Introduction
In nasopharyngeal carcinoma (NPC), tumors originate
from the epithelial cells that cover the surface of the
nasopharynx. The incidence of NPC is particularly high in Chinese
and Tunisian populations (1). At
diagnosis, 70% of patients have locally advanced, non-metastatic
stage III or IV NPC (2,3). Despite novel advances in
radiotherapy, chemotherapy and gene-targeting agents, the overall
survival rate of patients with aggressive phase NPC remains low
(4).
Although a number of chemotherapeutic drugs are
available for the treatment of cancer, a highly effective and less
toxic approach for treating NPC is required. One potential resource
for a new generation of therapeutic agents targeting the prevention
and treatment of NPC may be natural substances. For example,
epidemiological studies have indicated that the intake of broccoli,
cauliflower and other cruciferous vegetables can significantly
reduce the incidence of numerous types of cancer, including
bladder, pancreas, colon, lung and stomach (5,6).
Indole-3-carbinol (I3C) has been identified as an important
tumoricidal component in cruciferous vegetables, particularly in
members of the genus Brassica (7,8). A
number of studies have demonstrated that I3C induces cell cycle
arrest at the G1 phase in cancer cells (9,10),
promotes apoptosis (9–11) and prevents tumor invasion and
metastasis (11). I3C has been
shown to promote cell cycle arrest by downregulating
cyclin-proteins, including cyclin D1 and E. In addition, IC3 has
been hypothesized to induce apoptosis by mechanisms that depend on
the downregulation of the antiapoptotic genes Bcl-2, Bcl-xL and
survivin, as well as by enhancing the expression of Bax and by
functionally activating caspase-3 and 9 (10–13).
The phosphatidylinositol 3-kinase/serine-threonine
kinase (PI3K/Akt) signaling pathway is involved in the activation
of antiapoptotic mechanisms, glucose metabolism and protein
synthesis, all of which affect cell growth and proliferation
(14,15). Abnormal activation of the PI3K/Akt
pathway has been identified in a number of malignancies. In
addition, it is becoming apparent that tyrosine kinase-mediated
activation of PI3K may be important; for example, in the context of
phosphorylated tyrosine kinases interacting with the p85 subunit or
mutated Ras binding to PI3K, leading to the activation of PI3K
(4).
In addition, somatic mutations may also play an
important role. For example, a mutation in the PTEN
tumor-suppressor gene may disrupt the ability of PTEN to switch off
the PI3K pathway. In addition, PIK3CA gene mutations have been
shown to occur in ~30% of epithelial tumors. The abnormal
activation of PI3K and somatic mutations may collectively drive the
uncontrolled growth and cell proliferation observed during the
development of tumors, including ovarian, breast, pancreatic, lung
and colon cancer (14,16,17).
In the present study, the application of I3C for the
treatment of NPC was investigated in vivo and in
vitro, as well as the potential tumoricidal mechanism. In
addition, the preventive and treatment effects of I3C against NPC
were explored.
Materials and methods
Cell cultures and reagents
The poorly differentiated CNE2 NPC human cell line
was purchased from The Cell Bank of Type Culture Collection of
Chinese Academy of Sciences (Shanghai, China) and maintained in
RPMI-1640 medium containing 10% fetal bovine serum (HyClone
Laboratories, Inc., Logan, UT, USA), 100 U/ml penicillin and 100
mg/ml streptomycin at 37°C in a humidified atmosphere with 5%
CO2. I3C was purchased from Sigma-Aldrich (St. Louis,
MO, USA) and specific pathogen-free (SPF) female BALB/c nude mice
(age, 4 weeks; weight, 18–20 g) were obtained from Beijing HFK
Bioscience Co., Ltd. (Beijing, China).
Cell treatment
I3C was dissolved in dimethyl sulfoxide (DMSO) to
reach a stock concentration of 1 M, which was subsequently diluted
to concentrations of 100, 200 and 300 μM for use in the
experiments. The final concentration of DMSO in the I3C
preparations was <0.5%. In order to demonstrate that the final
concentration of DMSO did not affect the NPC cells, CNE2 cells
cultured in complete medium alone, without exposure to I3C (as the
untreated control) or DMSO (<0.5%), were used as the negative
control group (0 μM).
Cell proliferation and cytotoxicity
assays
CNE2 cells in complete culture medium were seeded
into 96-well plates at a density of 1×103 cells/ml. The
cells were stimulated with I3C at concentrations of 0, 100, 200 or
300 μM in replicates of six wells per I3C concentration. An
additional six wells contained culture medium alone as a blank
control. After 0, 24, 48 and 72 h, cells were treated with 10 μl
cell counting kit (CCK)-8 reagent (Dojindo, Kumamoto, Japan) and
incubated at 37°C for 1 h. An automatic microtiter plate reader was
set to zero using the blank control wells and the absorbance of the
wells was measured at a wavelength of 450 nm. The percentage of
cell proliferation inhibition was calculated using the following
formula: Cell proliferation inhibition (%) = (1 - average
absorbance of the experimental group/average absorbance of the
control group) × 100.
Flow cytometric analysis of apoptotic
cells using annexin V-fluorescein isothiocyanate (FITC) and
propidium iodide (PI)
CNE2 cells in complete culture medium containing 0,
100, 200 or 300 μM I3C, were seeded into 6-well plates. Additional
wells were seeded with culture medium alone as an untreated control
group and each group was seeded in triplicate. After 48 h, the
cells were harvested by centrifugation, resuspended in binding
buffer and successively incubated with 5 μl annexin V-FITC and 5 μl
PI (Multi Sciences Biotech Co., Ltd., Hangzhou, China) at room
temperature for 15 min. Apoptosis was determined by flow cytometric
analysis using a FACSCanto II flow cytometer (BD Biosciences, San
Jose, CA, USA). To construct the flow cytometric dot plot, annexin
V staining was set as the horizontal axis and PI staining was set
as the vertical axis. Mechanically damaged cells were located in
the upper left quadrant, apoptotic or necrotic cells were located
in the upper right quadrant, dual negative and normal cells were
present in the lower left quadrant and early apoptotic cells were
observed in the lower right quadrant of the flow cytometric dot
plot.
Western blot analysis
After 48 h of treatment with various concentrations
of I3C, cells were lysed in radioimmunoprecipitation assay (RIPA)
buffer to extract total cellular protein. Protein concentrations
were determined using the bicinchoninic acid (BCA) quantitative
method and 30-μg samples of each protein were resolved by SDS-PAGE.
Next, the protein bands were transferred to a nitrocellulose
membrane. Following protein transfer, the membrane was blocked for
1 h in the presence of 5% skimmed milk proteins and incubated at
4°C overnight with primary antibodies (Cell Signaling Technology,
Inc., Beverly, MA, USA) targeted against PI3K p110α, PI3K p85,
phosphorylated-Akt (p-Akt), Akt, phosphorylated-glycogen synthase
kinase (p-GSK)3-β, GSK3-β and GAPDH (as an internal reference
housekeeping protein). The blots were then incubated with a
secondary antibody at room temperature for 1 h and specific protein
bands were visualized using an enhanced chemiluminescence assay kit
(Pierce Biotechnology, Inc., Rockford, IL, USA).
Animal selection and breeding
SPF female BALB/c nude mice (age, 4 weeks; weight,
18–20 g) were used. All animal husbandry procedures and experiments
were conducted under SPF conditions. Prior to the experiment,
animals were provided with a conventional diet for 1 week.
Following this adaptive feeding, I3C was incorporated into the
conventional feed at a ratio of 0.5% (w/w). All experimental
procedures in the study were approved by the local Ethics and
Animal Care and Use Committee of Wuhan University (Wuhan,
China).
Establishing tumor-bearing animal models
in BALB/c nude mice
A total of 24 female BALB/c mice were randomly
assigned to pretreatment, treatment and control groups (n=8 mice
per group). CNE2 cells were cultured to a confluence of 80–90%,
harvested by treatment with trypsin and, following centrifugation,
were resuspended in plasma. The cells, at a density of
1×107 cells/ml, were then adoptively transferred into
the mice by the administration of 200-μl subcutaneous injections of
the cell suspension in plasma into the right side of the back of
the nude mice. The pretreatment group received a conventional diet
supplemented with I3C at a ratio of 0.5% (w/w) for 2 weeks prior to
the adoptive CNE2 cell transfer; following injection of the tumor
cells, the mice in this group received conventional feed without
supplementation. The treatment group received a conventional diet
supplemented with I3C at a 0.5% (w/w) ratio following the transfer
of CNE2 cells. The mice continued to receive the I3C supplemented
diet until the experiment had reached the endpoint at week 8. Mice
in the control group were fed conventional feed without
supplementation. For statistical analysis, at the end of each week
following the adoptive transfer of CNE2 cells, the maximum (a) and
minimum (b) diameters of the tumor were measured and the volume was
calculated using the formula: Volume = 1/2ab2. After 8
weeks, the mice were sacrificed by cervical dislocation and
specimens were collected from the tumor, heart, liver and
kidney.
Protein extraction and expression
assays
Tumor specimens from the sacrificed mice were cut
into regularly sized sections and minced using a tissue
homogenizer. Immediately following this preparation, the cells were
lysed in RIPA buffer and the extracted proteins were quantified
using the BCA method. Western blot analysis was applied to detect
the specific protein expression levels of PI3K, Akt and GSK3-β.
Morphological observation of the heart,
liver and kidney tissue sections
Biopsy specimens of the heart, liver and kidney
obtained from the nude mice were fixed in 10% formalin, dehydrated
and embedded in wax. The samples were sectioned into 5–8-μm slices
and pasted onto slides. Next, the specimens were dewaxed and
stained with hematoxylin and eosin (H&E) for final histological
evaluation using a standard light microscope.
Statistical analysis
Statistical analysis was conducted using SPSS
statistical software, version 16.0 (SPSS, Inc., Chicago, IL, USA)
for Microsoft Windows. Results are expressed as the mean ± SD.
Differences between multiple groups were compared by analysis of
variance and P<0.05 was considered to indicate a statistically
significant difference.
Results
I3C treatment markedly inhibits NPC cell
growth
Following treatment of the CNE2 cells with I3C at
concentrations of 100, 200 and 300 μM for various times (24, 48 and
72 h), concentration- and time-dependent inhibition of cell growth
was observed. Statistically significant differences in cell growth
were observed between the treated and negative control groups
(P<0.05; Fig. 1A). In the group
treated with 100 μM I3C, at the indicated time points of 24, 48 and
72 h, the percentage of CNE2 cell proliferation inhibition was
16.1, 20.2 and 18.8%, respectively. In addition, in the group
treated with 200 μM I3C, the percentage of cell proliferation
inhibition at 24, 48 and 72 h was 22.8, 26.0 and 34.2%,
respectively. In the 300 μM I3C group, the percentage of cell
proliferation inhibition at 24, 48 and 72 h was 29.4, 48.4 and
63.9%, respectively (Fig. 1B).
Therefore, inhibiting the proliferation of CNE2 NPC cells with I3C
was highly concentration-dependent.
I3C treatment induces NPC CNE2 cell
apoptosis
Analysis of CNE2 cell apoptosis with annexin V-PI
double staining revealed that after 48 h of treatment with 0, 100,
200 and 300 μM I3C, the apoptotic rates were 0.4±0.25, 2.3±1.35,
18.6±2.32 and 21.0±5.28%, respectively. In addition, when comparing
the 100 and 0 μM concentrations, there was no statistically
significant difference observed in the rate of apoptosis. However,
statistically significant increases were observed in the apoptotic
rates of the 200 and 300 μM groups when compared with those in the
0 μM group (P<0.05). Therefore, a concentration of 100 μM I3C
induced apoptosis of CNE2 cells, while a concentration of 300 μM
increased the proportion of apoptotic cells and exhibited an
apoptotic rate >20% (Fig. 2).
Thus, the results indicated that the proportion of cells undergoing
apoptosis following treatment with I3C was concentration-dependent
(Fig. 2).
I3C treatment significantly reduces the
expression levels of PI3K/Akt-associated proteins in CNE2
cells
The expression levels of key signaling proteins
involved in the PI3K/Akt pathway and downstream signaling proteins
associated with cancer cell proliferation and apoptosis were
analyzed. In the untreated control and negative control groups, the
protein expression of PI3K p110α, PI3K class III, p-Akt,
phosphorylated-c-Raf and GSK3-β was clearly observed. Treatment
with I3C markedly decreased the protein expression levels of PI3K
p110α, PI3K p85, p-Akt, Akt, p-GSK3-β and GSK3-β when compared with
the levels in the untreated groups. In addition, the decreased
expression levels of the signaling proteins was identified to be
I3C concentration-dependent (Fig.
3).
Tumoricidal effects of I3C in a BALB/c
nude mouse model
The tumoricidal effects of I3C were further
investigated in animal model experiments. The control mouse group
received a conventional diet only and these mice subsequently
developed large tumors. At week 8, the average volume of the tumors
was 2,236.7±255.2 mm3. By contrast, the mean volumes of
the tumors in the I3C treatment and pretreatment groups were
1,523.6±143.6 and 1,135.3±236.2 mm3, respectively
(P<0.05). These results indicated that I3C significantly
inhibited tumor growth (P<0.05). In addition, the administration
of I3C prior to tumor transplantation was more effective than I3C
administration following tumor transplantation (P<0.05).
I3C treatment significantly reduces the
expression of PI3K/Akt and downstream signaling proteins in the
transplanted tumors
Expression levels of proteins associated with the
PI3K/Akt pathway and downstream signaling pathways were analyzed in
the transplanted tumors. Pretreatment and treatment with I3C was
found to be associated with reductions in the expression levels of
PI3K p110α, PI3K p85, p-Akt and p-GSK3-β signaling proteins. The
lowest expression levels of signaling proteins were detected in the
pretreatment group, which was followed by the treatment group. The
highest expression levels of signaling proteins were identified in
the control group (Fig. 4).
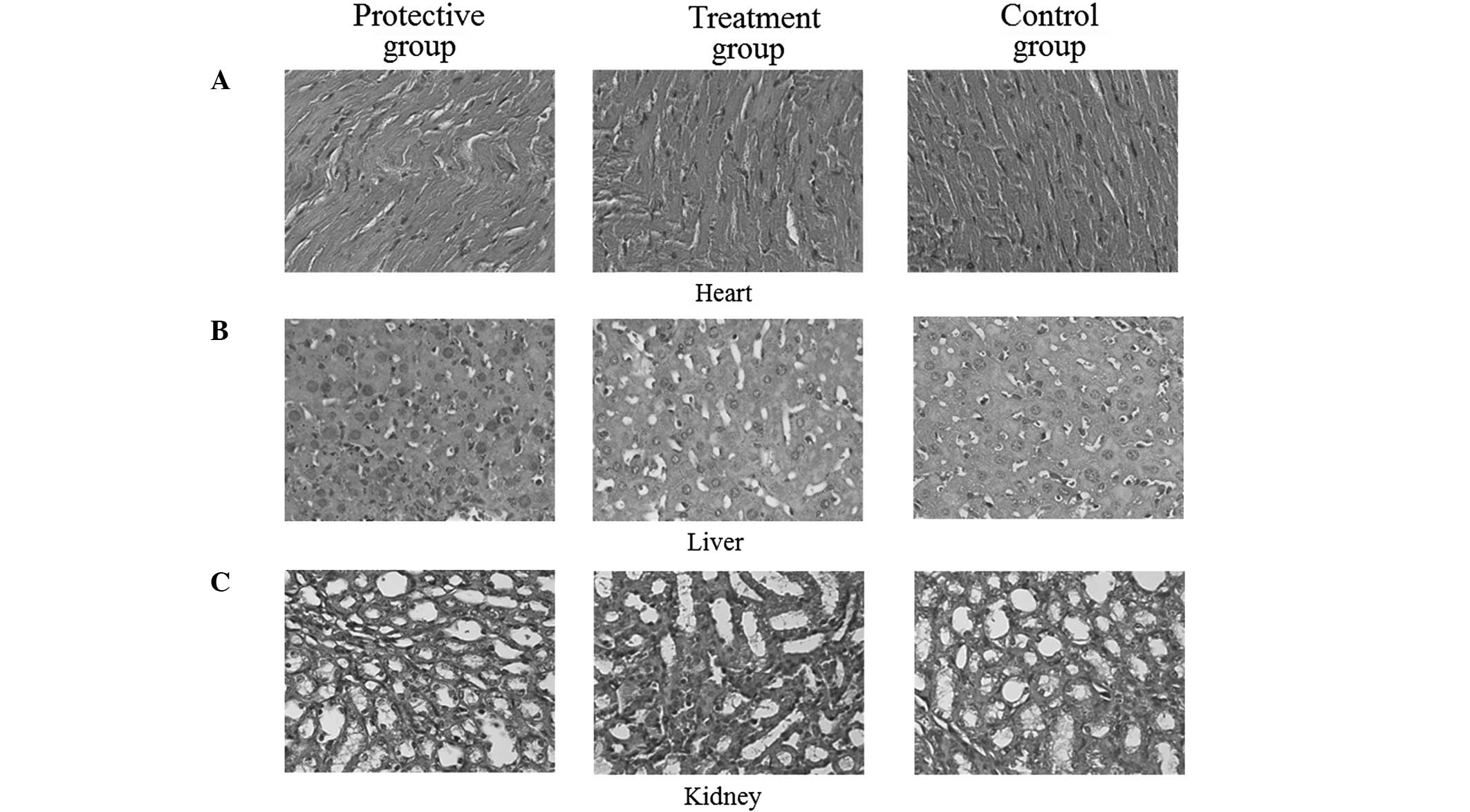
I3C does not produce harmful side-effects
in the heart, liver and kidney of nude mice
Specimens from the heart, liver and kidney obtained
from the nude mice were sectioned and stained with H&E.
Pathological evaluation revealed that treatment with I3C exerted no
adverse effects on the heart, liver or kidney in the nude mice. No
tissue damage, including structural degeneration or tissue
necrosis, was observed in any of the tissue specimens. These
results indicated that supplementing the feed with 0.5% (w/w) I3C
did not provoke any toxic side-effects in the examined organs of
the experimental mice (Fig.
5).
Discussion
I3C is extracted from cruciferous vegetables by the
hydrolysis of glucosinolates (7).
A number of previous in vitro studies have demonstrated that
I3C causes significant growth inhibition in a variety of tumor cell
lines by inducing cell cycle arrest and apoptosis (9,11,13,18,19).
However, the role of I3C in NPC has remained largely unexplored.
The observations of the present study revealed that I3C
significantly inhibited the proliferation of CNE2 cells with an
optimal concentration of 300 μM; at this concentration, the
inhibition rate of tumor cell proliferation was 29.4, 48.4 and
63.9% observed at 24, 48 and 72 h, respectively. The inhibitory
mechanism of I3C involves altering the proliferation of cancer
cells and the induction of apoptosis. In the present study, 200 μM
I3C was shown to induce an apoptotic effect. In addition, the
apoptotic rate increased in an I3C concentration-dependent manner.
Rahman et al (11) reported
that treatment of breast cancer cells with 30–100 μM I3C for 24–72
h induced apoptosis.
The PI3K/Akt pathway is associated with receptor
tyrosine kinase signaling pathways. Mutational activation of
pathway components may inappropriately activate PI3K and the
downstream target proteins, Akt and p-Akt, subsequently resulting
in the phosphorylation and activation of mTOR/GSK3, mouse mdm2, Bad
and members of the caspase family that collectively play an
important role in promoting tumor cell growth, proliferation,
suppression of apoptosis, promotion of cellular invasion, tumor
metastasis and angiogenesis (20).
Abnormalities in the PI3K/Akt signaling pathway and in particular,
abnormal phosphorylation of Akt, have been found to be closely
associated with the development of tongue carcinoma and head and
neck squamous cell carcinomas (17,21).
To further clarify the potential association between
I3C-induced apoptosis and the PI3K/Akt signaling pathway in
nasopharyngeal cancer, the differential expression of PI3K/Akt
pathway-specific signaling proteins and their cognate downstream
proteins were analyzed prior to and following I3C treatment.
Markedly decreased expression levels of PI3K p110α, PI3K p85, p-Akt
and p-GSK3-β were observed in CNE2 cells following treatment with
I3C, as compared with the levels in the untreated cells. This
observation indicates that the inhibition of NPC cell proliferation
and the induction of apoptosis are closely associated with the
PI3K/Akt signaling pathway. The results of the present study are in
accordance with previous studies of breast and prostate cancer
(9,21).
Previous animal studies (17,18)
have demonstrated that I3C inhibits the occurrence and development
of solid tumors. In addition, previous studies on cervical cancer
have revealed that the antitumor mechanism of I3C is partly
dependent on an increase in permeability of the tumor cell
membrane, activation of caspase-3 and, thus, induction of cancer
cell apoptosis (22). When mice
with prostate cancer (TRAMP model) were fed diets containing 1%
(w/w) I3C over a period of 10 weeks, tumor cell apoptosis was
induced (23) and Nrf2, which is
involved in a pathway known to regulate antioxidant signaling
pathways, was activated. Newfield et al (24) reported that the immune response of
mice fed I3C may be triggered to produce the antiproliferative
agent, 2-hydroxy estrone. The animal experiments of the present
study confirmed that I3C effectively inhibited the growth of NPC
and that I3C intervention significantly decreased NPC growth in
nude mice when compared with the controls. The graft tumor growth
rate was slower in the pretreatment group compared with that in the
treated mouse group, indicating that I3C effectively prevents the
occurrence and development of solid tumors. This observation also
indicates that I3C may be considered as an alternative therapeutic
approach for the prevention and treatment of NPC.
Consistent with the results obtained from the in
vitro experiments, in the pretreated and treated nude mice, the
expression levels of PI3K p110α, PI3K p85, p-Akt and p-GSK3-β were
shown to be significantly downregulated when compared with those in
the control group. This observation indicates that abnormal
activation of the PI3K/Akt pathway may play a key role in the
process of NPC. The animal experiments also confirmed that I3C
downregulated the protein expression of PI3K/Akt signaling pathway
proteins in the transplanted tumors.
Using I3C to treat MCF10CA1a breast cancer cells and
homologous CF10A mammary epithelial cells, Rahman et al
(11) found that I3C induced
apoptosis only in the MCF10CA1a breast cancer cells, indicating
that I3C was without risk to nontumor cells. Consistent with this,
the current study also confirmed that I3C caused growth inhibition
in the transplanted tumors of nude mice only and did not provoke
any degenerative harm to the heart, liver and kidney, with low
cytotoxicity to normal cells.
In summary, the in vivo and in vitro
experiments of the present study have demonstrated that I3C
significantly inhibits NPC proliferation and induces apoptosis. We
hypothesize that the observed effects of I3C are likely to be
associated with regulation of the PI3K/Akt signaling pathway and
downstream protein expression. Due to the effective, non-toxic and
natural antitumor properties of I3C, the compound should be
considered as a likely preventive and curative candidate for NPC.
Additional studies are required to determine the underlying
mechanisms by which I3C suppresses NPC at a molecular level,
providing a theoretical basis for the tumoricidal and clinical
utility of I3C.
Acknowledgements
The study was supported by grants from the National
Natural Science Foundation of China (no. 30901662) and the Science
and Technology Program of Wuhan City (nos. 200951199455 and
200950431168).
References
|
1
|
Brennan B: Nasopharyngeal carcinoma.
Orphanet J Rare Dis. 1:232006. View Article : Google Scholar
|
|
2
|
Afqir S, Ismaili N and Errihani H:
Concurrent chemoradiotherapy in the management of advanced
nasopharyngeal carcinoma: current status. J Cancer Res Ther. 5:3–7.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Razak AR, Siu LL, Liu FF, Ito E,
O’Sullivan B and Chan K: Nasopharyngeal carcinoma: the next
challenges. Eur J Cancer. 46:1967–1978. 2010. View Article : Google Scholar
|
|
4
|
Zhou JX, Han JB, Chen SM, Xu Y, Kong YG,
Xiao BK and Tao ZZ: γ-secretase inhibition combined with cisplatin
enhances apoptosis of nasopharyngeal carcinoma cells. Exp Ther Med.
3:357–361. 2012.
|
|
5
|
Annema N, Heyworth JS, McNaughton SA,
Iacopetta B and Fritschi L: Fruit and vegetable consumption and the
risk of proximal colon, distal colon, and rectal cancers in a
case-control study in Western Australia. J Am Diet Assoc.
111:1479–1490. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Larsson SC, Håkansson N, Näslund I,
Bergkvist L and Wolk A: Fruit and vegetable consumption in relation
to pancreatic cancer risk: a prospective study. Cancer Epidemiol
Biomarkers Prev. 15:301–305. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Keck AS and Finley JW: Cruciferous
vegetables: cancer protective mechanisms of glucosinolate
hydrolysis products and selenium. Integr Cancer Ther. 3:5–12. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shertzer HG and Senft AP: The
micronutrient indole-3-carbinol: implications for disease and
chemoprevention. Drug Metabol Drug Interact. 17:159–188. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chinni SR, Li Y, Upadhyay S, Koppolu PK
and Sarkar FH: Indole-3-carbinol (I3C) induced cell growth
inhibition, G1 cell cycle arrest and apoptosis in prostate cancer
cells. Oncogene. 20:2927–2936. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brew CT, Aronchik I, Hsu JC, et al:
Indole-3-carbinol activates the ATM signaling pathway independent
of DNA damage to stabilize p53 and induce G1 arrest of human
mammary epithelial cells. Int J Cancer. 118:857–868.
2006.PubMed/NCBI
|
|
11
|
Rahman KM, Aranha O and Sarkar FH:
Indole-3-carbinol (I3C) induces apoptosis in tumorigenic but not in
nontumorigenic breast epithelial cells. Nutr Cancer. 45:101–112.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meng Q, Qi M, Chen DZ, et al: Suppression
of breast cancer invasion and migration by indole-3-carbinol:
associated with up-regulation of BRCA1 and E-cadherin/catenin
complexes. J Mol Med (Berl). 78:155–165. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aggarwal BB and Ichikawa H: Molecular
targets and anticancer potential of indole-3-carbinol and its
derivatives. Cell Cycle. 4:1201–1215. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang F, Lee JT, Navolanic PM, et al:
Involvement of PI3K/Akt pathway in cell cycle progression,
apoptosis, and neoplastic transformation: a target for cancer
chemotherapy. Leukemia. 17:590–603. 2003. View Article : Google Scholar
|
|
15
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004.PubMed/NCBI
|
|
16
|
Watanabe S, Sato K, Okazaki Y, Tonogi M,
Tanaka Y and Yamane GY: Activation of PI3K-AKT pathway in oral
epithelial dysplasia and early cancer of tongue. Bull Tokyo Dent
Coll. 50:125–133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oganesian A, Hendricks JD and Williams DE:
Long term dietary indole-3-carbinol inhibits
diethylnitrosamine-initiated hepatocarcinogenesis in the infant
mouse model. Cancer Lett. 118:87–94. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Plate AY and Gallaher DD: Effects of
indole-3-carbinol and phenethyl isothiocyanate on colon
carcinogenesis induced by azoxymethane in rats. Carcinogenesis.
27:287–292. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pappa G, Lichtenberg M, Iori R, Barillari
J, Bartsch H and Gerhäuser C: Comparison of growth inhibition
profiles and mechanisms of apoptosis induction in human colon
cancer cell lines by isothiocyanates and indoles from Brassicaceae.
Mutat Res. 599:76–87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brognard J, Clark AS, Ni Y and Dennis PA:
Akt/protein kinase B is constitutively active in non-small cell
lung cancer cells and promotes cellular survival and resistance to
chemotherapy and radiation. Cancer Res. 61:3986–3997.
2001.PubMed/NCBI
|
|
21
|
Rahman KM, Li Y and Sarkar FH:
Inactivation of akt and NF-kappaB play important roles during
indole-3-carbinol-induced apoptosis in breast cancer cells. Nutr
Cancer. 48:84–94. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen DZ, Qi M, Auborn KJ and Carter TH:
Indole-3-carbinol and diindolylmethane induce apoptosis of human
cervical cancer cells and in murine HPV16-transgenic preneoplastic
cervical epithelium. J Nutr. 131:3294–3302. 2001.
|
|
23
|
Wu TY, Saw CL, Khor TO, Pung D,
Boyanapalli SS and Kong AN: In vivo pharmacodynamics of
indole-3-carbinol in the inhibition of prostate cancer in
transgenic adenocarcinoma of mouse prostate (TRAMP) mice:
involvement of Nrf2 and cell cycle/apoptosis signaling pathways.
Mol Carcinog. 51:761–770. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Newfield L, Goldsmith A, Bradlow HL and
Auborn K: Estrogen metabolism and human papillomavirus-induced
tumors of the larynx: chemo-prophylaxis with indole-3-carbinol.
Anticancer Res. 13:337–341. 1993.PubMed/NCBI
|