Introduction
The recent development of osteosynthesis has
revealed a close association between bone fracture treatment and
open reduction and internal fixation (ORIF). A previous study
demonstrated that the potential for nonunion in patients who have
undergone surgical treatment is four times greater than that in
patients who have undergone conservative treatment (1). A general surgical incision results in
excessive damage to the local blood supply (for example, periosteal
stripping), which is a negative factor for fracture healing
(2); however, there are few
studies that have considered the effect of the timing of surgery.
The authors of the present study have observed in the clinic that
delayed bone fracture healing or nonunion occurred in certain
patients who underwent early surgery, whereas satisfactory healing
occurred in patients who received delayed surgery. Thus, the
effects of surgical treatment may be hypothesised to be closely
associated with the local blood supply and the timing of
surgery.
The present study was conducted as a preliminary
investigation into the molecular basis for the secondary
damage-accelerated fracture-healing phenomenon (3,4) and
the optimal time at which to perform surgery following fracture was
determined.
Materials and methods
Animals
A total of 192 Wistar rats (weight, ~220 g) were
used in the present study and provided by the Zhejiang Chinese
Medical University Experimental Animal Centre (Hangzhou, China).
The rats were subjected to consistent feeding conditions at room
temperature (24±2°C) and provided with standard feed (Anritsu mime;
Nanjing Science and Technology Co., Ltd., Nanjing, China), with
access to food and drinking water ad libitum. The rats were
subjected to adaptive feeding for more than one week without
exception prior to their use in the study. The present study was
conducted in strict accordance with the recommendations in the
Regulations for the Administration of Affairs Concerning
Experimental Animals (Ministry of Science and Technology of China,
1988). The animal use protocol was reviewed and approved by the
Institutional Animal Care and Use Committee of Zhejiang Chinese
Medical University (Hangzhou, China).
Closed fracture model
The rats were anaesthetised with 10% sodium
pentobarbital (Shanghai Kefeng Chemical Reagent Co., Ltd.,
Shanghai, China) at a dose of 40 mg/kg via an intraperitoneal
injection. The Einhorn fracture modelling method (5,6) for
animals was employed to create a closed fracture model in the right
femur of the rats. The thumb and index finger were used to assess
the fracture site and determine the type of fracture. The middle
femur fracture type and short oblique fractures were identified to
be common.
Animal grouping and operation
The 192 closed fracture model rats that complied
with the inclusion criteria were randomly divided into seven groups
as follows: Group A, no surgery (n=36); group B, surgery one day
following fracture (n=36); group C, surgery three days following
fracture (n=32); group D, surgery five days following fracture
(n=28); group E, surgery seven days following fracture (n=24);
group F, surgery 11 days following fracture (n=20); and group G,
surgery 14 days following fracture (n=16).
In group A, four modelled rats were sacrificed by
cervical dislocation and observed at 8 h and 3, 5, 7, 11, 14, 21,
35 and 49 days following fracture. ORIF was performed on the rats
in groups B, C, D, E, F and G at 1, 3, 5, 7, 11 and 14 days after
fracture, respectively. The surgery was conducted as follows: The
rats were anaesthetised with 10% sodium pentobarbital at a dose of
40 mg/kg by intraperitoneal injection. They were placed in the
prone position and their right hind limbs were prepared for
surgery. Following a routine disinfection, a posterolateral
incision was made on the right femur, and the subcutaneous tissue
and right rear muscle were separated to reveal the femur fracture.
The periosteum was protected and the fracture was reduced. An
incision was made on the knee joint capsule to expose the lateral
condyle of the femur with an intercondylar diameter of l.5 mm. A
Kirschner wire was inserted to fix the fracture fragments, and the
incision was sutured and bandaged with gauze. The rats were
administered penicillin injections for three days to prevent the
occurrence of postoperative infections.
Four rats from each group (B, C, D, E, F and G) were
sacrificed by breaking of the neck and were observed at different
time points. For group B, the time points were 8 h after surgery,
and 3, 5, 7, 11, 14, 21, 35 and 49 days following fracture. For
group C, the time points were 3 (8 h after surgery), 5, 7, 11, 14,
21, 35 and 49 days following fracture. For group D, the time points
were 5 (8 h after surgery), 7, 11, 14, 21, 35 and 49 days following
fracture. For group E, the time points were 7 (8 h after surgery),
11, 14, 21, 35 and 49 days following fracture. For group F, the
time points were 11 (8 h after surgery), 14, 21, 35 and 49 d
following fracture. For group G, the time points were 14 (8 h after
surgery), 21, 35 and 49 days following fracture.
After the rats were sacrificed, the 1 cm bone tissue
with intact periosteum and bone callus at fracture region was
taken.
Immunohistochemical staining
Immunohistochemical staining for VEGF and BMP-2 was
performed. The bone tissue was fixed in 10% neutral buffered
formalin (Guangzhou Wexis Biotech Co., Ltd., Guangzhou, China),
washed with distilled water, placed in 5% ethylene diamine
tetraacetic acid solution (Beijing Century Aoke Biotech Co., Ltd.,
Beijing, China) and decalcified for 10–15 days. Following
decalcification, the specimens were flushed for 24 h, dehydrated
and embedded in paraffin in 5-μm thick serial sections. The samples
were immunohistochemically stained to detect the expression of VEGF
and BMP-2 (streptavidin biotin complex kit reagent; Wuhan Boster
Biological Technology, Ltd., Wuhan, China) using the
diaminobenzidine chromogenic method; a brown stain indicated a
positive result. The VEGF and BMP-2 content in the callus was
determined using an internet printing protocol computer with a
colour image analysis system (Image-Pro Plus 6.0; Media Cybernetics
Inc., Bethesda, MD, USA). Under a microscope (magnification, ×400;
B×50; Olympus Corp., Tokyo, Japan), five areas were randomly
selected for each specimen to perform the expression counts, and
the count values of the VEGF and BMP-2 expression were taken as the
measured indicators (7,8); the results were statistically
analysed.
Statistical analysis
SPSS software, version 13.0 (SPSS Inc., Chicago, IL,
USA) was used to perform the statistical analysis. The data are
expressed as means ± standard deviation and differences between the
groups were compared using Student’s t-test. P<0.05 was
considered to indicate a statistically significant result.
Results
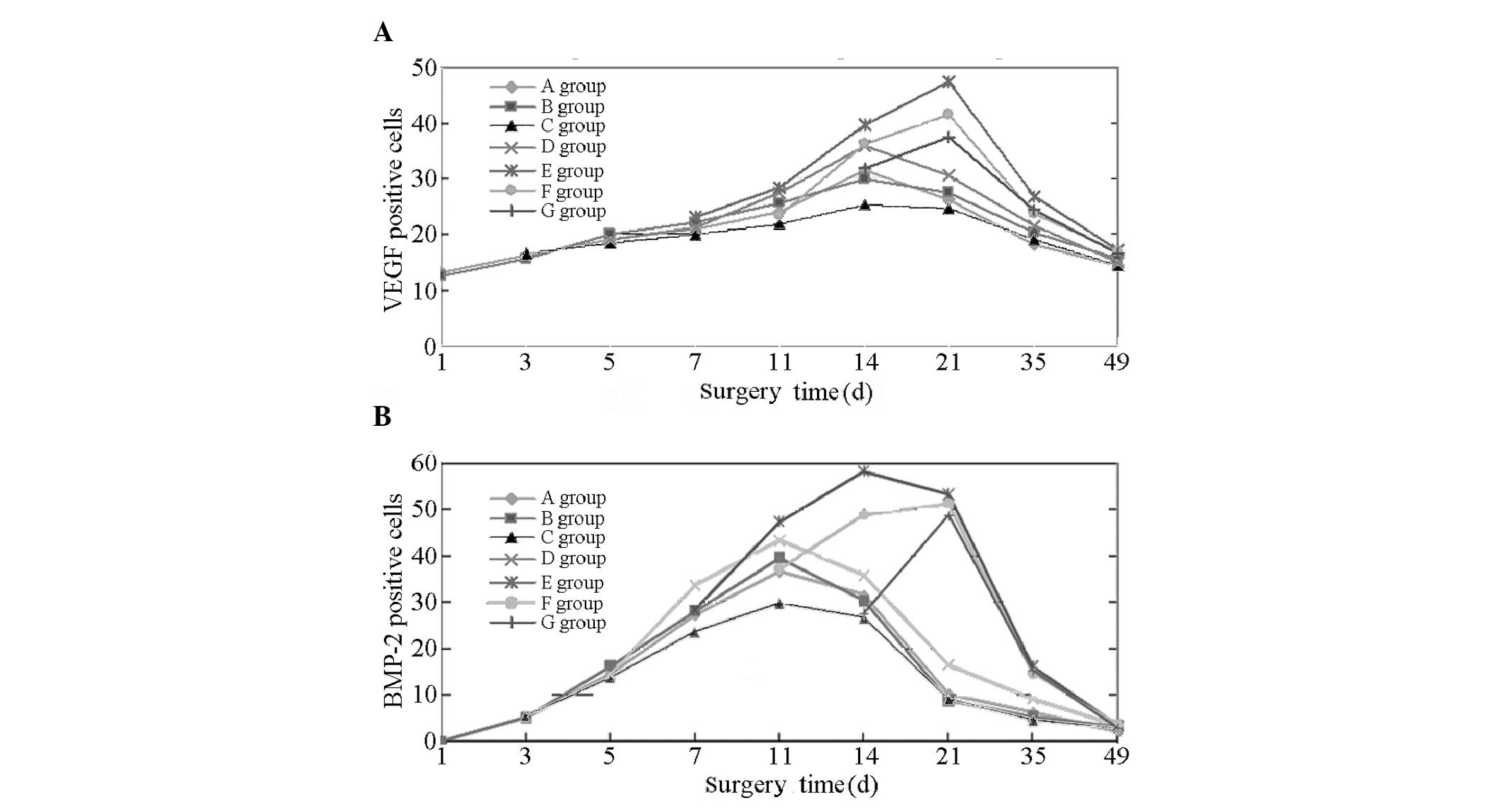
VEGF-positive cells
At 14 days following fracture, the VEGF expression
in groups A, B, C and D peaked and gradually decreased thereafter.
In groups E, F and G, the VEGF expression continued to increase
between 14 and 21 days following fracture and peaked at day 21,
followed by a gradual decline. However, the peak values for groups
F and G demonstrated no significant difference (P>0.05). At day
35 for each group VEGF expression was marginally elevated; however,
the VEGF expression levels in groups E, F and G were highest.
At 14 days, the results of the comparison indicated
that the VEGF expression levels decreased in the order: E, F and D
> A, B and G > C. No significant differences (P>0.05) were
observed in VEGF expression between groups A, B and G. Significant
differences were observed between groups E, F and D; A, B and G;
and C (P<0.05 or P<0.01). At 21 days, the results of the
comparison demonstrated that VEGF expression decreased in the
order: E, F and G > D > A, B and C. No significant
differences were observed between groups A, B and C (P>0.05).
Significant differences were observed between groups E, F and G; D;
and A, B and C (P<0.05 or P<0.01). At 35 days, the results of
the comparison show that the VEGF expression decreased in the
order: E > F and G > A, B, C and D. No significant difference
was observed between groups A, B, C and D (P>0.05). Significant
differences were observed between groups E; F and G; and A, B, C
and D (P<0.05 or P<0.01). At 49 days, the fractures exhibited
gradual healing. A marginal quantity of VEGF expression remained in
each group and the differences observed between the groups were not
identified to be statistically significant (P>0.05; Fig. 1A and Table I).
 | Table IVEGF-positive immunohistochemical
staining cell counts at various times following surgery conducted
at different times after fracture (means ± standard deviation;
n=4). |
Table I
VEGF-positive immunohistochemical
staining cell counts at various times following surgery conducted
at different times after fracture (means ± standard deviation;
n=4).
| Group | Day 1 | Day 3 | Day 5 | Day 7 | Day 11 | Day 14 | Day 21 | Day 35 | Day 49 |
|---|
| A | 13.0±1.52 | 16.2±2.04 | 19.0±2.27 | 21.0±2.86 | 24.2±2.03c,e | 31.5±1.88b,c,f,g | 26.2±3.01f,h,j | 18.1±2.91f,g,i | 14.5±2.40 |
| B | 12.6±1.70 | 15.5±2.30 | 20.1±2.30 | 22.1±2.15 | 25.5±1.95 | 30.0±2.67a,c,f,g | 27.5±2.71f,h,j | 20.3±2.43e | 15.6±2.47 |
| C | - | 16.5±1.40 | 18.3±1.55 | 20.1±2.24 | 22.0±2.34d,f | 25.2±2.50d,f,h | 24.6±3.03f,h,j | 19.2±2.17f,g,i | 14.4±2.44 |
| D | - | - | 19.0±1.97 | 21.2±1.77 | 27.5±1.72 | 35.8±2.78b | 30.5±2.97a,f,h,i | 21.5±2.59e | 15.0±2.33 |
| E | - | - | - | 23.0±2.45 | 28.5±2.50 | 39.8±3.92b | 47.6±4.10 | 26.9±2.66 | 17.2±2.59 |
| F | - | - | - | - | 23.5±2.57c,e | 36.2±3.22b | 41.5±3.80 | 23.8±2.59 | 16.8±2.45 |
| G | - | - | - | - | - | 32.0±3.29a,e | 37.4±3.00f | 24.5±2.83 | 16.6±2.16 |
BMP-2-positive cells
After 11 days, the BMP-2 expression levels in groups
A, B, C and D peaked and gradually decreased thereafter. For groups
E and F, the curve of BMP-2 expression continued to increase 14
days following fracture. Furthermore, BMP-2 expression peaked in
group E on day 14 and began to decline after day 21. BMP-2
expression peaked in groups F and G at 21 days and then gradually
decreased. At 35 days in each group, BMP-2 expression was detected;
however, the expression levels were highest in groups E, F and G.
At day 49, the fractures exhibited gradual healing and only a
marginal quantity of BMP-2 expression was evident in each
group.
On day 1, the expression levels in groups A and B
were not significantly different. On day 3, a low expression level
of BMP-2 was observed in groups A, B and C, with no significant
difference identified between the groups (P>0.05). On day 5, the
expression levels in groups A, B, C and D increased further, with
no significant differences identified between the groups
(P>0.05). By day 7, the expression levels in groups A, B, C, D
and E were increased significantly, in the order: D >A, B and E
> C. A comparison between groups A, B and E indicated no
significant differences (P>0.05). The differences among the
remaining groups were identified to be statistically significant
(P<0.05 or P<0.01). On day 11, the results of the comparison
demonstrated that BMP-2 expression was increased in the order: E
> D >A, B and F > C. The differences between groups A, B
and F were not identified to be statistically significant
(P>0.05); however, the differences were statistically
significant for the remaining groups (P<0.05 or P<0.01). At
day 14, a comparison of BMP-2 expression revealed that: E > F
> D > A, B and G > C. The differences among groups A, B
and G were not identified to be statistically significant
(P>0.05); however, the differences were statistically
significant among the remaining groups (P<0.05 or P<0.01). At
day 21, the comparison of BMP-2 shows the following: E, F and G
> D > A, B and C. The value in group E remained the highest.
Comparisons between two groups (E and F; F and G) indicated no
statistically significant differences (P>0.05). Furthermore, no
significant difference was observed between groups A, B and C
(P>0.05); however, the differences among the remaining groups
were statistically significant (all P<0.05 or P<0.01). At day
35, the expression levels observed in each group decreased and
those in groups E, F and G fell markedly; however, they were
greater than those in the other groups; the expression levels in
groups A, B and C were the lowest. A comparison of BMP-2 expression
levels indicated the following: E, F and G > D >A, B and C.
The differences between groups A, B and C were not identified to be
statistically significant (P>0.05); however, those among the
remaining groups were statistically significant (P<0.05 or
P<0.01). On day 49, a marginal quantity of BMP-2 expression
remained in each group and the differences between the groups were
not identified to be significant (P>0.05; Fig. 1B and Table II).
 | Table IIBMP-2-positive immunohistochemical
staining cell counts at various times following surgery conducted
at different times after fracture (means ± standard deviation;
n=4). |
Table II
BMP-2-positive immunohistochemical
staining cell counts at various times following surgery conducted
at different times after fracture (means ± standard deviation;
n=4).
| Group | Day 1 | Day 3 | Day 5 | Day 7 | Day 11 | Day 14 | Day 21 | Day 35 | Day 49 |
|---|
| A | 0 | 5.2±0.62 | 14.6±1.10 | 27.2±1.12a,d | 36.5±1.88b,d,f | 31.5±1.57b,d,f,h | 10.2±1.10d,f,h,j | 6.2±1.20a,f,h,j | 3.2±0.43 |
| B | 0 | 5.1±0.38 | 16.2±1.72 | 28.1±1.00b,d | 39.4±2.43b,c,f | 30.2±1.66a,d,f,h | 8.9±1.50d,f,h,j | 5.3±1.10d,f,h,j | 3.2±0.52 |
| C | - | 5.4±0.59 | 13.8±1.93 | 23.5±1.72 | 29.8±1.54 | 26.6±1.44 | 9.1±1.16 | 4.5±1.28 | 2.8±0.59 |
| D | - | - | 14.5±1.41 | 33.6±1.75b | 43.2±1.36b,f,h | 35.7±1.25b,f,h | 16.5±1.44b,f,h,j | 9.2±1.30b | 3.5±0.89 |
| E | - | - | - | 28.4±1.30b,d | 47.3±1.72b,h | 58.2±2.38b,d,h | 53.2±2.54b,i | 16.1±1.47bd | 3.3±0.81 |
| F | - | - | - | - | 37.1±1.31b | 48.8±2.11 | 51.1±2.16b | 14.7±1.37bd | 3.6±0.71 |
| G | - | - | - | - | - | 29.6±2.07d,f | 48.7±2.46b | 15.3±0.96bd | 2.9±0.99 |
Discussion
The histological healing process may be accompanied
by a complex biological regulatory mechanism. A large number of
cytokines in the various stages of fracture healing have different
functions. Angiogenesis, which is the premise of fracture repair,
is the restoration of blood flow and VEGF is the predominant
regulator of this process. Furthermore, binding of the vascular
endothelial cell membrane receptor specifically promotes vascular
endothelial cell proliferation and angiogenesis (9). The function of oxygen in angiogenesis
is significant in the fracture segment areas as it provides
nutrients for metabolic waste transport. In addition, it provides a
favourable microenvironment for local bone regeneration and
metabolism. Spector et al (10) found that VEGF in vitro did
not result in osteoblast proliferation, but led to migration and
osteoblast differentiation. Furthermore, the desired concentration
of VEGF which leads to osteoblast migration and differentiation is
100 times lower than that of BMP-2. Another study showed that VEGF
in original osteoblasts has a chemotactic effect on bone formation
and reconstruction in the endochondral bone, particularly in the
coupling of bone formation in the cartilage absorption process
(11). Connoly (12) indicated that fracture healing
relied on the revascularization process and the evaluation of
fracture healing was also entirely dependent on the
revascularization procedures. BMP has a major function in the
differentiation of original bone cells and is significant in
promoting osteogenesis (13–15).
BMP is one of the cell factors of the transforming growth factor 2β
superfamily, which is a group of multifunctional cytokines that
induce the migration, proliferation and differentiation of
mesenchymal cells resulting in cartilage and bone formation. The
majority of studies have focused on the osteogenic effect of BMP-2.
In vivo and in vitro experiments have shown that
BMP-2 is able to induce osteoblast differentiation and bone
formation, and is a potent cytokine (16–18).
Furthermore, the presence of BMP-2 has been observed in various
stages of bone formation (19).
Bouletreau et al (20)
confirmed that hypoxia or the use of exogenous VEGF promotes BMP-2
mRNA and protein expression after 24–48 h of the fracture-healing
process. This finding indicates that vascular endothelial cells
have a function in angiogenesis and BMP-2 expression results in
osteogenesis in the fractured region. Therefore, VEGF and BMP-2
were used as indicators in the present study to observe the
different fracture healing effects following surgery conducted at
various time points after fracture.
The results indicated that the VEGF and BMP-2
expression levels in groups E, F and G were significantly higher
and remained high for longer than the levels in groups A, B, C and
D. In groups E, F and G, the strength of the expression levels were
in the following order: E > F > G. Group D exhibited
relatively low expression levels, which were lower than those
observed in groups E, F and G, and marginally higher than those in
groups A and B; group C demonstrated the lowest expression levels.
The VEGF and BMP-2 expression levels during the fracture healing
process in each group were in the following order: E > F > G
> D > B = A > C. Thus, surgery during the different stages
of fracture healing in rats was shown to affect the VEGF and BMP-2
expression levels and the duration of the expression. Although the
timing of surgery showed no beneficial effects, the 7-day surgery
group demonstrated the highest VEGF and BMP-2 expression levels
during fracture healing, and the effects endured for longer. In
addition, in the 11-day surgery group, the VEGF and BMP-2
expression levels were promoted and the effects endured for a long
time. The expression levels of the 14-day surgery group were
comparable with those in the 7- and 11-day surgery groups. In the
5-day surgery group, the expression intensity was higher than that
observed in the group with natural healing and no surgery. There
was no significant difference between the 1-day surgery group and
non-surgery group, and no significant effect on the VEGF and BMP-2
expression levels at fracture was observed. The three-day surgery
group showed low levels of VEGF and BMP-2 at all stages of fracture
healing and these levels were lower than those of the non-surgery
group.
VEGF was positively expressed during the entire
process of fracture healing. The VEGF expression became visible on
the first day following fracture, whereas BMP-2 expression became
visible in the positively stained cells on day 3. The findings of
the present study were consistent with the study by Bouletreau
et al (20); VEGF may have
induced or promoted the expression of BMP-2. After 7–28 days, the
peak in VEGF expression was accompanied by a peak in angiogenesis;
thus, it was hypothesized that VEGF and visible vascular
reconstruction in the fracture were closely associated. Previous
studies (7,21,22)
have indicated that fractures caused by trauma result in the
partial interruption of blood flow and weak VEGF expression in the
following week. However, the process of fracture repair accelerates
the growth of granulation tissue, reduces the blood supply and
decreases the partial pressure of oxygen. In addition, hypoxia
strongly induces VEGF expression. In the present study, 2–3 weeks
after fracture, VEGF mRNA expression was observed to peak, which
indicated that the synthesis of cartilage cells was occurring. In
the endochondral ossification process, VEGF is involved in the
coordination of cartilage cells for bone cell transformation
(7,21,22).
In the present study, surgery conducted several days after the
fracture procedure may have aggravated the trauma and damaged the
periosteum of the blood vessels, resulting in an interruption of
the increased blood flow and reducing the VEGF expression. In the
initial two weeks, granulation growth around the fracture was
apparent and periosteal thickening occurred. Although the
periosteum was completely cut during surgery, less damage was
observed in blood flow of the fracture end. Furthermore, the time
period of inflammation was extended and local capillary generation
was promoted, which further strengthened the local blood supply.
Thus, surgery contributed to fracture healing, which was closely
associated with VEGF secretion. In groups A and B, BMP-2 expression
was stronger in the initial stage of the fracture healing process
(5–14 days following fracture) and gradually decreased in the later
stage of fracture healing (21–49 days following fracture). The
other groups demonstrated different BMP-2 expression levels due to
the varied timing of the surgery. In the 7- and 14-day surgery
groups, the BMP-2 expression was observed to be more intense and
endured for longer; the BMP-2 expression was most apparent in the
7-day surgery group. The results indicated that performing a
surgical procedure 7 days after the fracture was optimal for
promoting callus BMP-2 release and regeneration.
In conclusion, post-fracture surgical procedures may
affect the VEGF and BMP-2 expression levels in rats. Surgery may
lead to the acceleration of secondary damage. The optimal timing of
surgery was identified to be within one to two weeks of fracture.
Conducting a surgical procedure several days after fracture may not
be conducive to fracture healing.
Acknowledgements
This study was supported by the Education Department
of Zhejiang Province (grant no. 20050869).
References
|
1
|
Borrelli J Jr, Prickett WD and Ricci WM:
Treatment of nonunions and osseous defects with bone graft and
calcium sulfate. Clin Orthop Relat Res. 411:245–254. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wolf JM, Athwal GS, Shin AY and Dennison
DG: Acute trauma to the upper extremity: what to do and when to do
it. Instr Course Lect. 59:525–538. 2010.PubMed/NCBI
|
|
3
|
Cornell CN and Lane JM: Newest factors in
fracture healing. Clin Orthop Relat Res. 227:297–311. 1992.
|
|
4
|
Hulth A: Current concepts of fracture
healing. Clin Orthop Relat Res. 249:265–284. 1989.
|
|
5
|
Makino T, Hak DJ, Hazelwood SJ, Curtiss S
and Reddi AH: Prevention of atrophic nonunion development by
recombinant human bone morphogenetic protein-7. J Orthop Res.
23:632–638. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shefelbine SJ, Simon U, Claes L, et al:
Prediction of fracture callus mechanical properties using micro-CT
images and voxel-based finite element analysis. Bone. 36:480–488.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaspar D, Neidlinger-Wilke C, Holbein O,
Claes L and Ignatius A: Mitogens are increased in systemic
circulation during bone callus healing. J Orthop Res. 21:320–325.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hughes-Fulford M and Li CF: The role of
FGF-2 and BMP-2 in regulation of gene induction, cell proliferation
and mineralization. J Orthop Surg Res. 6:82011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Millauer B, Wizigmann-Voos S, Schnürch H,
et al: High affinity VEGF binding and developmental expression
suggest Flk-1 as a major regulator of vasculogenesis and
angiogenesis. Cell. 72:835–846. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spector JA, Mehrara BJ, Greenwald JA, et
al: Osteoblast expression of vascular endothelial growth factor is
modulated by the extracellular microenvironment. Am J Physiol Cell
Physiol. 280:C72–C80. 2001.PubMed/NCBI
|
|
11
|
Mayr-Wohlfart U, Waltenberger J, Hausser
H, et al: Vascular endothelial growth factor stimulates chemotactic
migration of primary human osteoblasts. Bone. 30:472–477. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Connolly JF: Injectable bone marrow
preparations to stimulate osteogenic repair. Clin Orthop Relat Res.
313:8–18. 1995.PubMed/NCBI
|
|
13
|
Roberts AB, Sporn MB, Assoian RK, et al:
Transforming growth factor type beta: rapid induction of fibrosis
and angiogenesis in vivo and stimulation of collagen formation in
vitro. Proc Natl Acad Sci U S A. 83:4167–4171. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song I, Kim BS, Kim CS and Im GI: Effects
of BMP-2 and vitamin D3 on the osteogenic differentiation of
adipose stem cells. Biochem Biophys Res Commun. 408:126–131. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang H, Song TJ, Li X, et al: BMP
signaling pathway is required for commitment of C3H10T1/2
pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci
U S A. 106:12670–12675. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen D, Harris MA, Rossini G, et al: Bone
morphogenetic protein 2 (BMP-2) enhances BMP-3, BMP-4, and bone
cell differentiation marker gene expression during the induction of
mineralized bone matrix formation in cultures of fetal rat
calvarial osteoblasts. Calcif Tissue Int. 60:283–290. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barradas AM, Fernandes HA, Groen N, et al:
A calcium-induced signaling cascade leading to osteogenic
differentiation of human bone marrow-derived mesenchymal stromal
cells. Biomaterials. 33:3205–3215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bramono DS, Murali S, Rai B, et al: Bone
marrow-derived heparan sulfate potentiates the osteogenic activity
of bone morphogenetic protein-2 (BMP-2). Bone. 50:954–964. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Onishi T, Ishidou Y, Nagamine T, et al:
Distinct and overlapping patterns of localization of bone
morphogenetic protein (BMP) family members and a BMP type II
receptor during fracture healing in rats. Bone. 22:605–612. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bouletreau PJ, Warren SM, Spector JA, et
al: Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression
in microvascular endothelial cells: implications for fracture
healing. Plastic Reconstr Surg. 109:2384–2397. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park SH, O’Connor KM and McKellop H:
Interaction between active motion and exogenous transforming growth
factor Beta during tibial fracture repair. J Orthop Trauma.
17:2–10. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Szczesny G: Molecular aspects of bone
healing and remodeling. Pol J Pathol. 53:145–153. 2002.PubMed/NCBI
|