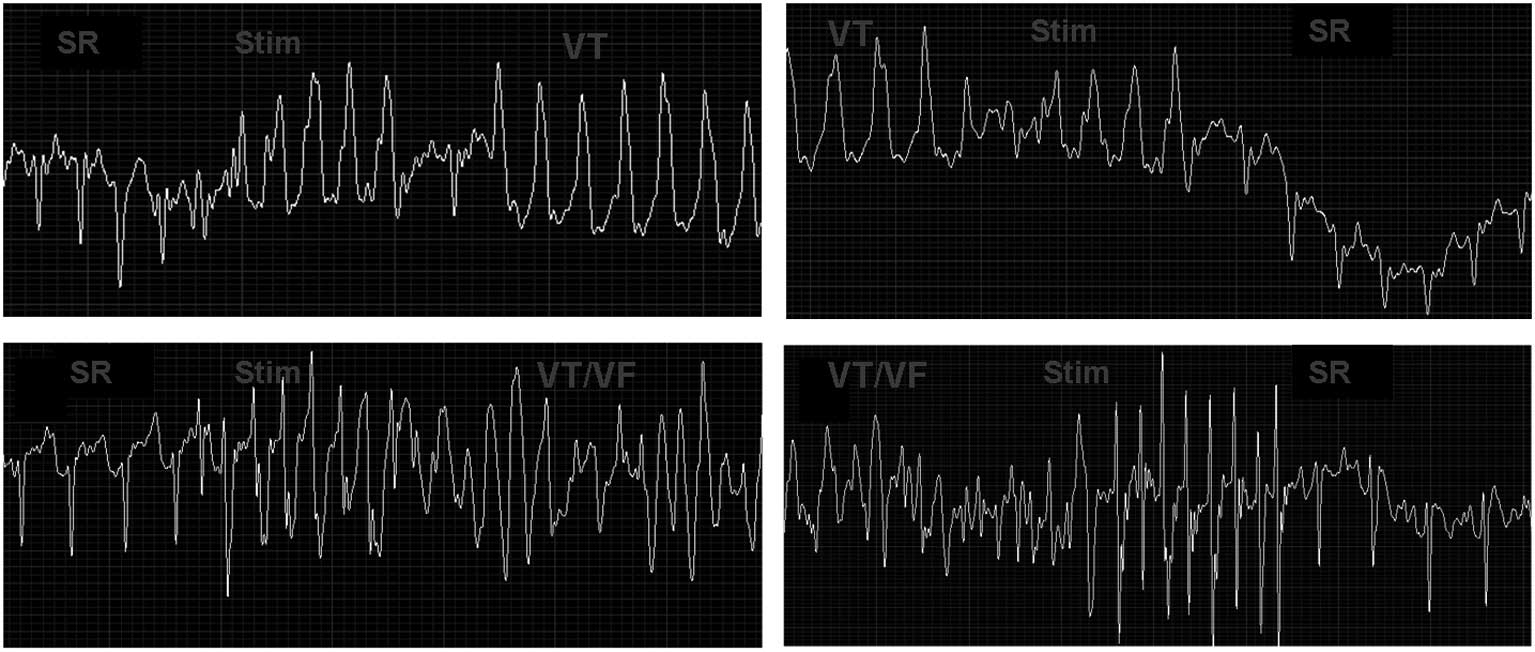
Induction and termination of VAs and
battery capacities
Sixteen rabbits were stimulated remotely by S1S1 and
S1S2 to induce VT and then terminate it afterwards. Sustained VT
was induced in five rabbits prior to the MI surgery (5/20, 25%) and
terminated successfully. Following the MI surgery, sustained VT was
induced in 12 rabbits (12/16, 75%), and four of these VTs
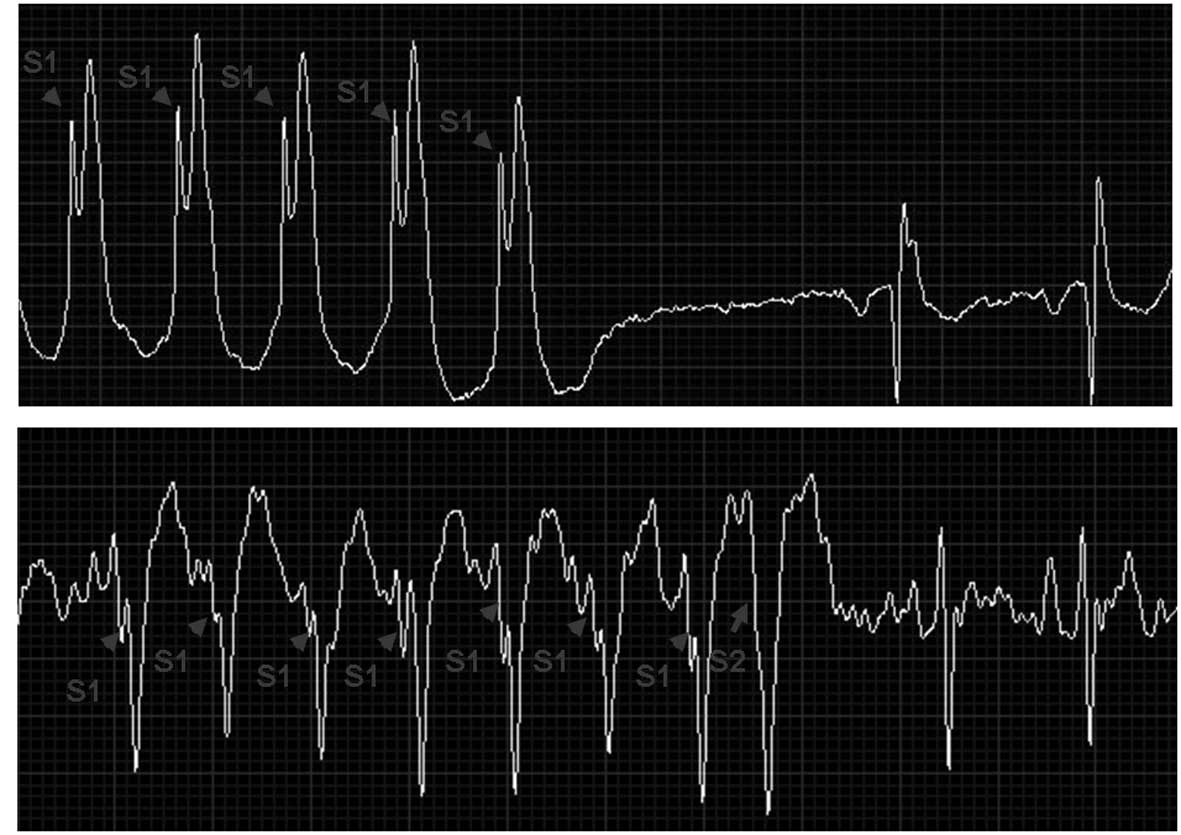
deteriorated into VF. The induced sustained VTs/VF were terminated
successfully in 11 of the 12 rabbits by S1S1 and S1S2 ventricular
stimulation and failed in one, which died of induced VT/VF. Three
of the induced VFs were also terminated successfully by ventricular
stimulation (Fig. 6). Overall, of
17 induced VTs, 16 (94%) were successfully terminated. The system
continued to work well with prolonged use, and high-fidelity and
stable wireless ECG signaling was observed for three months in the
two rabbits used for observing the battery capacities.
Discussion
The present study is the first report concerning the
feasibility of this novel IECD system in terms of remote
monitoring, and the induction and termination of VAs in a rabbit
model of MI, to the best of our knowledge. The results show that
this novel IECD system monitors, induces and terminates VAs in
rabbits with MI. Thus, this model of MI may be useful in studies on
novel drugs and devices for the management of VAs.
In the present study, real-time remote wireless and
bidirectional signal communication between the IECD implanted in
rabbits with MI and the extracorporeal system was achieved
effectively and satisfactorily. The remote ECG was able to identify
P waves, QRS complexes, T waves and arrhythmias, and to detect VTs
and VF correctly. High-fidelity and stable wireless ECG signaling
was observed at a remote workstation for three months. Real-time
and continuous ECG was saved as a compressed ECG signal file with
precise time stamps for offline analysis. Therefore, the novel
system is able to provide real-time VA monitoring and can be used
to analyze the stored ECG retrospectively for any type of
arrhythmia event and VA. In addition to ECG monitoring, the novel
system processes S1S1 and S1S2 stimulating programs with several
adjustable parameters. The continuous stimulation of IECD operates
for at least three months. VAs were readily induced (75%) in
rabbits with MI, and then terminated (94%) in rabbits pre- and
post-MI surgery using the multifunctional simulation system. The
novel system with high-quality ECG signals and advanced stimulation
functionality can be used to study the management of VAs in awake
and active animals without the requirement of anesthetics for acute
and chronic studies. Thus, the system may be useful for
experimental studies in terms of VAs in heart failure, and for
exploring the efficacy of novel drugs and testing the feasibilities
of using novel devices to manage VAs (14).
Telemedicine is one of the most attractive areas of
modern medicine (15). Thus far,
there have been relatively few research studies concerning the role
of web-based remote management of VAs, in contrast to the wealth of
studies on internet interventions to support the remote management
of other conditions (16,17). If it is possible to use the
web-based remote rescue method to save the lives of patients with
malignant VAs in the future, the mortality rate due to
cardiovascular events and SCD is likely to be reduced
significantly.
Therefore, the final goal of the new system is to
develop a novel remote clinical medical device for patients with
heart disease and arrhythmias. This novel system may be used for
round-the-clock real-time wireless ECG monitoring of patients with
VAs or with high risk of VAs, with unexplained syncope or
palpitations with possible arrhythmic origin, or to measure the
burden of atrial fibrillation, similarly to other types of
implantable subcutaneous cardiac monitors (6). In addition to the ECG monitoring
function, the ATP function is another method of managing VAs with
this system. Numerous clinical studies have shown that the majority
of VA events resulting from VT are terminated by ventricular pacing
(11,12,18,19).
In the present study, induced VAs were effectively terminated by
ATP in the rabbit model. The novel equipment may be used to monitor
and terminate VAs in patients at high risk in the future.
However, extensive improvements and further study
are required in order to achieve the clinical use of this device.
Presently, the novel equipment described in the present study is
under development and a number of drawbacks remain to be overcome.
The current system does not provide a defibrillator function. As VF
may be triggered by the ventricular simulation, it would be better
and safer to combine the defibrillator function in the implanted
system. In addition, only one pair of electrodes was used in the
present study. A single ECG monitoring channel occasionally limits
the diagnostic capability. Furthermore, beat-to-beat manual ECG
analysis is a time-consuming process (20,21)
and this system requires integration of an automatic analysis
system. The model switch with a wireless signal transceiver, a
signal transfer device of the extracorporeal system, should be
miniaturized to the size of a mobile phone to enable it to be
easily carried by the patient. Also, internet safety must be
guaranteed to prevent hacker manipulation on the stimulation system
and ensure the safety of patients.
In conclusion, the web-based newborn IECD system
with a real-time remote ECG monitoring and stimulation system
supplies a useful method of creating an animal model, which may be
used in acute and chronic experimental studies concerning the
development of novel drugs and devices for the management of VAs.
Extensive future studies are required to develop novel versions of
the IECD system, which may enable the diagnosis and termination of
VAs.
Acknowledgements
The authors thank Mr. Wen-Liang Gu and Shanghai
Yiliu Education Information Consulting Co., Ltd. (Shanghai, China)
for technical support. This study was supported by grants from the
National Natural Science Foundation of China (grant numbers:
81070154 and 81270258) and the Shanghai Committee of Science and
Technology (grant numbers: 11JC1408200 and 12411951900).
Abbreviations:
|
VA
|
ventricular arrhythmia
|
|
VT
|
ventricular tachycardia
|
|
VF
|
ventricular fibrillation
|
|
IECD
|
implantable electronic cardiovascular
device
|
|
MI
|
myocardial infarction
|
|
S1S1
|
regular stimuli
|
|
S1S2
|
regular stimuli with an added extra
stimulus
|
|
SCD
|
sudden cardiac death
|
|
ATP
|
anti-tachycardia pacing
|
References
|
1
|
Zipes DP, Camm AJ, Borggrefe M, et al;
American College of Cardiology/American Heart Association Task
Force; European Society of Cardiology Committee for Practice
Guidelines; European Heart Rhythm Association; Heart Rhythm
Society. ACC/AHA/ESC 2006 guidelines for management of patients
with ventricular arrhythmias and the prevention of sudden cardiac
death: a report of the American College of Cardiology/American
Heart Association Task Force and the European Society of Cardiology
Committee for Practice Guidelines (writing committee to develop
guidelines for management of patients with ventricular arrhythmias
and the prevention of sudden cardiac death): developed in
collaboration with the European Heart Rhythm Association and the
Heart Rhythm Society. Circulation. 114:e385–e484. 2006.
|
|
2
|
McNally B, Robb R, Mehta M, et al; Centers
for Disease Control and Prevention. Out-of-hospital cardiac arrest
surveillance - Cardiac Arrest Registry to Enhance Survival (CARES),
United States, October 1, 2005 - December 31, 2010. MMWR Surveill
Summ. 60:1–19. 2011.PubMed/NCBI
|
|
3
|
Hayes MM, Berg RA and Otto CW: Monitoring
during cardiac arrest: are we there yet? Curr Opin Crit Care.
9:211–217. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arzbaecher R, Hampton DR, Burke MC and
Garrett MC: Subcutaneous electrocardiogram monitors and their field
of view. J Electrocardiol. 43:601–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giada F, Gulizia M, Francese M, et al:
Recurrent unexplained palpitations (RUP) study: comparison of
implantable loop recorder versus conventional diagnostic strategy.
J Am Coll Cardiol. 49:1951–1956. 2007.PubMed/NCBI
|
|
6
|
Furukawa T, Maggi R, Bertolone C, et al:
Effectiveness of remote monitoring in the management of syncope and
palpitations. Europace. 13:431–437. 2011.PubMed/NCBI
|
|
7
|
De Ruvo E, Gargaro A, Sciarra L, et al:
Early detection of adverse events with daily remote monitoring
versus quarterly standard follow-up program in patients with CRT-D.
Pacing Clin Electrophysiol. 34:208–216. 2011.PubMed/NCBI
|
|
8
|
Perings C, Bauer WR, Bondke HJ, et al:
Remote monitoring of implantable-cardioverter defibrillators:
results from the Reliability of IEGM Online Interpretation (RIONI)
study. Europace. 13:221–229. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sacher F, Probst V, Bessouet M, et al:
Remote implantable cardioverter defibrillator monitoring in a
Brugada syndrome population. Europace. 11:489–494. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spencker S, Coban N, Koch L, et al:
Potential role of home monitoring to reduce inappropriate shocks in
implantable cardioverter-defibrillator patients due to lead
failure. Europace. 11:483–488. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wathen M: Implantable cardioverter
defibrillator shock reduction using new antitachycardia pacing
therapies. Am Heart J. 153(4 Suppl): 44–52. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sweeney MO, Wathen MS, Volosin K, et al:
Appropriate and inappropriate ventricular therapies, quality of
life, and mortality among primary and secondary prevention
implantable cardioverter defibrillator patients: results from the
Pacing Fast VT REduces Shock ThErapies (PainFREE Rx II) trial.
Circulation. 111:2898–2905. 2005. View Article : Google Scholar
|
|
13
|
Roya S and Wang X: Wireless multi-channel
single unit recording in freely moving and vocalizing primates. J
Neurosci Methods. 203:28–40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reek S, Bicknell JL, Walcott GP, et al:
Inducibility of sustained ventricular tachycardia in a closed-chest
ovine model of myocardial infarction. Pacing Clin Electrophysiol.
22:605–614. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Acosta-Lobos A, Riley JP and Cowie MR:
Current and future technologies for remote monitoring in cardiology
and evidence from trial data. Future Cardiol. 8:425–437. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sanchez-Ross M, Oghlakian G, Maher J, et
al: The STAT-MI (ST-Segment Analysis Using Wireless Technology in
Acute Myocardial Infarction) trial improves outcomes. JACC
Cardiovasc Interv. 4:222–227. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Crossley GH, Chen J, Choucair W, et al:
Clinical benefits of remote versus transtelephonic monitoring of
implanted pacemakers. J Am Coll Cardiol. 54:2012–2019. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gasparini M, Anselme F, Clementy J, et al;
ADVANCE CRT-D Investigators. BIVentricular versus right ventricular
antitachycardia pacing to terminate ventricular tachyarrhythmias in
patients receiving cardiac resynchronization therapy: the ADVANCE
CRT-D Trial. Am Heart J. 159:1116–1123. 2010. View Article : Google Scholar
|
|
19
|
Grimm W, Plachta E and Maisch B:
Antitachycardia pacing for spontaneous rapid ventricular
tachycardia in patients with prophylactic
cardioverter-defibrillator therapy. Pacing Clin Electrophysiol.
29:759–764. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singh Alvarado A, Lakshminarayan C and
Principe JC: Time-based compression and classification of
heartbeats. IEEE Trans Biomed Eng. 59:1641–1648. 2012.PubMed/NCBI
|
|
21
|
Ibaida A and Khalil I: Distinguishing
between ventricular tachycardia and ventricular fibrillation from
compressed ECG signal in wireless Body Sensor Networks. Conf Proc
IEEE Eng Med Biol Soc. 2010:2013–2016. 2010.PubMed/NCBI
|