Introduction
Affective disorders and obstructive diseases,
including asthma and chronic obstructive pulmonary disease (COPD),
are becoming more frequent (1).
The growth in the number of asthma sufferers has increased with the
incidence of mood disorders, including asthma and mood disorder,
both, dependent on genetic and environmental factors. There is a
significant prevalence of depression in daily life with 7–12% of
males and 20–25% of females affected, and an annual occurrence of
6–12% in the adult population. Females are two or three times more
likely to suffer from depression compared with males, and the
probability of depression recurring after treatment during a
lifetime is as high as 80% (1).
The relative occurrence of mood disorders in the course of
obstructive disease is 1.7 times greater in asthma sufferers [95%
confidence interval (CI), 1.1–2.3] and 1.9 times greater in
patients with COPD (95% CI, 1.2–2.1) (2). In the stable phase of COPD,
depression affects between 15 and 42% of patients, while mood
disorders affect 10–19% (3). Mild
depression is estimated to occur in 26% of mild asthma sufferers
and moderate symptoms of depression may be present in 36% of
individuals with moderate asthma (4).
Depression and anxiety, as components of
personality, are to a certain degree conditioned by temperament,
which is a natural predisposition of human emotional reactivity.
Temperament is manifested in the formal elements of behavior; the
reaction of a person to stress and their behavior in extreme
situations depends indirectly on their temperament. Certain
elements of temperament predispose the patient to the development
of disorders in their psyche, including depression and anxiety, and
behavior (5–7).
Psychosomatic factors play a significant role in the
pathogenesis of asthma (8). Mood
and anxiety disorders are more common in patients with obstructive
disorders compared with healthy control groups. Stress, such as
that encountered when not carrying an inhaler, may cause
psychogenic dyspnea, which is demonstrated by a positive
association existing between the functional parameters of the
flow-volume curve and the subjective sense of dyspnea and anxiety
(4).
There are several important mechanisms that induce
mood disorders in asthma, including common genetic factors, certain
drugs used to treat obstruction and inflammatory mediators
(cytokines) that modify the metabolism of the brain. Moreover,
asthma as a chronic inflammatory airway disease acts as a stressor
(4,9–13).
Asthma is a chronic inflammatory disease of the
respiratory tract that exacerbates mood disorders and is correlated
with the concentration of a number of inflammatory markers,
including C-reactive protein, interleukin (IL)-5, -6 and -12, tumor
necrosis factor-α and interferon-α (10–12).
Inflammatory mediators cause a secondary decrease in the activity
of the cAMP response element-binding and tyrosine kinase
transforming proteins, resulting in impaired secretion of
brain-derived neurotrophic factor in the frontal lobes and limbic
system (12,14–16).
As a consequence of these reactions, the hippocampus becomes
damaged and a there is a reduced concentration of monoamines in the
brain (14,17). Certain cytokines are able to
activate multiple signaling pathways with the activation of janus
kinase (JAK) 2 and STAT5. The JAK-STAT signaling pathway plays an
important role in cell proliferation and survival in the central
nervous system (CNS). The pathway also affects the cell response to
various hormones, growth factors and cytokines (18,19).
Activation of the JAK-STAT pathway leads to the development of
depressive effects via glucocorticoid (GC) signaling (20).
Hyperactivity of the well characterized
hypothalamic-pituitary-adrenal (HPA) axis, plays an important role
in the pathogenesis of affective disorders in asthma. In addition,
corticotrophin-releasing hormone (CRH) hyperactivity causes
disorders in the proper functioning of the CNS, due to impaired
negative regulation of CRH by GCs (21–23).
This altered regulation of CRH by GCs is caused by disturbances to
the structure and function of the GC receptor (GR) (23,24).
Numerous studies have demonstrated the crucial role played by
polymorphic forms of the gene coding for the GR, NR3C1, in
the regulation of CRH (25–29).
Changes in the nucleotide sequence by single nucleotide
polymorphisms (SNPs) may influence expression and lead to changes
in RNA assembly. Polymorphisms are responsible for the modification
of the secondary and tertiary structure of GR domains, and cause
disorders in the initiation and stability of mRNA transcription for
GRs (25,26,28,30,31).
The BclI (rs41423247) and N363S (rs6195) polymorphisms of the
NR3C1 gene increase the sensitivity of the GCs, while the
ER22/23EK SNP (rs6189/rs6190) is associated with resistance to GCs
(28,32,33).
Therefore, the aims of the present study were
twofold: Firstly, to determine whether correlations exist between
the levels of depression and anxiety and more objective measures of
airflow obstruction in asthma patients, and secondly, to confirm
whether the genetic determinant of NR3C1 significantly
affects these factors.
Patients and methods
Ethical approval
The study was approved by the local Ethics Committee
(Consent of Research Review Board of the Medical University of
Łódź, Łódź, Poland; no. RNN/133/09/KE). At the start of the study,
participants were invited to attend voluntarily and prior to
enrollment, written informed consent was obtained from every
patient.
Patient selection
A total of 235 patients with bronchial asthma were
recruited for the study. Asthma diagnosis was established according
to the Global Initiative For Asthma recommendations, based on
clinical asthma symptoms and lung function tests. The level of
asthma severity and control was determined according to the
American Thoracic Society (ATS) guidelines (34). Apart from a subjective examination,
structured anamnesis was performed and a number of factors were
examined, including gender, obesity, tobacco smoking, duration of
bronchial asthma, allergy to house dust mites, animal fur, mould
spores, cockroach allergens and hypersensitivity to nonsteroidal
anti-inflammatory drugs (25,26).
The exclusion criteria included subjects suffering
from clinically significant exacerbations, or who were using drugs,
such as rifampicin, phenobarbital, phenytoin or ephedrine, which
may induce resistance to GCs. Subjects with signs of viral
infections, generalized or affecting the respiratory tract, as well
as those failing to comply with the recommendations of their
doctor, were also excluded. The control arm included a group of 216
healthy adults who met the following criteria: No history or
symptoms of bronchial asthma, other pulmonary diseases, allergy,
atopic dermatitis and hypersensitivity to aspirin; negative skin
tests results for 12 common allergens; and no first-degree
relatives with bronchial asthma or atopic disorders. Healthy
volunteers were selected on a random basis from the general
population (25,26).
In the bronchial asthma group, 62.6% (147) of the
patients were female and 37.4% (88) were male. The average age was
48.8±16.0 years (range, 19–82 years; median, 51 years; mode, 52
years). The average forced expiratory volume in 1 sec (FEV1) was
2.2±0.9 litres (72.7%; median, 2.2 litres; mode, 2.4 litres) and
the average forced vital capacity (FVC) was 3.3±1.1 litres (91.4%;
median, 3.2 litres; mode, 2.3 litres).
The control group comprised 216 healthy individuals:
65.7% (142) females and 34.3% (74) males. The average age was
45.7±16.3 years (range, 18–85 years; median, 47 years; mode, 23
years). The average FEV1 was 3.0±0.8 litres (96.1%; median, 2.9
litres; mode, 2.7 litres) and the average FVC was 3.8±1.0 litres
(102.7%; median, 3.6 litres; mode, 3.5 litres).
Detailed descriptive statistics for age and
spirometric parameters for the cases and controls are presented in
Table I.
 | Table IDescriptive statistics for age and
spirometric parameters in the healthy control subjects and asthma
patients. |
Table I
Descriptive statistics for age and
spirometric parameters in the healthy control subjects and asthma
patients.
| Parameter | Bronchial asthma
group | Control group |
|---|
| Subjects (n) | 235 | 216 |
| Females (%) | 62.6 | 65.7 |
| Males (%) | 37.4 | 34.3 |
| Age (years) |
| Average | 48.8 | 45.7 |
| SD | ±16.0 | ±16.3 |
| Minimum | 19 | 18 |
| Maximum | 82 | 85 |
| Median | 51.0 | 47.0 |
| Mode | 52.0 | 23.0 |
| FEV1 |
| Average
(litre) | 2.2 | 3.0 |
| Average (%) | 72.7 | 96.1 |
| SD (litre) | ±0.9 | ± 0.8 |
| Median
(litre) | 2.2 | 2.9 |
| Mode (litre) | 2.4 | 2.7 |
| FVC |
| Average
(litre) | 3.3 | 3.8 |
| Average (%) | 91.4 | 102.7 |
| SD (litre) | ±1.1 | ±1.0 |
| Median
(litre) | 3.2 | 3.6 |
| Mode (litre) | 2.3 | 3.5 |
Functional assessments
Functional assessments of the respiratory system
were conducted according to the European Respiratory Society and
ATS standards (34).
In all the tests, the Polish language version of the
Beck Depression Inventory was used (35–37),
where results were expressed as a total number of the points
obtained. Trait and state anxieties were measured using the Polish
language adaptation of Spielberg’s State and Trait Anxiety
Inventory (STAI) (37,38), where the results were expressed as
absolute numbers of the points obtained. The level of declared
breathlessness was estimated on the 10-point Borg scale of
subjective feelings (37,39). Each patient recorded their
subjective impression of breathlessness at the time of the test on
a scale of 0–10 (37).
Sample collection
Venous blood samples were collected from the
participants and placed into K3-EDTA tubes. DNA was obtained from
the peripheral blood leukocyte fraction and isolated using a QIAamp
DNA Blood Mini kit (Qiagen, Hilden, Germany), according to the
manufacturer’s instructions. Polymorphisms were analyzed using
polymerase chain reaction-restriction fragment length polymorphism
(PCR-RFLP).
PCR
Exponential amplification of the DNA segments for
the ER22/23EK polymorphism was conducted using forward (5′-TGC ATT
CGG AGT TAA CTA AAA AG-3′) and reverse (5′-ATC CCA GGT CAT TTC CCA
TC-3′) primers, according to a standard PCR protocol. Starter
binding to complementary DNA matrix sites was conducted at 56°C.
Amplified DNA sequences of 448 bp were obtained. The genetic
material was incubated with the MnlI restriction enzyme
(Fermentas International, Inc., Burlington, Canada) at 37°C for 20
h (40). DNA fragments of 149 and
163 bp, and shorter fragments containing 50, 49 and 35 bp, were
obtained as a set of representative, typical (wild type) alleles,
whereas segments of 163 and 184 bp, and shorter fragments
containing 50 and 49 bp, constituted the set of polymorphic alleles
(26,40).
Exponential amplification of the DNA segments for
the N363S polymorphism was performed using forward (5′-CCA GTA ATG
TAA CAC TGC CCC-3′) and reverse (5′-TTC GAC CAG GGG AAG TTC AGA-3′)
primers, according to a standard PCR protocol (41). Starter binding to complementary DNA
matrix sites was conducted at 56°C and amplified DNA sequences of
357 bp were obtained. The material was incubated with
FastDigest® Tsp509I (TasI) restriction
enzyme (Fermentas International, Inc., Burlington, Canada) at 65°C
for 1 h (40). DNA fragments of
135, 73, 70, 60 and 19 bp were obtained as a set of representative,
typical (wild type) alleles, whereas segments of 135, 92 (73 and 19
bp), 70 and 60 bp constituted the set of polymorphic alleles
(25,41).
Amplification of the DNA fragment containing the
BclI polymorphism of the NR3C1 gene was conducted using
starters with the following sequences: Forward, 5′-GAG AAA TTC ACC
CCT ACC AAC-3′ and reverse (5′-AGA GCC CTA TTC TTC AAA CTG-3′,
according to a standard PCR protocol (42). Starter binding to complementary DNA
matrix sites was conducted at 56°C. The BclI restriction
enzyme (Fermentas International, Inc.) was used for the digestion
of the amplification product containing the Bc1I
polymorphism (40). Hydrolysis of
the PCR product with the restriction enzyme was conducted for 24 h
at 55°C. DNA fragments containing 263 and 151 bp were identified as
a set of representative, typical (wild type) alleles, as well as
segments with 418, 263 and 155 bp. An RFLP product, 418 bp in
length, was identified as a set of polymorphic alleles (40).
For each of the SNP tests, representative, typical
homozygotes and heterozygotes were sequenced and used as internal
controls. Following restriction enzyme digestion, 4 ml indicator
dye was added to the test tube. Electrophoresis was performed using
an 8% polyacrylamide gel with 1:20 Tris-acetate-EDTA buffer at 120
V for 60 min. The gel was stained with 0.5 mmol/ml ethidium bromide
and imaged under ultraviolet light using a camera and Image Master
software (Pharmacia Biotech, Tokyo, Japan). Electropherograms of
the amplified products following restriction enzyme digestion were
photographed and saved on digital media. Images were analyzed using
Image Master software.
Statistical analysis
Statistical analysis was performed using univariate
analysis of variance. An advanced regression model (general linear
model) was used to evaluate the dependencies between multiple
variables. Statistical analysis was performed using STATISTICA data
analysis software system, version 10 (AXAP202E504303AR-A; StatSoft,
Inc., Tulsa, OK, USA). In addition, the Bonferroni correction was
used for the three tested polymorphisms. P<0.05 was considered
to indicate a statistically significant difference. Differences and
linear trends between the three tested genotypes were identified. A
haplotype effect model (additive and dominant) was used to analyze
the haplotypes and haplotype-specific scores with the R statistics
package (http://www.r-project.org/).
Genotyping was performed by two investigators who were unaware of
the phenotypes.
Results
In the test and control groups, the following values
were identified for the studied variables: Beck (depression),
STAI-I (state anxiety), STAI–II (trait anxiety) and Borg
(breathlessness) scales, as presented in Table II.
 | Table IIDescriptive statistics of depression,
anxiety and breathlessness in the healthy control and asthma
patients. |
Table II
Descriptive statistics of depression,
anxiety and breathlessness in the healthy control and asthma
patients.
| A, Controls |
|---|
|
|---|
| Parameter | Mean | Median | Mode | Min. | Max. | SD |
|---|
| Beck scale | 8.34 | 6.00 | 2.00 | 0.00 | 33.00 | 7.14 |
| STAI–I | 36.95 | 35.00 | 1.00 | 20.00 | 62.00 | 9.29 |
| STAI–II | 41.54 | 41.00 | 1.00 | 21.00 | 63.00 | 9.06 |
| Borg scale | 1.74 | 1.00 | 0.00 | 0.00 | 8.00 | 1.89 |
|
| B, Cases |
|
| Parameter | Mean | Median | Mode | Min. | Max. | SD |
|
| Beck scale | 10.28 | 9.00 | 1.00 | 0.00 | 45.00 | 7.65 |
| STAI–I | 38.19 | 37.00 | 1.00 | 20.00 | 74.00 | 10.60 |
| STAI–II | 42.91 | 43.00 | 1.00 | 1.00 | 64.00 | 9.17 |
| Borg scale | 3.48 | 3.00 | 5.00 | 0.00 | 9.00 | 2.48 |
The frequencies of occurrence of the polymorphic
forms of NR3C1 are presented in Table III. The AA ER22/23EK, GG N363S
and CC BclI alleles were identified to be rare forms of
NR3C1 polymorphisms, while GG ER22/23EK, AA N363S and GG
BclI were more frequent. Univariate analysis of the tested
parameters revealed the existence of significant differences
between the test and control groups, as shown in Table IV.
 | Table IIIFrequencies of polymorphic forms of
NR3C1 in the healthy controls and asthma patients. |
Table III
Frequencies of polymorphic forms of
NR3C1 in the healthy controls and asthma patients.
| NR3C1
polymorphic form | Controls (%) | Cases (%) |
|---|
| ER22/23EK G | 96.74 | 97.22 |
| ER22/23EK A | 3.26 | 2.78 |
| N363S A | 86.74 | 87.45 |
| N363S G | 13.26 | 12.55 |
| BclI G | 58.80 | 57.05 |
| BclI C | 41.20 | 42.95 |
 | Table IVAssociations between the variables in
the test and control groups using univariate analysis of
variance. |
Table IV
Associations between the variables in
the test and control groups using univariate analysis of
variance.
| Variable | SS | DF | MS | F | P-value |
|---|
| Beck scale | 1285.840 | 2 | 642.920 | 12.107 | <0.001 |
| STAI-I | 307.600 | 2 | 153.800 | 1.526 | 0.218 |
| STAI–II | 443.500 | 2 | 221.700 | 2.676 | 0.070 |
| Borg scale | 387.139 | 2 | 193.569 | 40.220 | <0.001 |
In the studied populations, a complex association
was identified between the analyzed variables (depression, state
and trait anxieties and breathlessness) and the results of the
respiratory function tests. Table
V presents detailed correlations divided into the two
subgroups: Healthy subjects and asthma patients.
 | Table VAdvanced multivariate general
regression models for depression, anxiety and breathlessness in the
group of asthma patients compared with the healthy control
group. |
Table V
Advanced multivariate general
regression models for depression, anxiety and breathlessness in the
group of asthma patients compared with the healthy control
group.
| A, Depression (Beck
scale) |
|---|
|
|---|
| Spirometric
parameter | Healthy
subjects | Asthma
patients |
|---|
| FEV1 (litre) | r=−0.2544 | r=−0.3759 |
| P=0.0007 | P<0.0001 |
|
r2=0.0647 |
r2=0.1413 |
| FVC (litre) | r=−0.2300 | r=−0.3495 |
| P=0.0023 | P<0.0001 |
|
r2=0.0529 |
r2=0.1221 |
| FEV1:FVC (%) | r=−0.1069 | r=−0.1876 |
| P=0.1615 | P=0.0052 |
|
r2=0.0114 |
r2=0.0352 |
|
| B, State-anxiety
(STAI-I) |
|
| Spirometric
parameter | Healthy
subjects | Asthma
patients |
|
| FEV1 (litre) | r=−0.1430 | r=−0.1517 |
| P=0.0605 | P=0.0244 |
|
r2=0.0205 |
r2=0.0230 |
| FVC (litre) | r=−0.1488 | r=−0.2217 |
| P=0.0507 | P=0.0009 |
|
r2=0.0222 |
r2=0.0491 |
| FEV1:FVC (%) | r=−0.0147 | r=0.0893 |
| P=0.8482 | P=0.1867 |
|
r2=0.0002 |
r2=0.0080 |
|
| C, Trait-anxiety
(STAI–II) |
|
| Spirometric
parameter | Healthy
subjects | Asthma
patients |
|
| FEV1 (litre) | r=−0.2651 | r=−0.2810 |
| P=0.0004 | P<0.0001 |
|
r2=0.0703 |
r2=0.0789 |
| FVC (litre) | r=−0.2618 | r=−0.3397 |
| P=0.0005 | P<0.0001 |
|
r2=0.0686 |
r2=0.1154 |
| FEV1:FVC (%) | r=−0.0500 | r=0.0248 |
| P=0.5133 | P=0.7142 |
|
r2=0.0025 |
r2=0.0006 |
|
| D, Breathlessness
(Borg scale) |
|
| Spirometric
parameter | Healthy
subjects | Asthma
patients |
|
| FEV1 (litre) | r=−0.1948 | r=−0.3202 |
| P=0.0107 | P<0.0001 |
|
r2=0.0379 |
r2=0.1025 |
|
| Spirometric
parameter | Healthy
subjects | Asthma
sufferers |
|
| FVC (litre) | r=−0.1506 | r=−0.3048 |
| P=0.0493 | P<0.0001 |
|
r2=0.0227 |
r2=0.0929 |
| FEV1:FVC (%) | r=−0.2194 | r=−0.1512 |
| P=0.0039 | P=0.0249 |
|
r2=0.0481 |
r2=0.0229 |
Functional assessments
Associations between the variables (depression,
anxiety and breathlessness) and objective, measurable and
repeatable spirometric parameters were analyzed. These results
illustrated the degree of airway obstruction in the asthma patients
with regard to the severity of the illness, as assessed by the ATS
criteria, compared with the control group. A detailed analysis is
presented in Table VI.
 | Table VIAdvanced multivariate general
regression models for depression, anxiety and breathlessness in the
asthma patient group compared with the control group, with regard
to the severity of asthma. |
Table VI
Advanced multivariate general
regression models for depression, anxiety and breathlessness in the
asthma patient group compared with the control group, with regard
to the severity of asthma.
| A, Depression (Beck
scale) |
|---|
|
|---|
| Spirometric
parameter | Healthy | ATS 0 | ATS 1 |
|---|
| FEV1 (litre) | r=−0.2544 | r=−0.3630 | r=−0.2273 |
| P=0.0007 | P<0.0001 | P=0.0483 |
|
r2=0.0647 |
r2=0.1318 |
r2=0.0517 |
| FVC (litre) | r=−0.2300 | r=−0.3687 | r=−0.2099 |
| P=0.0023 | P<0.0001 | P=0.0688 |
|
r2=0.0529 |
r2=0.1360 |
r2=0.0441 |
| FEV1:FVC (%) | r=−0.1069 | r=−0.0686 | r=−0.1422 |
| P=0.1615 | P=0.4139 | P=0.2204 |
|
r2=0.0114 |
r2=0.0047 |
r2=0.0202 |
|
| B, State anxiety
(STAI-I) |
|
| Spirometric
parameter | Healthy | ATS 0 | ATS 1 |
|
| FEV1 (litre) | r=−0.1430 | r=−0.1552 | r=−0.0805 |
| P=0.0605 | P=0.0633 | P=0.4895 |
|
r2=0.0205 |
r2=0.0241 |
r2=0.0065 |
| FVC (litre) | r=−0.1488 | r=−0.2183 | r=−0.1899 |
| P=0.0507 | P=0.0086 | P=0.1003 |
|
r2=0.0222 |
r2=0.0476 |
r2=0.0361 |
| FEV1:FVC (%) | r=0.0767 | r=0.1753 | r=0.1775 |
| P=0.3156 | P=0.0356 | P=0.1250 |
|
r2=0.0059 |
r2=0.0307 |
r2=0.0315 |
|
| C, Trait anxiety
(STAI–II) |
|
| Spirometric
parameter | Healthy | ATS 0 | ATS 1 |
|
| FEV1 (litre) | r=−0.2651 | r=−0.2724 | r=−0.2186 |
| P=0.0004 | P=0.0010 | P=0.0578 |
|
r2=0.0703 |
r2=0.0742 |
r2=0.0478 |
| FVC (litre) | r=−0.2618 | r=−0.3221 | r=−0.3210 |
| P=0.0005 | P<0.0001 | P=0.0047 |
|
r2=0.0686 |
r2=0.1038 |
r2=0.1031 |
| FEV1:FVC (%) | r=0.0519 | r=0.1093 | r=0.1400 |
| P=0.4977 | P=0.1923 | P=0.2276 |
|
r2=0.0027 |
r2=0.0119 |
r2=0.0196 |
|
| D, Breathlessness
(Borg scale) |
|
| Spirometric
parameter | Healthy | ATS 0 | ATS 1 |
|
| FEV1 (litre) | r=−0.1948 | r=−0.3043 | r=−0.1254 |
| P=0.0107 | P=0.0002 | P=0.2773 |
|
r2=0.0379 |
r2=0.0926 |
r2=0.0157 |
| FVC (litre) | r=−0.1506 | r=−0.2907 | r=−0.1909 |
| P=0.0493 | P=0.0004 | P=0.0963 |
|
r2=0.0227 |
r2=0.0845 |
r2=0.0365 |
| FEV1:FVC (%) | r=−0.2194 | r=−0.1256 | r=0.0500 |
| P=0.0039 | P=0.1350 | P=0.6656 |
|
r2=0.0481 |
r2=0.0158 |
r2=0.0025 |
Haplotype analysis
The results of more advanced tests concerning the
polymorphisms of the NR3C1 gene, which is located on the
long arm of chromosome 5 at position q31–q32, are shown in Tables VII–X (42).
The gene is inherited as a set of associated alleles, together with
depression, anxiety and breathlessness. Haplotype analysis
performed using the additive haplotype effects model [global
statistic, 0.913; degrees of freedom (DF), 6; P=0.988] did not
reveal any correlations between the NR3C1 gene haplotypes
and depression, as shown in Table
VII. In addition, haplotype analysis performed using the
additive haplotype effects model (global statistic, 1.237; DF, 6;
P=0.974) revealed that no combinations of alleles were
significantly associated with state-anxiety (Table VIII). However, haplotype
analysis using the haplotype-specific stores did confirm a
statistically significant association (P=0.026) with trait anxiety
for one of the combinations of alleles, as shown in Table IX. Haplotype analysis performed
using the additive haplotype effects model (global statistic,
6.195; DF, 6; P=0.401) did not reveal an association between
NR3C1 haplotypes and breathlessness, as shown in Table X. Therefore, haplotype analysis
revealed a correlation between polymorphic forms of NR3C1
and the level of trait-anxiety.
 | Table VIIHaplotype-specific scores for
depression using the haplotype effects model. |
Table VII
Haplotype-specific scores for
depression using the haplotype effects model.
| Genotype | Haplotype | |
|---|
|
| |
|---|
| ER22/23EK | N363S | BclI | Frequency | Score | P-value |
|---|
| GG | GG | GG | 0.05797 | −0.88383 | 0.37679 |
| GG | AA | CC | 0.39565 | −0.71309 | 0.47579 |
| GG | GG | CC | 0.02783 | −0.24739 | 0.80461 |
| AA | AA | GG | 0.02235 | 0.36511 | 0.71503 |
| AA | AA | CC | 0.00725 | 0.63020 | 0.52856 |
| GG | AA | GG | 0.48776 | 0.97533 | 0.32940 |
 | Table XHaplotype-specific stores for
breathlessness using the haplotype effects model. |
Table X
Haplotype-specific stores for
breathlessness using the haplotype effects model.
| Genotype | Haplotype | |
|---|
|
| |
|---|
| ER22/23EK | N363S | BclI | Frequency | Score | P-value |
|---|
| GG | GG | GG | 0.05998 | −1.20047 | 0.22995 |
| AA | AA | CC | 0.00720 | −0.89380 | 0.37143 |
| AA | AA | GG | 0.02255 | −0.76096 | 0.44668 |
| GG | AA | CC | 0.39722 | −0.67443 | 0.50004 |
| GG | GG | CC | 0.02741 | 0.92493 | 0.35500 |
| GG | AA | GG | 0.48451 | 1.33597 | 0.18156 |
 | Table VIIIHaplotype-specific scores for STAI-I
using the haplotype effects model. |
Table VIII
Haplotype-specific scores for STAI-I
using the haplotype effects model.
| Genotype | Haplotype | |
|---|
|
| |
|---|
| ER22/23EK | N363S | BclI | Frequency | Score | P-value |
|---|
| GG | AA | CC | 0.39565 | −0.63527 | 0.52525 |
| GG | GG | GG | 0.05797 | −0.57593 | 0.56466 |
| AA | AA | CC | 0.00725 | −0.29875 | 0.76513 |
| AA | AA | GG | 0.02235 | −0.22619 | 0.82106 |
| GG | GG | CC | 0.02783 | 0.01604 | 0.98720 |
| GG | AA | GG | 0.48776 | 0.98623 | 0.32402 |
 | Table IXHaplotype-specific scores for STAI–II
using the haplotype effects model. |
Table IX
Haplotype-specific scores for STAI–II
using the haplotype effects model.
| Genotype | Haplotype | |
|---|
|
| |
|---|
| ER22/23EK | N363S | BclI | Frequency | Score | P-value |
|---|
| GG | AA | CC | 0.39565 | −1.21509 | 0.22433 |
| GG | GG | GG | 0.05797 | −1.0306 | 0.30273 |
| AA | AA | GG | 0.02235 | −1.01922 | 0.30810 |
| GG | GG | CC | 0.02783 | 0.63846 | 0.52317 |
| GG | AA | GG | 0.48776 | 1.53739 | 0.12420 |
| AA | AA | CC | 0.00725 | 2.21614 | 0.02668 |
Correlation analysis
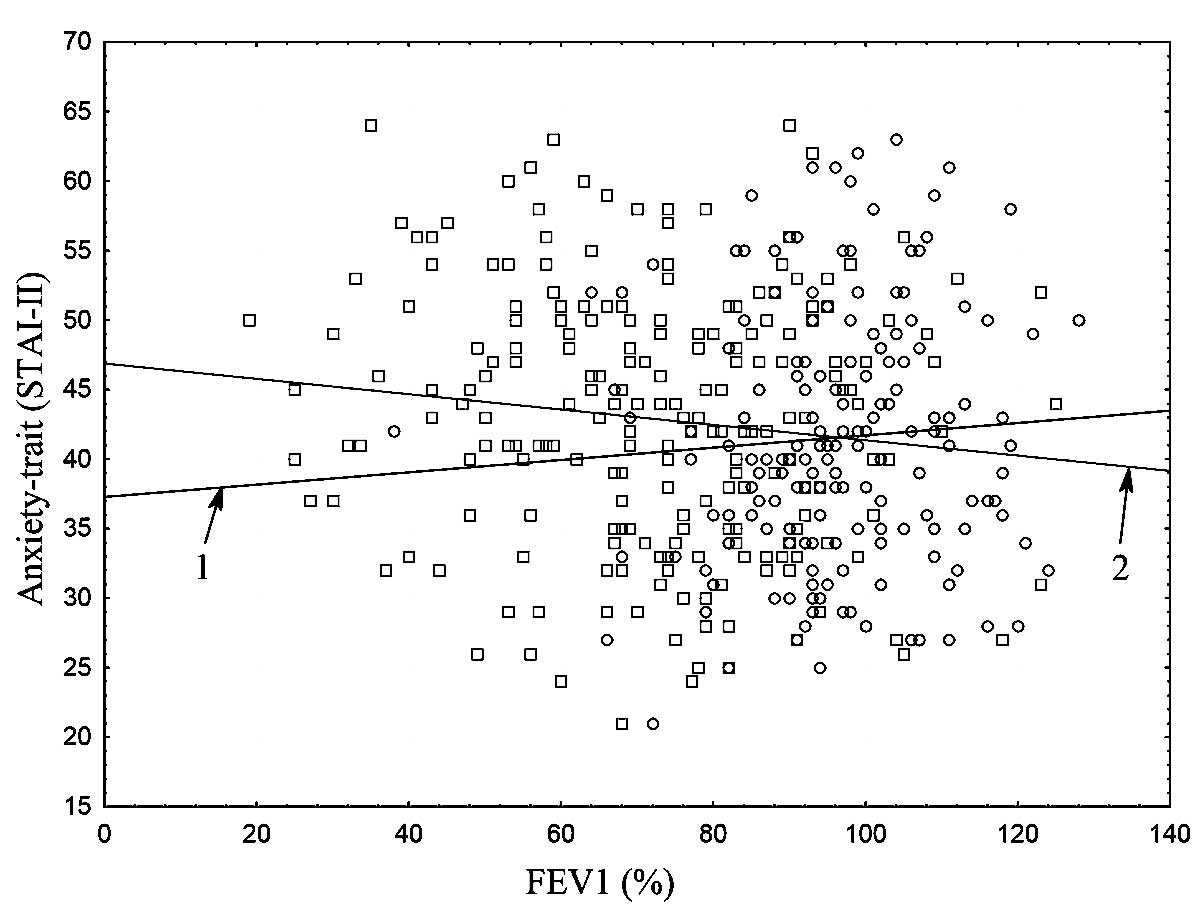
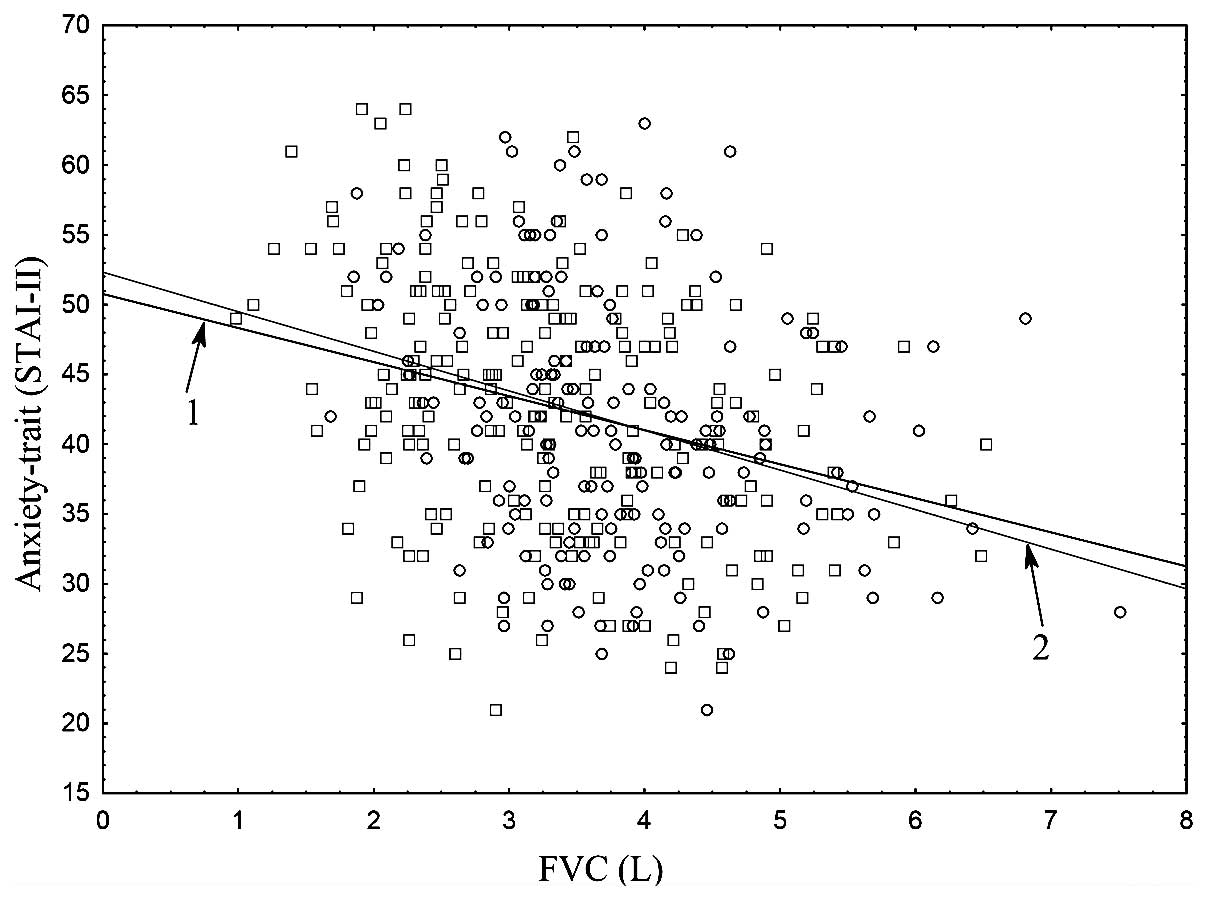
Scatter plots (Figs.
1 and 2) presented a graphical
interpretation of the key correlations between trait-anxiety
(STAI–II) and the spirometric parameters, FEV1 and FVC, for the
following groups: Cases vs. controls.
Discussion
Research on the interaction between GCs and
receptors is important for improving the understanding of asthma
therapy. The use of GCs was a turning point in the history of the
treatment of this disease. The NR3C1 gene and its
transcripts are key factors involved in the inflammation of asthma
and are responsible for alterations in the functioning of the GR
(22–24). GCs are able to alter the regulation
of CRH, thus, affect the development of depression and anxiety
disorders (22–24). Therefore, polymorphic forms of
NR3C1, which replicate the effect of the regulatory gene and
affect the function of the promoter and the encoded protein (GR),
can lead to the induction of depressive and anxiety disorders, and
modify their intensity.
In the present study, a statistically significant
correlation was observed between the levels of depression, anxiety
(state and trait) and dyspnea with the spirometric parameters FEV1
and FVC. In addition, a correlation was observed between the
FEV1:FVC respiratory function with depression and shortness of
breath. These associations were observed in the asthma patients and
control group.
Advanced analysis concerning the severity of asthma
was performed by dividing the group of patients into two subgroups
based on the ATS criteria. A correlation was observed between the
level of depression in patients with severe asthma refractory to
treatment and FEV1 (r=−0.227, P=0.048) and between the level of
trait anxiety and FVC (r=−0.321, P=0.004). Similar observations
were identified in asthma patients who did not meet the criteria of
severe asthma refractory to treatment (depression vs. FEV1;
depression vs. FVC; state-anxiety vs. FVC; state-anxiety vs.
FEV1:FVC; trait-anxiety vs. FEV1, trait-anxiety vs. FVC; dyspnea
vs. FEV1; dyspnea vs. FEV1; dyspnea vs. FVC; depression vs.
FEV1:FVC).
A total of 24 NR3C1 gene variants were
detected for each of the tested variables (depression, state and
trait anxiety and breathlessness). Only one of the NR3C1
haplotypes exhibited a correlation with state-anxiety (P=0.026),
which was AA ER22/23EK (rare), AA N363S (common) and CC BclI
(rare).
The ER22/23EK polymorphism is composed of two
transition nucleotides at the 22nd and 23rd
codons, which are connected with each other. SNPs may alter the
secondary structure of the GR mRNA, and may consequently initiate
translation from the 1st or the 27th
methionine, as well as affect the stability of the mRNA (26,32,33,43,44).
The presence of the N363S polymorphism promotes
structural changes in the A/B region of the GR, affecting
activation function-1 domain, which interacts with multiple
transcription factors, as well as within the activator protein-1
functional domain (25,26,45).
The N363S polymorphism modulates numerous regulatory protein
groups, decreases the activity of nuclear factor-κB and stimulates
the production of IκBα, thus, interfering with the suppression of
IL-2 (25,26,45).
The BclI SNP is coupled with two other polymorphic
forms of NR3C1: Intron B 33389 (rs33389) and Intron B 33388
(rs33388) (27,46). These three polymorphisms modify the
recognition site by alternative splicing of the NR3C1
factors: SR (serine/arginine-rich protein) and SF2/ASF (splicing
factor 2/alternative splicing factor) (27,47).
The results of the present study confirm previous
observations that changes, including depression, anxiety and
breathlessness, significantly correlate with spirometric parameters
and have an influence on the severity of the course of asthma
(4,37). The present study characterizes and
precisely describes the haplotypes of the NR3C1 gene, while
identifying the haplotype responsible for the changes occurring in
trait-anxiety in asthma sufferers. The results demonstrated that
trait-anxiety significantly lowered a number of spirometric
parameters (FEV1, FVC, FEV1:FVC), causing a more severe course of
illness. Previous studies on NR3C1 gene polymorphisms have
failed to confirm that the ER22/23EK and BclI SNPs play any role in
the etiopathogenesis of asthma. The results of the current study
confirmed that all NR3C1 polymorphisms (haplotype,
ER22/23EK, N363S and BclI) participate in the regulation of the
intensity of trait-anxiety in asthma patients, which significantly
correlates with increases in airflow obstruction (4,25,26,37,48).
The association between NR3C1 haplotypes with depression and
anxiety may therefore be complex. The same polymorphism which
influences the development of asthma and the degree of severity can
function indirectly as a stressor (airway obstruction), inducing
depression and anxiety disorders. This observed phenomenon may also
explain the differences in the functioning of the GR associated
with the decreased expression of multiple proteins in the brain.
Co-occurrence of asthma, mood disorders and anxiety may stem from a
common genetic cause; this has been indirectly confirmed by the
evidence that gene polymorphisms are associated with the occurrence
of NR3C1 mood disorders, and also that the HPA axis is
involved in the pathogenesis of depression.
Therefore, the gene encoding NR3C1 GR
activity is an important regulator of the biochemical and molecular
mechanisms involved in the changes of the GR, thus, affects the
degree of airway obstruction. The haplotype, ER22/23EK, N363S and
BclI, materially affects, directly or indirectly,
psychopathological and personality variables, including depression,
anxiety and shortness of breath (4,25,26,37,48).
Furthermore, the present study highlights the under
diagnosis of affective and anxiety disorders among asthma
sufferers.
However, the present study has limitations
associated with asthma medication treatment. This group of
medicines comprises inhaled and systematic glucocorticosteroids,
which can induce depression. Therefore, patients experiencing
asthma attacks, which required the use of glucocorticosteroids,
were excluded from the study. In addition, the test and the control
group did not include subjects who were receiving long-term
systematic glucocorticosteroid treatment for any other medical
conditions.
For the subjects included in the study, the
diagnosis of depression and anxiety disorders was performed for the
first time. Thus, it was difficult to further assess the clinical
course of the affective and anxiety disorders. Patients who had
previously been treated for anxiety disorders and depression were
not included in the study.
In conclusion, a multivariate study analyzing the
role of genetic variants in the NR3C1 gene was performed in
patients with asthma, with particular emphasis on the intensity of
their depression, anxiety and shortness of breath.
The results demonstrated that the haplotypic
variations in the regulatory regions of the NR3C1 gene
significantly correlated with trait-anxiety. In addition, important
molecular mechanisms leading to the development of depression and
anxiety in asthma patients were identified, and correlations that
existed with functional spirometry parameters were indicated.
NR3C1 polymorphisms and haplotypes are
general modulating factors of the level of depression, shortness of
breath and anxiety, which significantly predispose patients with
asthma to the development of affective disorders and anxiety.
Acknowledgements
The study was supported by a grant from the Minister
of Science and Higher Education of the Polish Republic (no. N N402
374638). In addition, the study was supported by a grant within the
framework of a project supporting innovative doctoral studies,
entitled: ‘Scholarships supporting innovative doctoral studies’,
based on funds provided by the EU European Social Fund and Polish
state budget allocation for the Integrated Operational Plan for
Regional Development according to Lodz Region Innovation
Strategy-RSI LORIS (program title, ZPORR; sector, 2.6 Regional
Innovation Strategies and Transfer of Knowledge; project no.
Z/2.10/II/2.6/1/09). This project was financed 75% by the European
Social Fund and 25% by the state budget. The study was also
financed by a fund from the Department of Internal Medicine, Asthma
and Allergy, 2nd Chair of Internal Medicine, Medical
University of Lodz, Poland (no. 503/1-095-03/503-01). The authors
thank Damian Tworek MD-PhD for assistance with patient recruitment
and blood collection, Joanna Molinska MA for assistance with
administration and Ms. Beata Małachowska for assistance with
statistical analysis. Beata Małachowska was supported by the TEAM
project financed by the Innovative Economy Operational Program and
coordinated by the Foundation for Polish Science. Finally, the
authors thank all those who provided assistance during the
completion of the study.
References
|
1
|
Kessler RC, McGonagle KA, Zhao S, et al:
Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders
in the United States. Results from the National Comorbidity Survey.
Arch Gen Psychiatry. 51:8–19. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Miguel Diez J, García RJ, Hernandez
Barrera V, et al: Mental health among adults with asthma and
chronic bronchitis. A population-based study in Spain. Respir Med.
106:924–932. 2012.PubMed/NCBI
|
|
3
|
Wang ZL: Evolving role of systemic
inflammation in comorbidities of chronic obstructive pulmonary
disease. Chin Med J (Engl). 123:3467–3478. 2010.PubMed/NCBI
|
|
4
|
Pietras T, Panek M, Witusik A, et al:
Analysis of the correlation between level of anxiety, intensity of
depression and bronchial asthma control. Post Dermatol Alergol.
28:15–22. 2011.
|
|
5
|
Strelau J: Psychology of temperament. PWN;
Warsaw: 2009, (In Polish).
|
|
6
|
Strelau J: Temperament, personality,
activity. Academic Press; London: 1983
|
|
7
|
Strelau J: The Psychology of individual
differences. Scholar; Warsaw: 2006, (In Polish).
|
|
8
|
Sęk H: Introduction to Clinical
Psychology. Scholar; Warsaw: 2001, (In Polish).
|
|
9
|
Marques AH, Silverman MN and Sternberg EM:
Glucocorticoid dysregulations and their clinical correlates. From
receptors to therapeutics. Ann N Y Acad Sci. 1179:1–18. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Margaretten M, Julian L, Katz P and Yelin
E: Depression in patients with rheumatoid arthritis: description,
causes and mechanisms. Int J Clin Rheumtol. 6:617–623. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jacobson L:
Hypothalamic-pituitary-adrenocortical axis regulation. Endocrinol
Metab Clin North Am. 34:271–292. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Elomaa AP, Niskanen L, Herzig KH, et al:
Elevated levels of serum IL-5 are associated with an increased
likelihood of major depressive disorder. BMC Psychiatry. 12:22012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Favreau H, Bacon SL, Joseph M, et al:
Association between asthma medications and suicidal ideation in
adult asthmatics. Respir Med. 106:933–941. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pace TW, Hu F and Miller AH:
Cytokine-effects on glucocorticoid receptor function: relevance to
glucocorticoid resistance and the pathophysiology and treatment of
major depression. Brain Behav Immun. 21:9–19. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Irwin MR and Miller AH: Depressive
disorders and immunity: 20 years of progress and discovery. Brain
Behav Immun. 21:374–383. 2007.PubMed/NCBI
|
|
16
|
Khairova RA, Machado-Vieira R, Du J and
Manji HK: A potential role for pro-inflammatory cytokines in
regulating synaptic plasticity in major depressive disorder. Int J
Neuropsychopharmacol. 12:561–578. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Denayer E, Ahmed T, Brems H, et al: Spred1
is required for synaptic plasticity and hippocampus-dependent
learning. J Neurosci. 28:14443–14449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heim MH: The Jak-STAT pathway: specific
signal transduction from the cell membrane to the nucleus. Eur J
Clin Invest. 26:1–12. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zambrano A, Otth C, Maccioni RB and Concha
II: IL-3 controls tau modifications and protects cortical neurons
from neurodegeneration. Curr Alzheimer Res. 7:615–624. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu F, Pace TW and Miller AH:
Interferon-alpha inhibits glucocorticoid receptor-mediated gene
transcription via STAT5 activation in mouse HT22 cells. Brain Behav
Immun. 23:455–463. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Owens MJ and Nemeroff CB: Physiology and
pharmacology of corticotropin-releasing factor. Pharmacol Rev.
43:425–473. 1991.PubMed/NCBI
|
|
22
|
Raison CL and Miller AH: When not enough
is too much: The role of insufficient glucocorticoid signaling in
the pathophysiology of stress-related disorders. Am J Psychiatry.
160:1554–1565. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Raison CL, Capuron L and Miller AH:
Cytokines sing the blues: inflammation and the pathogenesis of
depression. Trends Immunol. 27:24–31. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pariante CM and Miller AH: Glucocorticoid
receptors in major depression: relevance to pathophysiology and
treatment. Biol Psychiatry. 49:391–404. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Panek M, Pietras T, Antczak A, et al: The
N363S and I559N single nucleotide polymorphisms of the h-GR/NR3C1
gene in patients with bronchial asthma. Int J Mol Med. 30:142–150.
2012.PubMed/NCBI
|
|
26
|
Panek M, Pietras T, Antczak A, et al: The
role of functional single nucleotide polymorphisms of the human
glucocorticoid receptor gene NR3C1 in Polish patients with
bronchial asthma. Mol Biol Rep. 39:4749–4757. 2012. View Article : Google Scholar
|
|
27
|
Stevens A, Ray DW, Zeggini E, et al:
Glucocorticoid sensitivity is determined by a specific
glucocorticoid receptor haplotype. J Clin Endocrinol Metab.
89:892–897. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nicolaides NC, Galata Z, Kino T, et al:
The human glucocorticoid receptor: molecular basis of biologic
function. Steroids. 75:1–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bray PJ and Cotton RG: Variations of the
human glucocorticoid receptor gene (NR3C1): pathological and in
vitro mutations and polymorphisms. Hum Mutat. 21:557–568. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
DeRijk RH, Schaaf M and de Kloet ER:
Glucocorticoid receptor variants: clinical implications. J Steroid
Biochem Mol Biol. 81:103–122. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ito K, Chung KF and Adcock IM: Update on
glucocorticoid action and resistance. J Allergy Clin Immunol.
117:522–543. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
van Rossum EF, Voorhoeve PG, te Velde SJ,
et al: The ER22/23EK polymorphism in the glucocorticoid receptor
gene is associated with a beneficial body composition and muscle
strength in young adults. J Clin Endocrinol Metab. 89:4004–4009.
2004.PubMed/NCBI
|
|
33
|
Bulas M, Trelińska J, Stolarska M, et al:
Association of steroid receptor gene (NR3C1) polymorphism with
clinical course of lymphoproliferative disorders in children -
preliminary results. Onkol Pol. 2:77–81. 2008.
|
|
34
|
Miller M, Hankinson J, Brusasco V, et al:
ATS/ERS Task Force: Standardisation of spirometry. Eur Respir J.
26:319–338. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Beck AT, Ward CH, Mendelson M, et al: An
inventory for measuring depression. Arch Gen Psychiatry. 4:561–571.
1961. View Article : Google Scholar
|
|
36
|
Pużyński S and Wciórka J: The tools of
mental state assessment. Bilikiewicz A, Pużyński S and Rybakowski
J: Psychiatry. Medical Publisher Urban & Partner; Wrocław: pp.
453–538. 2002, (In Polish).
|
|
37
|
Pietras T, Panek M, Witusik A, et al:
Analysis of the correlation between anxiety, depression, intensity
of dyspnoea and severity of the bronchial asthma disease process.
Post Dermatol Alergol. 27:390–399. 2010.
|
|
38
|
Wrześniewski K, Sosnowski T and Matusik D:
Inventory of State and Trait Anxiety STAI. Polish adaptation of
STAI. Handbook. Laboratory of Psychological Tests of The Polish
Psychological Association; Warsaw: 2002, (In Polish).
|
|
39
|
Grammatopoulou E, Skordilis E, Koutsouki D
and Baltopoulos G: An 18-item standardized asthma quality of life
questionnaire-AQLQ(S). Qual Life Res. 17:323–332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Szabó V, Borgulya G, Filkorn T, et al: The
variant N363S of glucocorticoid receptor in steroid-induced ocular
hypertension in Hungarian patients treated with photorefractive
keratectomy. Mol Vis. 13:659–666. 2007.PubMed/NCBI
|
|
41
|
Majnik J, Patócs A, Balogh K, et al: A
rapid and simple method for detection of Asn363Ser polymorphism of
the human glucocorticoid receptor gene. J Steroid Biochem Mol Biol.
92:465–468. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kino T, De Martino MU, Charmandari E, et
al: Tissue glucocorticoid resistance/hypersensitivity syndromes. J
Steroid Biochem Mol Biol. 85:457–467. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
van Rossum EF and Lamberts SW:
Polymorphisms in the glucocorticoid receptor gene and their
associations with metabolic parameters and body composition. Recent
Prog Horm Res. 59:333–357. 2004.PubMed/NCBI
|
|
44
|
de Lange P, Koper JW, Huizenga NA, et al:
Differential hormone-dependent transcriptional activation and
-repression by naturally occurring human glucocorticoid receptor
variants. Mol Endocrinol. 11:1156–1164. 1997.
|
|
45
|
Grzanka A and Rogala B: Molecular
mechanism of glucocorticoids and difficult asthma. Allerg Asthma
Immunol. 5:247–252. 2000.
|
|
46
|
Derijk R and de Kloet E: Corticosteroid
receptor polymorphisms: determinants of vulnerability and
resilience. Eur J Pharmacol. 583:303–311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pietras T, Panek M, Tworek D, et al: The
BclI single nucleotide polymorphism of the human glucocorticoid
receptor gene h-GR/NR3C1 promoter in patients with bronchial
asthma: pilot study. Mol Biol Rep. 38:3953–3958. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Panek M, Pietras T, Fabijan A, et al:
Effect of glucocorticoid receptor gene polymorphisms on asthma
phenotypes. Exp Ther Med. 5:572–580. 2013.PubMed/NCBI
|