Introduction
Adult stem cells are important in the research and
clinical treatment of numerous diseases (1). Adult stem cells are capable of
trans-system and trans-mesoderm differentiation, and, thus, these
cells have been extensively studied for a variety of diseases,
including erectile dysfunction (ED) (2,3).
However, the characterization and distribution of endogenous stem
cells in cavernosum tissue have not been fully elucidated, and
instead exogenous stem cells are utilized for investigations into
cellular therapeutics for ED (4,5).
These exogenous cells include embryonic stem cells, mesenchymal
stem cells, adipose stem cells, muscle-derived stem cells (MDSCs)
and neural crest stem cells (6).
Although there are several different types of exogenous cells that
may be used, the development of these cells has been limited due to
the complexity and invasiveness of the treatment process (7,8).
Non-invasive methods and investigations into the expression of
endogenous stem cells in the cavernosum are therefore paramount for
the development of successful cellular ED interventions (9).
The functional unit for erection is smooth muscle
fibers and the sinusoidal endothelial cell system (10). Smooth muscle accounts for 40–50% of
cavernosum tissue and is important in erectile function (11,12).
Due to their ability to undergo multipotent differentiation,
cavernosum MDSCs were selected as the target cells in the present
study. MDSCs have been widely used in studies investigating the
treatment of muscular dystrophy (8), heart disease (13), stress urinary incontinence
(14), neurogenic bladder
(15) as well as bone and nervous
system diseases (16,17). However, despite the widespread use
of MDSCs, treatments for organic ED by interfering with and
regulating the expression of cavernosum MDSCs have not been
reported.
The aim of the present study was to investigate the
existence of cavernosum MDSCs. MDSCs are primarily obtained through
short-cycle enzymatic digestion, which has the advantage of easy
access and low contamination rates. The pre-plate differential
adhesion technique was used for separation and purification
(4,18). Since specific markers for the
identification of MDSCs remain unavailable, frequently used stem
cell markers, for example, stem cell antigen-1 (Sca-1), Oct4 and
Desmin, were utilized (4,19,20).
Immunohistochemistry and reverse transcription polymerase chain
reaction (RT-PCR) were used to detect the expression of Sca-1 and
Desmin in the cavernosum of rats of varying ages, as well as to
investigate the distribution of MDSCs in the cavernosum tissue. The
enzymatic digestion method and an improved pre-plate differential
adhesion method were used to separate and purify cavernosum cells
of rats. Immunofluorescence cytochemistry, flow cytometry and
western blot analysis were performed to detect the expression of
Sca-1, Oct4 and Desmin in adherent cells, and to further examine
the associated techniques for initial separation of MDSCs. The
results from the present study may provide an experimental basis
for further subculture of MDSCs and induced differentiation, and
provide potential therapeutic strategies for stem cell regulation
therapy of organic ED.
Materials and methods
Animals and treatments
A total of 10 male Sprague-Dawley (SD) rats (clean
grade), aged 2, 5 and 20 months were randomly selected from
different nests, with average body weights of 180, 370 and 520 g,
respectively. The rats were purchased from the Center of
Experimental Animals at Soochow University (Suzhou, Jiangsu, China)
and they were divided into young, middle-aged and old groups
according to their age. The rats were subjected to anesthesia by
ether inhalation and an incision was then made in the inferior
portion of the abdomen. The penile tissues were carefully separated
and dissected. The penis head (including the penis cartilage) and
urethral sponge were removed and the corpus cavernosum was
collected. Following rinsing with normal saline, the tissues were
divided into two parts. The first part was immersed in 10% neutral
formalin solution for fixation, hematoxylin and eosin (H&E)
staining and immunohistochemical analysis. The second part was
immediately placed in liquid nitrogen for storage and further
analysis using RT-PCR. The current study was performed in
accordance with the approved animal protocols and guidelines
established by Medicine Ethics Review Committee of The Second
Affiliated Hospital of Soochow University for the care and use of
studied animals. All animals were given humane care in compliance
with the Guide for the Care and Use of Laboratory Animals, National
Institutes of Health (NIH Publication No. 85-23, revised 1996).
Immunohistochemical analysis
Three serial sections (4 μm thick) of the cavernosum
tissues were dehydrated and embedded in paraffin. The EnVision
method was used for single immunohistochemical labeling of Sca-1
and Desmin, as well as for double immunohistochemical labeling of
Sca-1/Desmin. Phosphate-buffered saline (PBS) was used instead of
the primary antibody as the blank control.
RT-PCR
The primer sequences were designed using Primer
Premier 5.0 software (Premier Biosoft International, Palo Alto, CA,
USA) and the housekeeping gene β-actin was used as an internal
reference (Table I). The RT-PCR
reaction system was prepared as follows: 15 μl 2X RT buffer, 1 μl
primers (100 pmol/μl), 1 μl RTase, 6 μl RNA template and 7 μl
diethylpyrocarbonate (DEPC)-treated water (the total volume was 30
μl). The reaction conditions were as follows: 25°C for 10 min, 40°C
for 60 min and 70°C for 10 min. Preparation of the fluorescence
quantitative PCR reaction system was as follows: 25 μl 2X PCR
buffer, 0.6 μl primers (25 pmol/μl, ×2), 1 μl cDNA template and
22.8 μl DEPC-treated water (the total volume was 50 μl). The
amplification conditions were as follows: 94°C pre-denaturation for
3 min, 94°C for 25 sec, 60°C for 25 sec and 72°C for 25 sec for 35
cycles. Agarose gel electrophoresis (2%) was used for product
analysis.
 | Table IPrimer sequences for the detection of
mRNA expression level and product length. |
Table I
Primer sequences for the detection of
mRNA expression level and product length.
| Gene | Sense | Anti-sense | Product length
(bp) |
|---|
| β-actin |
CCCATCTATGAGGGTTACGC |
TTTAATGTCACGCACGATTTC | 150 |
| Sca-1 |
AACCATATTTGCCTTCCCGTCT |
CCAGGTGCTGCCTCCAGTG | 135 |
| Desmin |
CTTGATGAGGCAGATGAGGA |
AGCTTCCGGTAGGTGGCAAT | 192 |
Digestion and separation of corpus
cavernosum cells
Following anethesia, the penile tissue was collected
under sterile conditions from the 2-month-old male SD rats. All
surgical procedures were performed on an ultra-clean bench. PBS was
used to rinse the tissues three times. The penis skin, subcutaneous
fascia, urethra and albuginea were then carefully removed using
sterile ophthalmic scissors, and the corpus cavernosum tissues were
stored. The cavernous tissues were cut into small 1–2
mm3 sections and enzymes were used to digest and
separate the cells. The tissue sections were first digested with
type I collagenase (0.5%) at a constant temperature for 3 h.
Trypsin (0.1%) was then added in the same volume and the tissues
were digested for a further 30 min. The cells were then observed
under a microscope (Axiovert 100, Zeiss, Oberkochen, Germany) and
high glucose Dulbecco’s modified Eagle’s medium (DMEM) containing
10% fetal calf serum (FCS; volume fraction; volume fraction was
identical to volume concentration in ideal solutions, where the
constituents volumes are additive that is equal to the total
volumes of its ingredients) was added to terminate the digestion
when nearly all the cells were dispersed into single cells. The
solution was then filtered using a 200-mesh filter and 200 g of
filtrate was collected and centrifuged at 200 × g for 10 min. The
supernatant was discarded and the cell pellet was resuspended in
DMEM containing 20% FCS (volume fraction). The cell concentration
was adjusted and inoculated in the cell culture bottles and the
cells were incubated at 37°C and 5% CO2 (volume
fraction).
Purification and culture of cells
The contaminated cells were removed using the
pre-plate differential adhesion method (21). The cells were cultured for 1 h and
the adherent cells were considered pre-plate 1 (PP1). The
non-adherent cells were cultured in a new culture bottle for a
further 2 h and the adherent cells were considered pre-plate 2
(PP2). The non-adherent cell suspension was transferred into a new
bottle and cultured for a further 18 h, and the subsequent adherent
cells were considered pre-plate 3 (PP3). Following this, transfer
bottle cultures were then performed every 24 h and the cells were
successively identified as pre-plate 4, 5 and 6, respectively (PP4,
PP5 and PP6). PP6 cells began to adhere to the wall following 2–3
days and the culture solution was changed once a day. The cells
were observed under an inverted microscope and divided and cultured
in different bottles when the cell confluence approached 50%. These
cells were then used for the subsequent experiments.
Flow cytometric analysis
PP6 cells were collected and the cell concentration
was adjusted to 1×1010/l. Reagent A (100 μl) was added
for fixation for 15 min and the cells were rinsed once with PBS.
The cells were subsequently centrifuged and collected and the
primary antibodies (Sca-1, Oct4 and Desmin; 1:50) were added. The
immunoglobulin G (IgG) corresponding to the primary antibody was
added to the control group and fully mixed with the cells. The
cells were incubated at room temperature for 40 min and then washed
twice with PBS prior to the addition of fluorescein isothiocyanate
(FITC)-labeled secondary antibody corresponding to the primary
antibody (1:50). The cells were incubated at room temperature in
the dark for 30 min. Subsequently, the cells were washed once with
PBS and 500 μl PBS was added. A flow cytometer was then used to
detect the positive cell count.
Immunofluorescence cytochemical
identification
The PP6 cells were inoculated in a six-well plate
with a cover glass. After the cells adhered to the cover glass, the
growth cover glasses were prepared. A total of 40 g/l
paraformaldehyde was used to fix the cells for 2 h and the cells
were then immersed and rinsed with PBS. Triton X-100 (1%) was added
and the cells were incubated at room temperature for 15 min. The
cells were then immersed and rinsed again with PBS. The growth
cover glasses were treated with 3%
H2O2-methanol solution (volume fraction) for
15 min following which the cells were then immersed and rinsed with
PBS. A total of 100 μl primary antibody was added (Sca-1, embryonic
antigen and Desmin; 1:50) and the cells were incubated at 37°C for
2 h. The two types of primary antibodies were added simultaneously
at the same time as Sca-1/Oct4, Sca-1/Desmin and Oct4/Desmin double
immunofluorescence cytochemical analysis. PBS was used instead of
the primary antibody as the negative control and the cells were
immersed and rinsed with PBS. Then, 100 μl FITC-labeled secondary
antibody (1:200) corresponding to the primary antibody was added
and the cells were incubated at 37°C for 1 h. The two types of
secondary antibodies, corresponding to the primary antibody, were
added simultaneously at the same time as the double-label
immunofluorescence method. PBS was used to immerse and rinse the
cells and the mounting medium preventing fluorescence quenching was
used. The specimens were then observed and images were captured
under a fluorescence microscope (Carl Zeiss MicroImaging Inc.,
Thornwood, NY, USA). The images were processed using Image-Pro Plus
software (Media Cybernetics, Silver Spring, MD, USA).
Western blot analysis
PP1–PP6 cells were collected and a total protein
extraction kit was used to extract the proteins in the different
cell samples. The Bradford method was used to determine the protein
concentrations of different samples and the loading amount was
adjusted. Electrophoresis was performed using an SDS-PAGE gel. The
proteins were transferred onto a PVDF membrane and inhibited using
the blocking buffer. Following this, the primary antibody (Sca-1,
Oct4 and Desmin; 1:400) was added and the membranes were incubated
on a shaker at 4°C overnight. The secondary antibody [horseradish
peroxidase (HRP)-goat anti-rabbit IgG and HRP-sheep anti-mouse IgG;
1:5,000 dilution with blocking buffer] was then added after the
membrane was rinsed and exposure was performed which was
developed.
Determination of positive results
The immunohistochemical results were collected from
the different groups and analyzed under a microscope
(magnification, ×400; JEOL, Model JSM-7600F). The positive staining
result for single labeling was brown and the positive staining
result for double labeling was dark brown.
Statistical analysis
The data are presented as the mean ± standard
deviation. T-tests were used to determine differences between
groups and P<0.05 was considered to indicate a statistically
significant difference. Spearman rank correlation was used for the
correlation analysis and P<0.05 was considered to indicate a
statistically significant difference.
Results
H&E staining
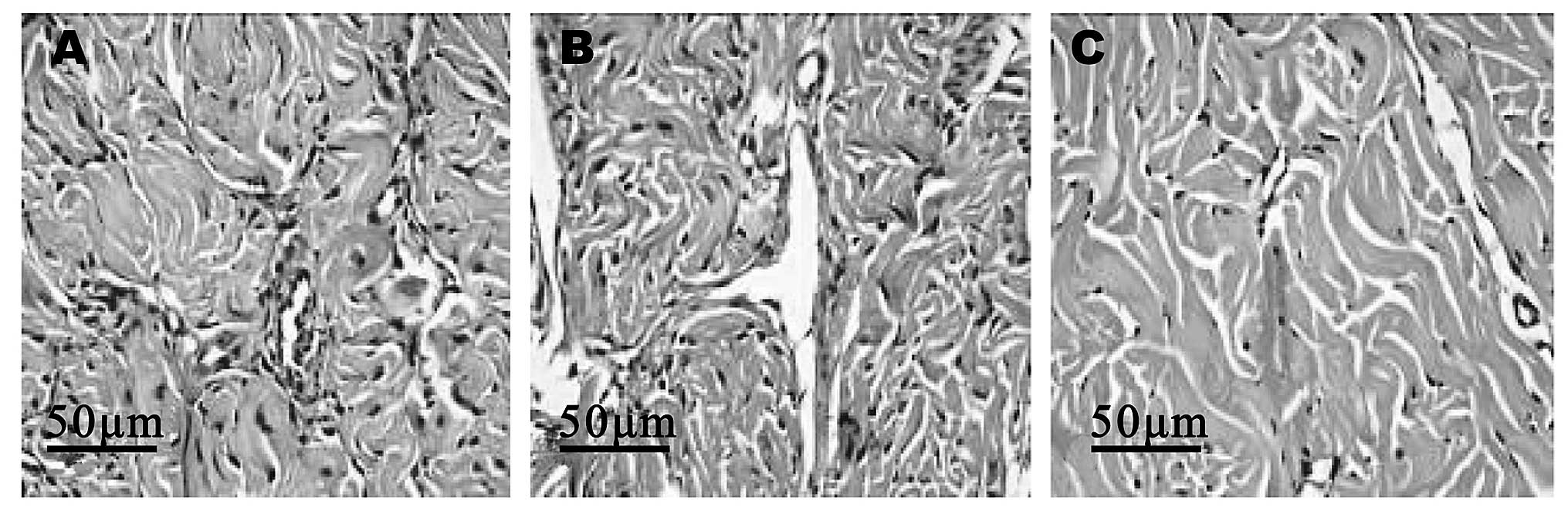
The corpus cavernosum of the rats gradually changed
from tightly organized structures to loosely organized structures
as the age of the rats increased. Similarly, the blood vessels were
found to be abundant, slightly decreased and significantly
decreased in the young, middle-aged and old rats, respectively
(Fig. 1).
Immunohistochemical results
The immunohistochemical results demonstrated that
Sca-1 was predominantly expressed in the blood vessels and
cavernous sinus, and demonstrated primarily cytoplasmic staining.
By contrast, Desmin was expressed primarily in muscular tissues and
staining demonstrated that Desmin was mainly expressed in the
cytoplasm, however, it was also partially expressed in the nuclei.
A small number of double positively stained cells (Sca-1/Desmin)
were also detected near the cavernous sinus. A statistically
significant difference in the expression of Sca-1 and Desmin
between the different age groups was also observed (P<0.05).
Expression of the markers was found to be negatively correlated
with the age of the rats (P<0.05; Fig. 2 and 3).
RT-PCR results
The results from the RT-PCR demonstrated that the
expression levels of Sca-1 and Desmin significantly decreased with
age (P<0.05). Significant differences in the concentrations of
the markers were identified between the different age groups
(P<0.05; Table II), which was
in accordance with the results from the immunohistochemical
analysis.
 | Table IIExpression levels of Sca-1 and Desmin
in the corpus cavernosum of rats from different age groups. |
Table II
Expression levels of Sca-1 and Desmin
in the corpus cavernosum of rats from different age groups.
| Group | Young group | Middle-aged
group | Old group |
|---|
| Sca-1 | 0.55±0.07 | 0.27±0.04a | 0.14±0.02a,b |
| Desmin | 3.40±0.31 | 2.10±0.23a | 1.10±0.24a,b |
Correlation analysis
The results from the correlation analysis indicated
that the expression of Sca-1 was significantly and negatively
correlated with the age of the rats (r=−0.929; P<0.05).
Similarly, the same result was observed for Desmin (r=−0.924;
P<0.05).
Cell isolation and flow cytometry
PP1 and PP2 cells adhered to the wall rapidly and
they were primarily long spindle-shaped fibrous cells. The adherent
capability of PP3–PP5 cells successively decreased and in these
plates, short spindle-shaped cells were observed. Several of these
cells were polygon-shaped and primarily consisted of vascular
endothelial and smooth muscle cells. A number of the PP6 cells were
small, round floating cells. These PP6 cells slowly adhered to the
wall and became round or spindle-shaped following 2–3 days
(Fig. 4).
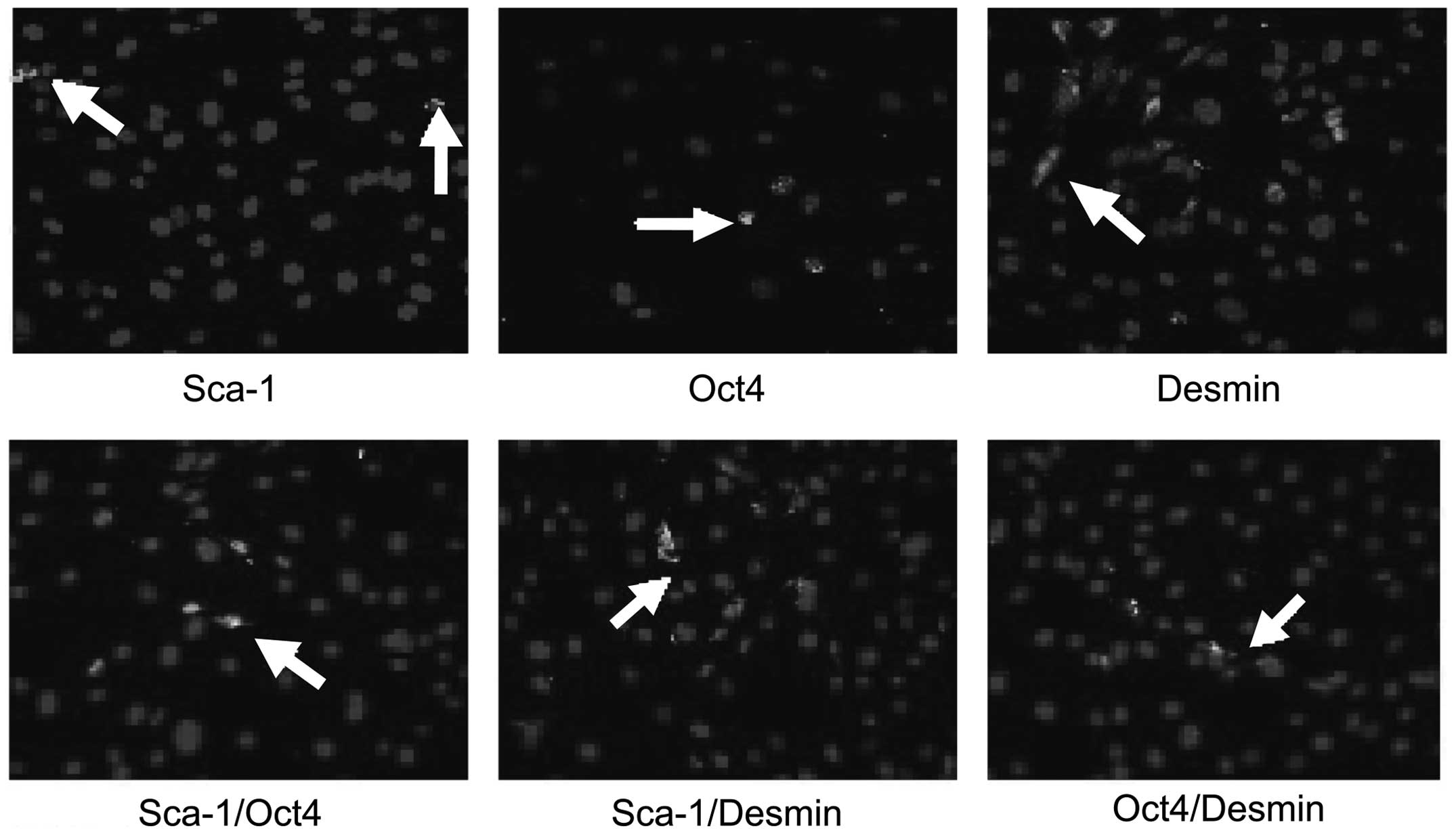
Immunofluorescence cytochemistry
The expression of Sca-1, Oct4 and Desmin was
detected in PP6 cells, however, the expression of Sca-1 and Oct4
was only detected in a few cells. The expression of Sca-1, Oct4 and
Desmin in PP6 cells was 5.7, 2.6 and 41.2% respectively (Fig. 5). Sca-1 and Desmin were primarily
expressed in the cytoplasm, whilst Oct4 was primarily expressed in
the nuclei (Fig. 6). A small
number of cells expressed Sca-1/Oct4, Sca-1/Desmin and Oct4/Desmin.
Sca-1/Oct4 were expressed in the cytoplasm and the nuclei, whilst
Sca-1/Desmin were primarily expressed in the cytoplasm. Oct4/Desmin
were mainly expressed in the nuclei and the cytoplasm.
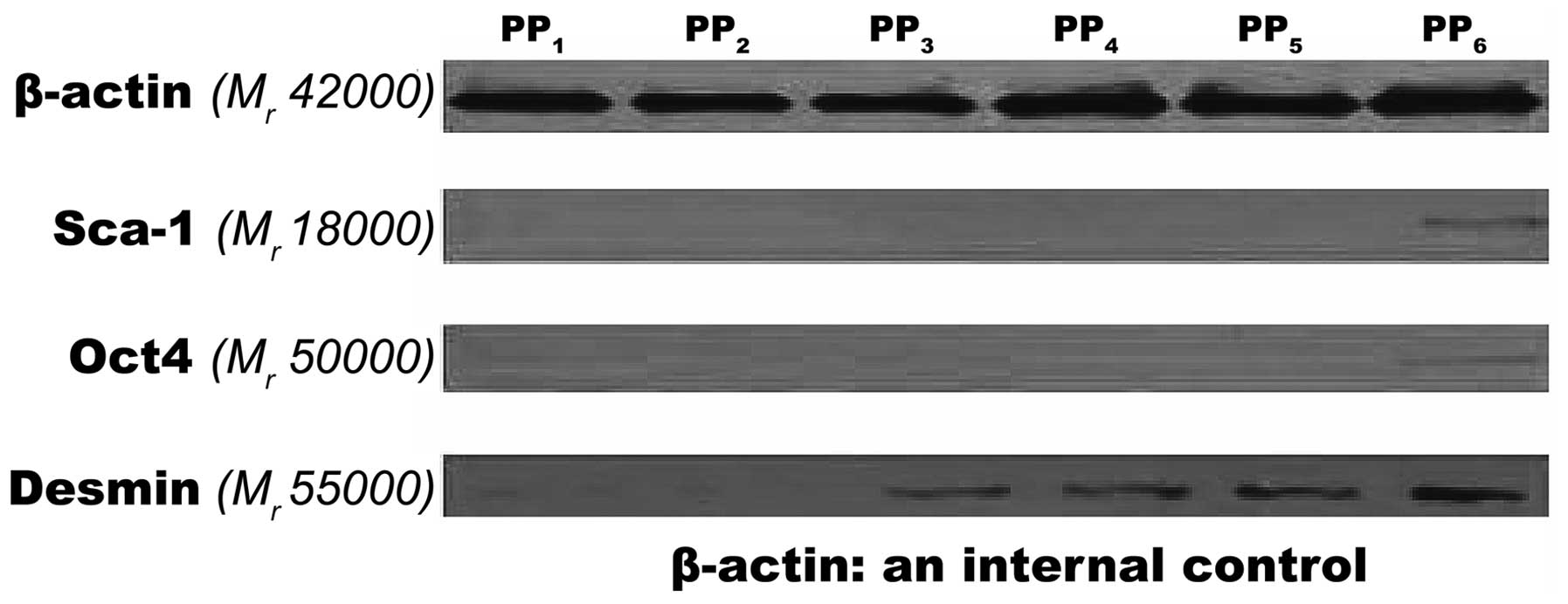
Western blot analysis
When the total amount of the proteins was
equivalent, significant expression levels of Sca-1 and Oct4 were
not detected in the PP1–PP5 cells. However, the expression of Sca-1
and Oct4 was detected in the PP6 cells and the concentrations were
lower than the internal reference. A very low expression level of
Desmin was detected in PP1–PP2 cells, while its expression level
significantly increased in PP3–PP6 cells (Fig. 6).
Discussion
The present study investigated the expression and
distribution of Sca-1, Desmin and Sca-1/Desmin in the corpus
cavernosum of rats. It was found that the expression levels were
significantly negatively correlated with the age of the rats,
suggesting that the expression of these markers gradually decreases
with age. As part of this investigation, the corpus cavernosum
cells were also isolated and purified to determine the expression
of Sca-1, Oct4, Desmin, Sca-1/Oct4, Sca-1/Desmin and Oct4/Desmin.
These data provide evidence for further subculture and
amplification of MDSCs, and also provide evidence for possible
therapeutic strategies for non-invasive stem cell regulatory
therapy for organic ED.
In the present study, important techniques were used
to isolate MDSCs in corpus cavernosum tissue. The corpus cavernosum
tissues were processed using enzymatic digestion. The modified
corpus pre-plate differential adhesion technique was further
utilized to isolate and purify the MDSCs as described previously by
Zuba-Surma et al (22).
This technique provides a homogenous sample of stem cells (23). Fibroblasts and collagen fiber cells
have the greatest adhesive capability; therefore, these cells were
primarily isolated on the plate. Since the adhesive capability of
vascular endothelial cells and smooth muscle cells is relatively
low, these cells adhere on later plates. MDSCs have the lowest
adhesion capability, therefore, following continuous six-step
differential adhesion, the majority of impure cells were removed.
Detection efficiency can be improved in the final sample of cells.
The isolated PP6 cells in the present study were small and round or
short fusiform-shaped cells and their adhesive capability was
relatively weak, which was consistent with the characteristics of
MDSCs.
In the present study, the expression of Sca-1 and
Oct4 was detected in cavernous tissue and cells, and the results
obtained were consistent with previous studies by Zuba-Surma et
al and Ho et al (22,24)
investigating non-corpus cavernosum-derived MDSCs. The techniques
presented in the present study may be used with previously
established methods to identify corpus cavernosum stem cells and
improve the identification rate of these stem cells.
Previous studies have indicated that that the
positive rate of Desmin in MDSCs can be as high as 90%. Therefore,
Desmin is frequently used for the identification of muscle-derived
MDSCs (25). In the present study,
very few double positive cells (Sca-1/Desmin) were detected near
the cavernous sinus. The double positive cells (Sca-1/Oct4,
Sca-1/Desmin and Oct4/Desmin) were successfully isolated, which
further confirmed that MDSCs existed in the stem cells from corpus
cavernosum. However, it is important to note that endothelial,
vascular, neurological and other factors are also important in the
pathogenesis of organic ED (26).
Whilst MDSCs possess the multiple differentiation capability of
stem cells, they not only differentiate into myogenic cells, but
also have the potential to differentiate into endothelial, vascular
and neural cells. Therefore, these cells may be important in future
clinical treatment approaches.
Another important finding from the present study was
the association between the expression of the markers and the age
of the rats. The expression of the markers was significantly
decreased in the old group compared with the young group of rats.
This suggests that the collection of stem cells from the corpus
cavernosum of rats should be performed on young rats. In addition,
the efficacy of regulatory therapy of ED using endogenous stem
cells may be closely associated with the age of patients. The
therapeutic efficacy may be higher in middle-aged and younger
patients compared with older patients.
The present study provides an important foundation
for future studies that target cellular treatments for ED. In the
present study, corpus cavernosum MDSCs were detected and isolated
on the tissue level, which is a promising first step for future
treatments for ED using endogenous stem cells. However, the
subculture amplification, multiple-direction induced
differentiation and functional tests on animal models require
further investigation before conclusions can be made regarding the
viability MDSCs. The incidence of diabetic-associated ED has
increased in recent years and it has been attributed to structural
impairments in endothelial cells, decreased smooth muscle cells and
cavernous nerve injury (27,28).
However, the potent self-renewing and proliferative capability of
MDSCs, as well as the multi-direction differentiation potency, may
have the potential to treat diabetic ED. Certain studies have
proposed that endogenous organ stem cells may be activated by
ultrasound treatments on the distribution region of stem cells
(29). This may provide a feasible
treatment paradigm for the non-invasive regulatory therapy of
organic ED using stem cells in the future.
Acknowledgements
The authors would like to thank their colleagues at
the Department of Urinary Surgery, The Second Affiliated Hospital
of Suzhou University.
References
|
1
|
Trounson A: New perspectives in human stem
cell therapeutic research. BMC Med. 7:292009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim BJ, Jin HK and Bae JS: Bone
marrow-derived mesenchymal stem cells improve the functioning of
neurotrophic factors in a mouse model of diabetic neuropathy. Lab
Anim Res. 27:171–176. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Albersen M, Fandel TM, Lin G, et al:
Injections of adipose tissue-derived stem cells and stem cell
lysate improve recovery of erectile function in a rat model of
cavernous nerve injury. J Sex Med. 7:3331–3340. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nolazco G, Kovanecz I, Vernet D, et al:
Effect of muscle-derived stem cells on the restoration of corpora
cavernosa smooth muscle and erectile function in the aged rat. BJU
Int. 101:1156–1164. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin G, Banie L, Ning H, Bella AJ, Lin CS
and Lue TF: Potential of adipose-derived stem cells for treatment
of erectile dysfunction. J Sex Med. 6(Suppl 3): 320–327. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jackson L, Jones DR, Scotting P and
Sottile V: Adult mesenchymal stem cells: differentiation potential
and therapeutic applications. J Postgrad Med. 53:121–127. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim EK, Li G, Lee TJ and Hong JP: The
effect of human adipose-derived stem cells on healing of ischemic
wounds in a diabetic nude mouse model. Plast Reconstr Surg.
128:387–394. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ambrosio F, Ferrari RJ, Fitzgerald GK,
Carvell G, Boninger ML and Huard J: Functional overloading of
dystrophic mice enhances muscle-derived stem cell contribution to
muscle contractile capacity. Arch Phys Med Rehabil. 90:66–73. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song YS, Lee HJ, Park IH, Kim WK, Ku JH
and Kim SU: Potential differentiation of human mesenchymal stem
cell transplanted in rat corpus cavernosum toward endothelial or
smooth muscle cells. Int J Impot Res. 19:378–385. 2007. View Article : Google Scholar
|
|
10
|
Andersson KE: Mechanisms of penile
erection and basis for pharmacological treatment of erectile
dysfunction. Pharmacol Rev. 63:811–859. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sullivan ME, Keoghane SR and Miller MA:
Vascular risk factors and erectile dysfunction. BJU Int.
87:838–845. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moreland RB, Hsieh G, Nakane M and Brioni
JD: The biochemical and neurologic basis for the treatment of male
erectile dysfunction. J Pharmacol Exp Ther. 296:225–234.
2001.PubMed/NCBI
|
|
13
|
Shibuya M, Miura T, Fukagawa Y, et al:
Tongue muscle-derived stem cells express connexin 43 and improve
cardiac remodeling and survival after myocardial infarction in
mice. Circ J. 74:1219–1226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Proaño AR, Medrano A, Garrido G and Mazza
O: Muscle-derived stem cell therapy for stress urinary
incontinence. Actas Urol Esp. 34:15–23. 2010.(In Spanish).
|
|
15
|
Nitta M, Tamaki T, Tono K, et al:
Reconstitution of experimental neurogenic bladder dysfunction using
skeletal muscle-derived multipotent stem cells. Transplantation.
89:1043–1049. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsumoto T, Cooper GM, Gharaibeh B, et
al: Cartilage repair in a rat model of osteoarthritis through
intraarticular transplantation of muscle-derived stem cells
expressing bone morphogenetic protein 4 and soluble Flt-1.
Arthritis Rheum. 60:1390–1405. 2009. View Article : Google Scholar
|
|
17
|
Xu Y, Song YF and Lin ZX: Transplantation
of muscle-derived stem cells plus biodegradable fibrin glue
restores the urethral sphincter in a pudendal nerve-transected rat
model. Braz J Med Biol Res. 43:1076–1083. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gharaibeh B, Lu A, Tebbets J, et al:
Isolation of a slowly adhering cell fraction containing stem cells
from murine skeletal muscle by the preplate technique. Nat Protoc.
3:1501–1509. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi G and Jin Y: Role of Oct4 in
maintaining and regaining stem cell pluripotency. Stem Cell Res
Ther. 1:392010. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lam ML, Hashem SI and Claycomb WC:
Embryonic stem cell-derived cardiomyocytes harbor a subpopulation
of niche-forming Sca-1+ progenitor cells. Mol Cell Biochem.
349:69–76. 2011.PubMed/NCBI
|
|
21
|
Qu-Petersen Z, Deasy B, Jankowski R, et
al: Identification of a novel population of muscle stem cells in
mice: potential for muscle regeneration. J Cell Biol. 157:851–864.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zuba-Surma EK, Abdel-Latif A, Case J, et
al: Sca-1 expression is associated with decreased cardiomyogenic
differentiation potential of skeletal muscle-derived adult
primitive cells. J Mol Cell Cardiol. 41:650–660. 2006. View Article : Google Scholar
|
|
23
|
Becker C and Jakse G: Stem cells for
regeneration of urological structures. Eur Urol. 51:1217–1228.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ho MH, Heydarkhan S, Vernet D, et al:
Stimulating vaginal repair in rats through skeletal muscle-derived
stem cells seeded on small intestinal submucosal scaffolds. Obstet
Gynecol. 114:300–309. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Danisovic L, Varga I, Polak S, Ulicna M,
Bohmer D and Vojtassak J: Morphology of in vitro expanded human
muscle-derived stem cells. Biomed Pap Med Fac Univ Palacky Olomouc
Czech Repub. 152:235–238. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lippi G, Plebani M, Montagnana M and
Cervellin G: Biochemical and genetic markers of erectile
dysfunction. Adv Clin Chem. 57:139–162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li WJ, Zhou J, Li B, Wang H, Peng YB and
Wang Z: PARP inhibition restores erectile function by suppressing
corporal smooth muscle apoptosis in diabetic rats. J Sex Med.
8:1072–1082. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Angeloni NL, Bond CW, Tang Y, et al:
Regeneration of the cavernous nerve by Sonic hedgehog using aligned
peptide amphiphile nanofibers. Biomaterials. 32:1091–1101. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang S, Li Y, Ji YC, Lin CM, Man C and
Zheng XX: Stem-cell-activated organ following ultrasound exposure:
better transplant option for organ transplantation. Med Hypotheses.
74:147–149. 2010. View Article : Google Scholar : PubMed/NCBI
|