Introduction
Urokinase-type plasminogen activator (uPA) is one of
the most important family members of the serine proteolytic enzymes
(1). In the human body, uPA
combines with the uPA receptor under physical and pathological
conditions, affecting cell migration, tissue reparation and
mediating the hydrolysis of extracellular matrix proteins, the
degradation of collagenase and the activation of other proteolytic
enzymes, while also directly degrading the extracellular matrix and
basilar membrane (2). uPA has also
been shown to be highly associated with the invasion and metastasis
of tumors and the inflammatory reaction of the synovium. A recent
study demonstrated that the serine protease plays an important role
in matrix metalloproteinase (MMPs)-mediated degradation of
cartilage tissue in arthritis (3).
Although uPA is an important member of the serine protease family,
the mechanisms underlying uPA function in the MMP signaling
transduction pathway remain unknown. Post-transcriptional gene
silencing is known as RNA interference technology (RNAi) and can be
used to specifically inhibit the regulation of gene expression
following transcription. This new method is used for quick
silencing of one specific gene (4). To date, the technique has been widely
applied in functional genomes and gene therapy. In the present
study, a lentiviral vector was constructed to specifically target
the uPA gene in the New Zealand rabbit. The aim of the study was to
establish the foundation for further research on the role of uPA in
the pathogenesis of osteoarthritis (OA).
Materials and methods
Materials and reagents
A total of 3 New Zealand rabbits (age, 2 years) were
purchased from the Experimental Animal Center of Xinjiang Medical
University (Ürümqi, China). Escherichia coli (E. coli) DH5α
and 293T cells had been preserved in the research center. The
lentivirus-negative control (NC) and pGLV-H1-green fluorescent
protein (GFP)+Puro vector were purchased from Zimmer Medical
International Trading Co., Ltd. (Shanghai, China). The restriction
enzymes, BamHI and EcoRI, were obtained from MBI
Fermentas (Amherst, NY, USA). The transfection reagent,
Lipofectamine 2000, as well as TRIzol reagent and Dulbecco’s
modified Eagle’s medium/F12 culture medium were purchased from
Invitrogen Life Technologies (Carlsbad, CA, USA). The Taq
enzyme and plasmid extraction kit were obtained from Takara Bio,
Inc. (Shiga, Japan), while the uPA antibody was purchased from
Abcam (Cambridge, MA, USA). All animals were maintained in the
Animal Facility of the Shihezi University School of Medicine. The
experimental protocol was reviewed and approved by the
Institutional Animal Care and Use Committee of the Shihezi
University, Shihezi, China.
Primer synthesis
Primers for uPA and the β-actin reference were
designed using Oligo 6.0 software (Molecular Biology Insights, Inc.
Cascade, CO, USA), which was conducted by Shanghai Sangon
Biological Engineering Technology & Services Co., Ltd.
(Shanghai, China). The sequences of the primers were as follows:
uPA upstream, 5′-ACTACATTG TCTACCTGGGTCGGTC-3′ and downstream,
5′-ATGCAA GATGAGTTGCTCCACTTC-3′ (amplification length, 86 bp);
β-actin upstream, 5′-CAGGTCATCACCATCGGCAAC-3′ and downstream,
5′-GGATGTCCACGTCGCACTTCA-3′ (amplification length, 133 bp).
Cell culture
Cartilage tissue was obtained from the knee-joint of
a two-year-old New Zealand rabbit. The cartilage cells were
collected from cartilage tissue by repeated digestion for four
times, then cultured at flask with culture medium at 37°C in a 5%
CO2 humidified incubator. The medium was changed every
two days. Cells were observed under an inverted microscope and
images and observations were recorded with regard to the cellular
morphology and adherence conditions. Further experiments were
conducted when the cells are 85–90% in a confluent state for later
use.
Designing and synthesizing uPA-siRNA
Using GenBank, the uPA gene sequence in rabbits was
identified (NM-001082011). Referring to the design principles of
the targeted point of siRNA, four targeted points were selected and
designed using GenePharma siRNA designer v 3.0 software (Shanghai
GenePharma, Shanghai, China), which were P1, P2, P3 and P4
(Table I). A pervasive disturbing
sequence was also designed as the NC. The structure of the small
hairpin RNA (shRNA) chain was positive-sense strand-loop-antisense
strand. Each group of sequences were designed and synthesized
according to the hairpin structural model. Restriction enzyme
digestion was conducted at the two ends of the molecules, thus, the
specific gene sequence was inserted into the vector directly
following digestion (Table II).
The sequencing fragments were synthesized by Zimmer Medical
International Trading Co., Ltd (Shanghai, China).
 | Table ITarget sequences for the uPA gene. |
Table I
Target sequences for the uPA gene.
| Vector | Gene locus | Effect | Sub-sequence |
|---|
| P1 | PLAU-Oc-795 | Interference target
gene |
GCGCCACACATTGCTTCATTA |
| P2 | PLAU-Oc-855 | Interference target
gene |
GGTCAAGGCTTAACTCCATGA |
| P3 | PLAU-Oc-901 | Interference target
gene |
GGAGCAACTCATCTTGCATGA |
| P4 | PLAU-Oc-676 | Interference target
gene |
GGGAGAATTCACCATCATTGA |
| NC | / | Negative control |
UUCUCCGAACGUGUCACGUTT |
 | Table IIDesign and synthesis of uPA shRNA
targeted sequences. |
Table II
Design and synthesis of uPA shRNA
targeted sequences.
| Vector | Sense | 5′ | STEMP | LOOP | STEMP | 3′ |
|---|
| P1 | S | GATCC |
GCGCCACACATTGCTTCATTA | TTCAAGAGA |
TAATGAAGCAATGTGTGGCGC | TTTTTTG |
| AS | AATTCAAAAAA |
GCGCCACACATTGCTTCATTA | TCTCTTGAA |
TAATGAAGCAATGTGTGGCGC | G |
| P2 | S | GATCC |
GGTCAAGGCTTAACTCCATGA | TTCAAGAGA |
TCATGGAGTTAAGCCTTGACC | TTTTTTG |
| AS | AATTCAAAAAA |
GGTCAAGGCTTAACTCCATGA | TCTCTTGAA |
TCATGGAGTTAAGCCTTGACC | G |
| P3 | S | GATCC |
GGAGCAACTCATCTTGCATGA | TTCAAGAGA |
TCATGCAAGATGAGTTGCTCC | TTTTTTG |
| AS | AATTCAAAAAA |
GGAGCAACTCATCTTGCATGA | TCTCTTGAA |
TCATGCAAGATGAGTTGCTCC | G |
| P4 | S | GATCC |
GGGAGAATTCACCATCATTGA | TTCAAGAGA |
TCAATGATGGTGAATTCTCCC | TTTTTTG |
| AS | AATTCAAAAAA |
GGGAGAATTCACCATCATTGA | TCTCTTGAA |
TCAATGATGGTGAATTCTCCC | G |
| NC | S | GATCC |
UUCUCCGAACGUGUCACGUTT | TTCAAGAGA |
AAACGTGACACGTTAGGAGAA | TTTTTTG |
| AS | AATTCAAAAAA |
UUCUCCGAACGUGUCACGUTT | TCTCTTGAA |
AAACGTGACACGTTAGGAGAA | G |
Interference lentiviral vector
construction
As shown in Table
II, following the dilution of the oligonucleotide fragments,
double stranded DNA fragments were formed in the annealing reaction
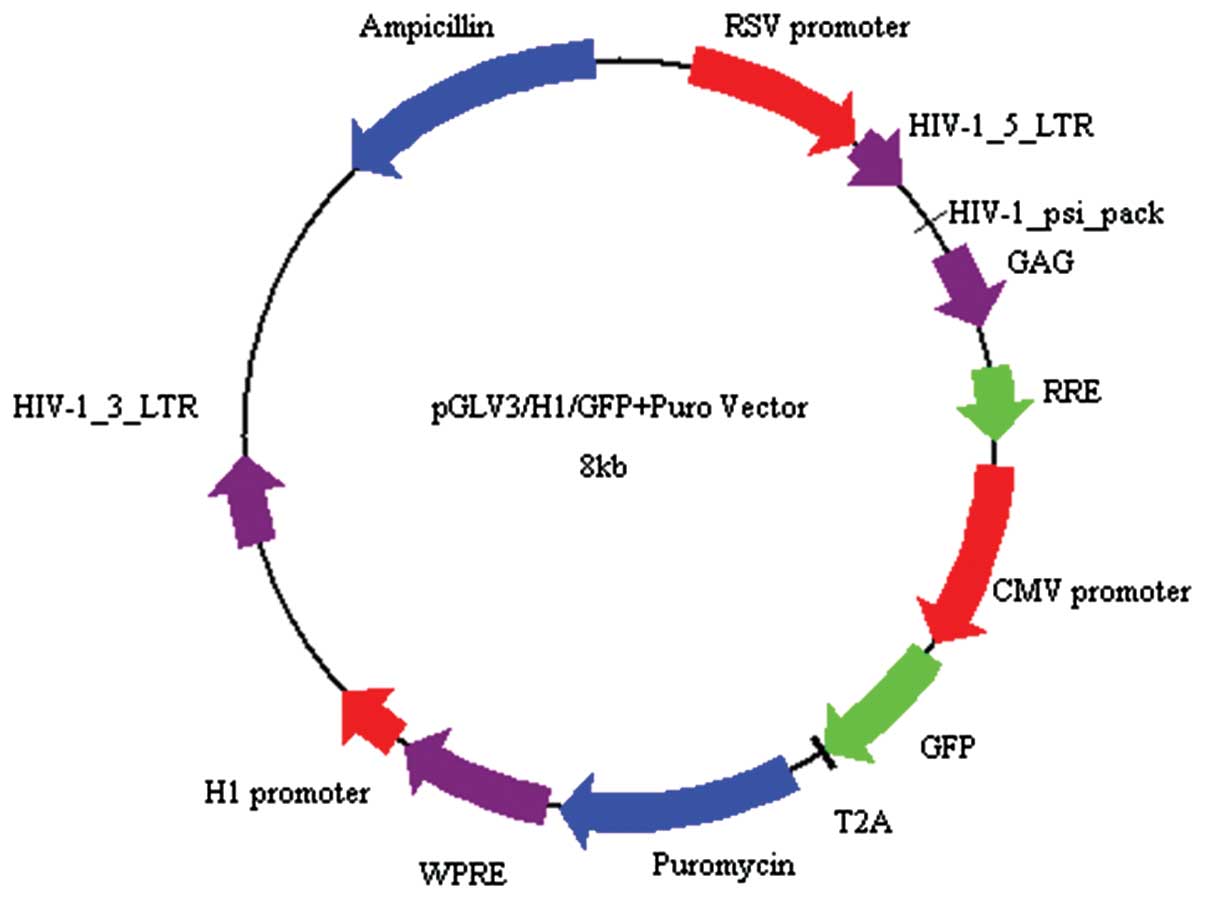
system. The pGLV-HI-GFP+Puro vector was linearized by BamHI
and EcoRI restriction enzyme digestion (Fig. 1). Pure linearized vector fragments,
double stranded DNA fragments and vector fragments were collected
and combined together during a 12-h reaction. The recombinant
vector loop was then transformed into the freshly prepared E.
coli competent cells. Following E. coli cell culture for
16 h at 37°C, bacterial colonies were selected randomly as
polymerase chain reaction (PCR) templates. Verification of the
positive clones was conducted using PCR technology. The sequencing
fragments were synthesized by Shanghai Sangon Biological
Engineering Technology & Services Co., Ltd.
Recombinant lentiviron packaging and
titering
Using uPA genes, RNAi lentiviral vectors and pMD2.G
plasmids were tranfected together in 293T cells. After 8 h, the
culture medium was changed to complete medium. The supernatant was
concentrated and collected after culturing for 48 h. The virus
titer of the 293T cells was determined using the dilution gradient
method and calculated as follows: Virus titer (TU/ml) = (counted
fluorescent cells/corresponding dilution times)/0.01. The
transfected cells were stored in a −80°C refrigerator for later
use.
Examination of cell transfection and the
transfection rate
Packaged recombinant lentivirons were classified
into three groups based on multiplicity of infection (MOI) values
(1, 10 or 100), and were transfected into the cartilage cells
separately. The culture medium was changed to complete medium at 6
h following transfection. Cells were cultured for 3 days, following
which the expression level of GFP in the cells was observed using
an inverted fluorescence microscope. The transfection rate was also
analyzed using Image-Pro Plus 6.0 software (Media Cybernetics,
Inc., Rockville, MD, USA).
Quantitative PCR (qPCR)
Total RNA was extracted at day 4 following
transfection using TRIzol reagent. Reverse transcription of the RNA
to cDNA was conducted using the RNA as a template, and then
PCR-amplification of the uPA gene was performed using the cDNA as a
template. The primers were designed by Shanghai Sangon Biological
Engineering Technology & Services Co., Ltd. The qPCR conditions
were as follows: Primary degeneration for 15 sec at 95°C,
degeneration for 5 sec at 95°C and annealing for 30 sec at 60°C,
which was repeated 40 times. Light absorption values were assayed
at each extension stage. Following PCR, degeneration of the PCR
product was conducted for 1 min at 95°C, which was then cooled-down
to 55°C in order for the DNA double strands to combine together
fully. A melting curve was constructed using the light absorption
values.
Western blot analysis
Total protein was extracted at day 4 following
transfection and gel-electrophoresis was conducted using 10%
SDS-PAGE following protein quantification. Next, the proteins were
transferred to a nitrocellulose membrane and then incubated with
primary antibodies including PLAU (1:5,000 dilute, GeneTex Inc.,
Irvine, CA, USA) and β-actin (1:10,000 dilute. Abcam Inc.,
Shanghai, China) overnight at 4°C followed with anti-rabbit second
antibodies (1:10,000 dilute, GeneTex Inc.) at room-temperature for
one hour. Enhanced chemiluminescence was used for detection.
β-actin was used as the internal reference.
Statistical analysis
All the results are expressed as the mean ± standard
deviation. Data were analyzed by single factor analysis of variance
and the t-test using SPSS 13.0 software (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Vector construction and verification of
the positive clone using PCR
The size of the PCR amplification product, which was
from the positive clone of the inserted shRNA sequence, was 384 bp
according to the primer size. By contrast, the size of the PCR
amplification product for the empty vector clone was 322 bp
(Fig. 2). These results
demonstrated that all five random clones were positive clones. The
sequencing results of the shRNS carrier vector revealed the
expected sequence, indicating that the shRNA vector was constructed
successfully. DNA sequencing further demonstrated that the sequence
was correct and showed no mutations.
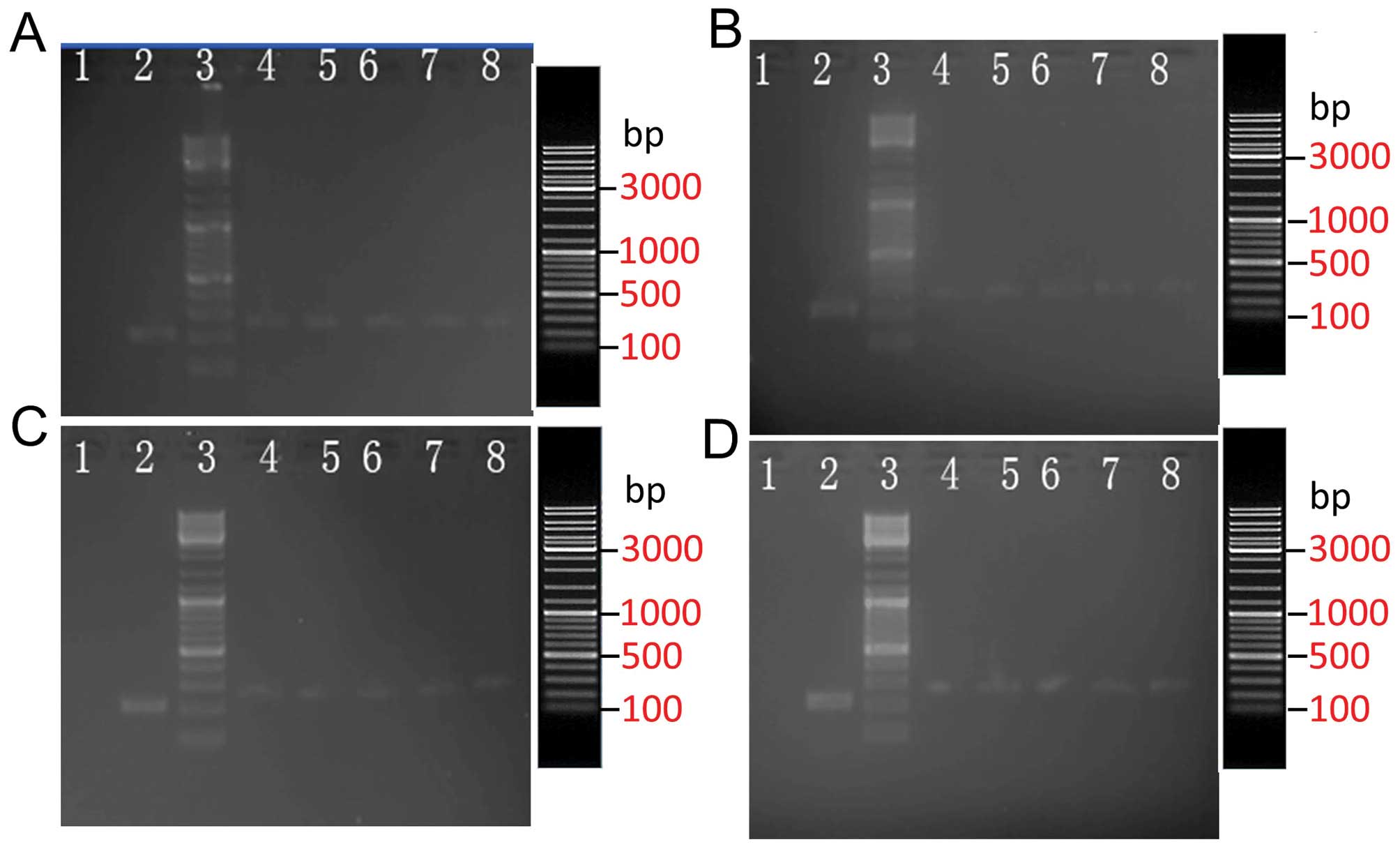 | Figure 2Electrophores of (A) P1, (B) P2, (C)
P3 and (D) P4 sequence vectors and PCR products. Lanes: 1,
ddH2O NC; 2, control; 3, markers (19.3, 7.7, 6.2, 4.2,
3.4, 2.6, 1.8, 1.4 and 0.9 Kb); 4–8, positive colony group. PCR,
polymerase chain reaction; NC, negative control. |
Lentivirus packaging and viral titer
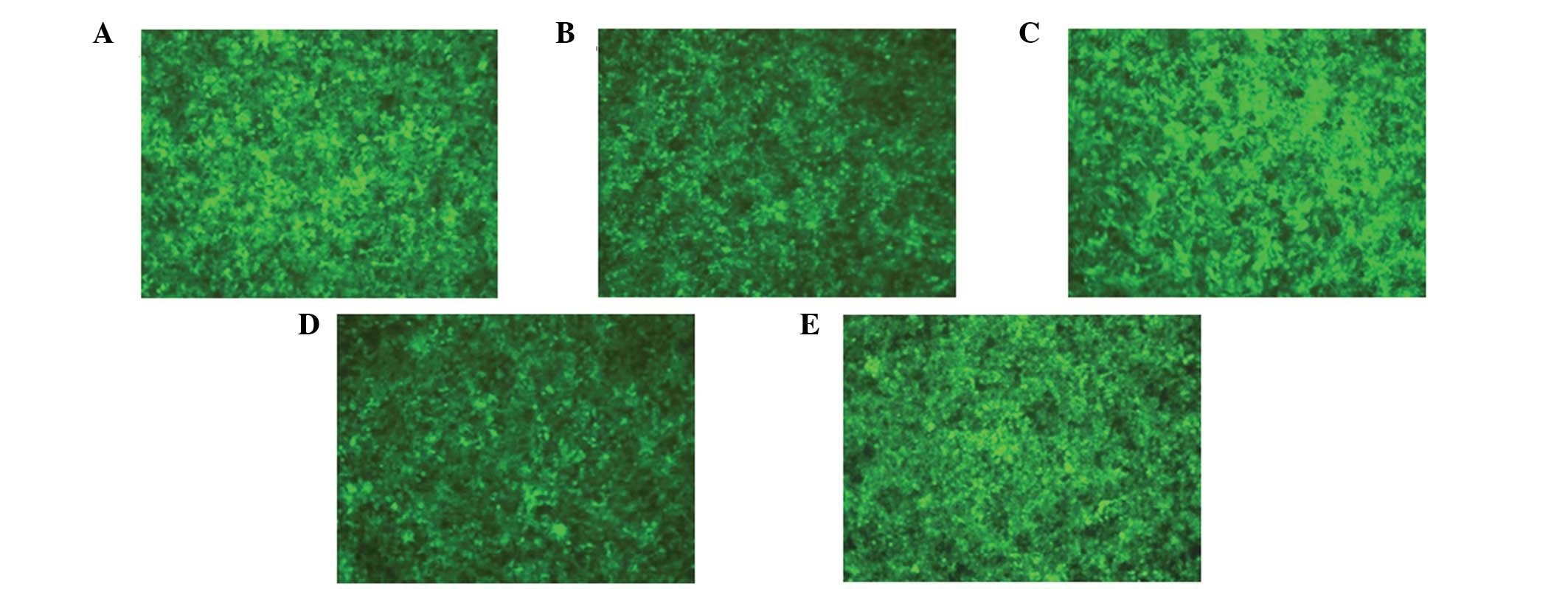
293T cells were transfected using the successfully
constructed uPA RNAi lentiviral vectors and pMD2.G coated plasmids.
Significant expression of GFP was observed under the fluorescence
microscope after 40 h (Fig. 3),
and the biological titer for the collected and concentrated virus
was 1×108 TU/ml, as determined by the hole dilution
method.
Examination of the lentivirus
transfection rate
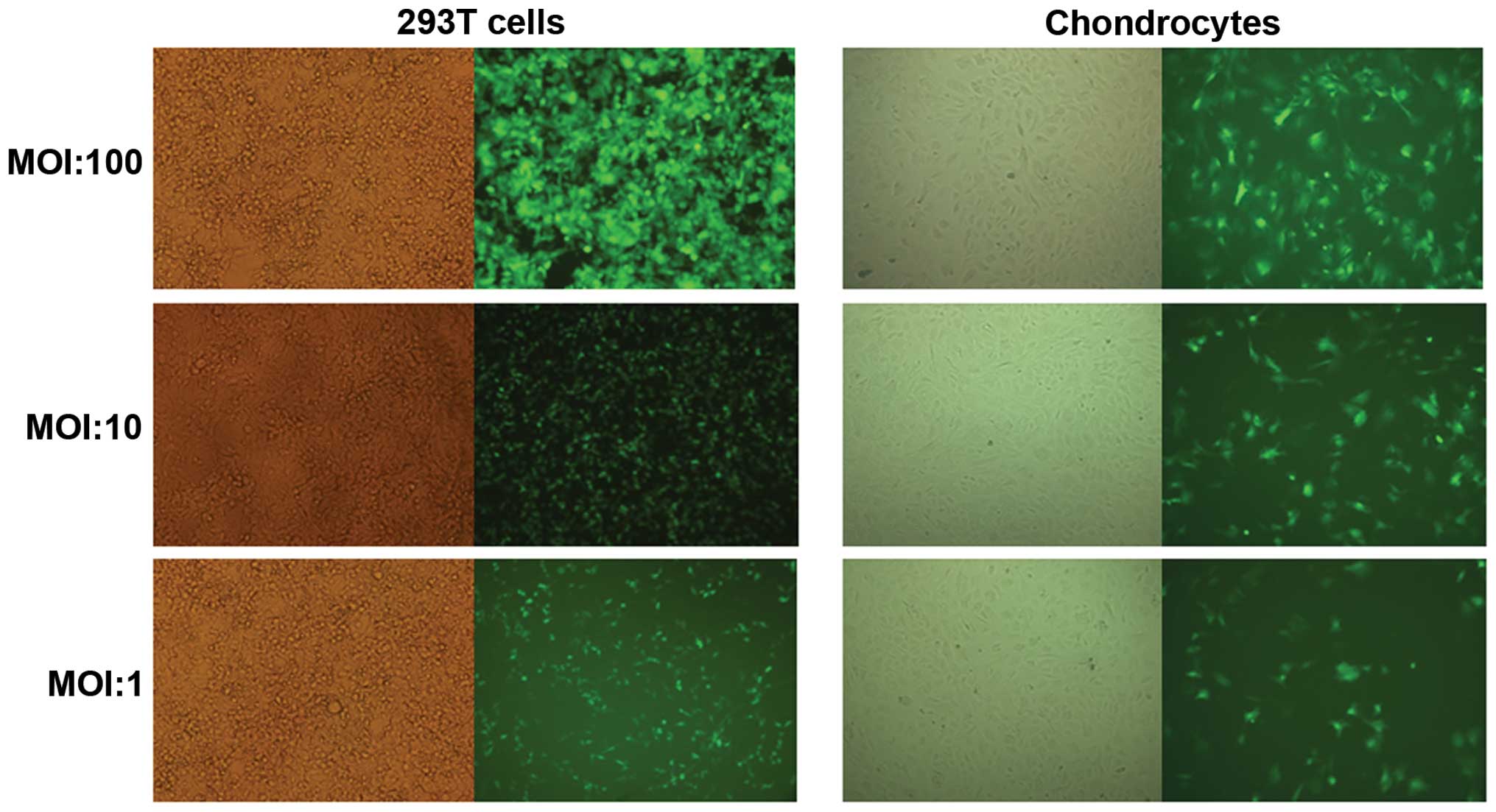
Transfection rates of the four lentiviral vectors in
293T cells and cartilage cells were observed under the various MOI
values. The results demonstrated that with increasing MOI, the
lentivirus transfection rate also improved. When the MOI was 100,
the transfection rate was >85% (Fig. 4), which was which was used for the
following experiments.
Examination of the interference rate of
uPA-siRNA
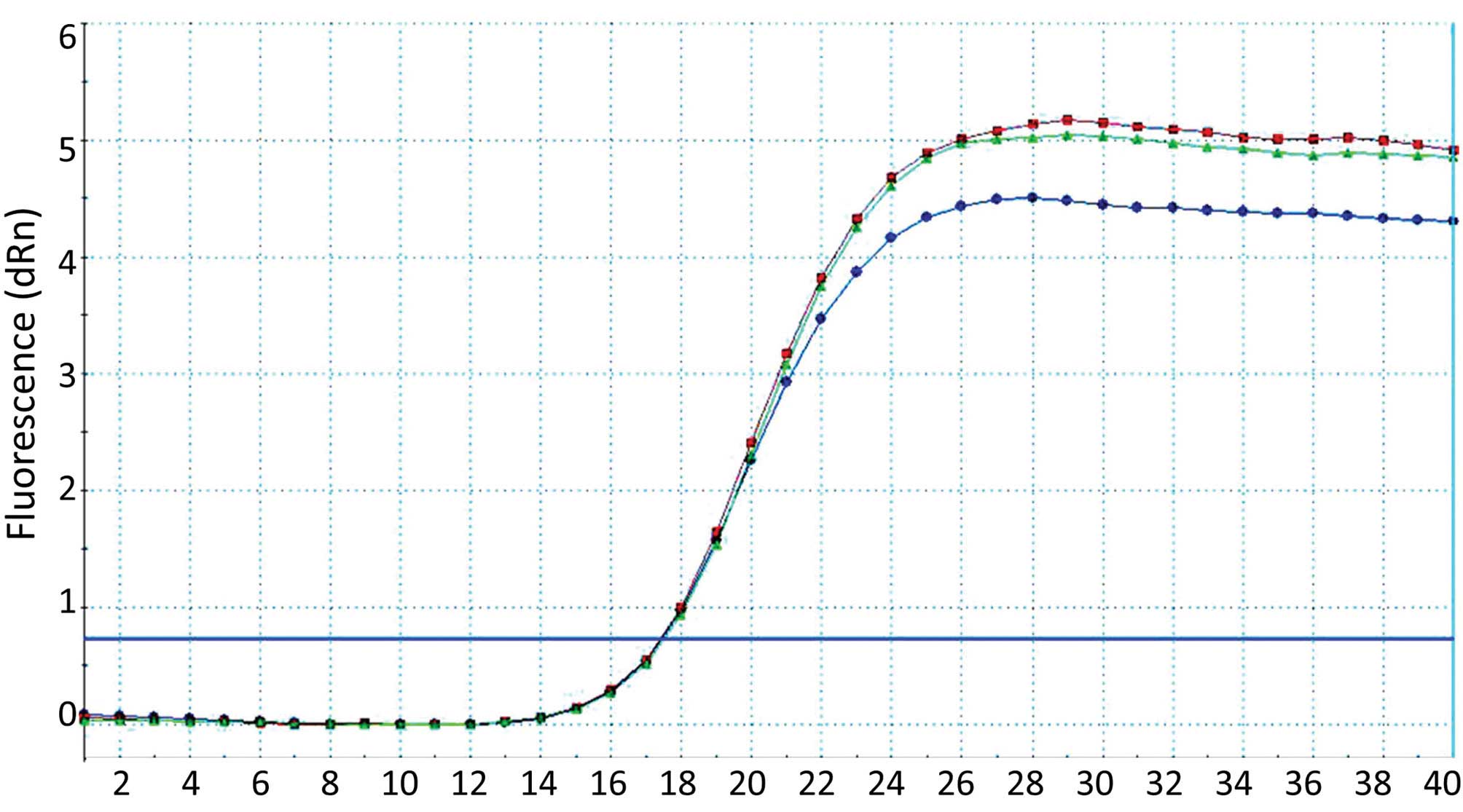
Relative qPCR was performed with β-actin as the
internal reference and the results were analyzed using the standard
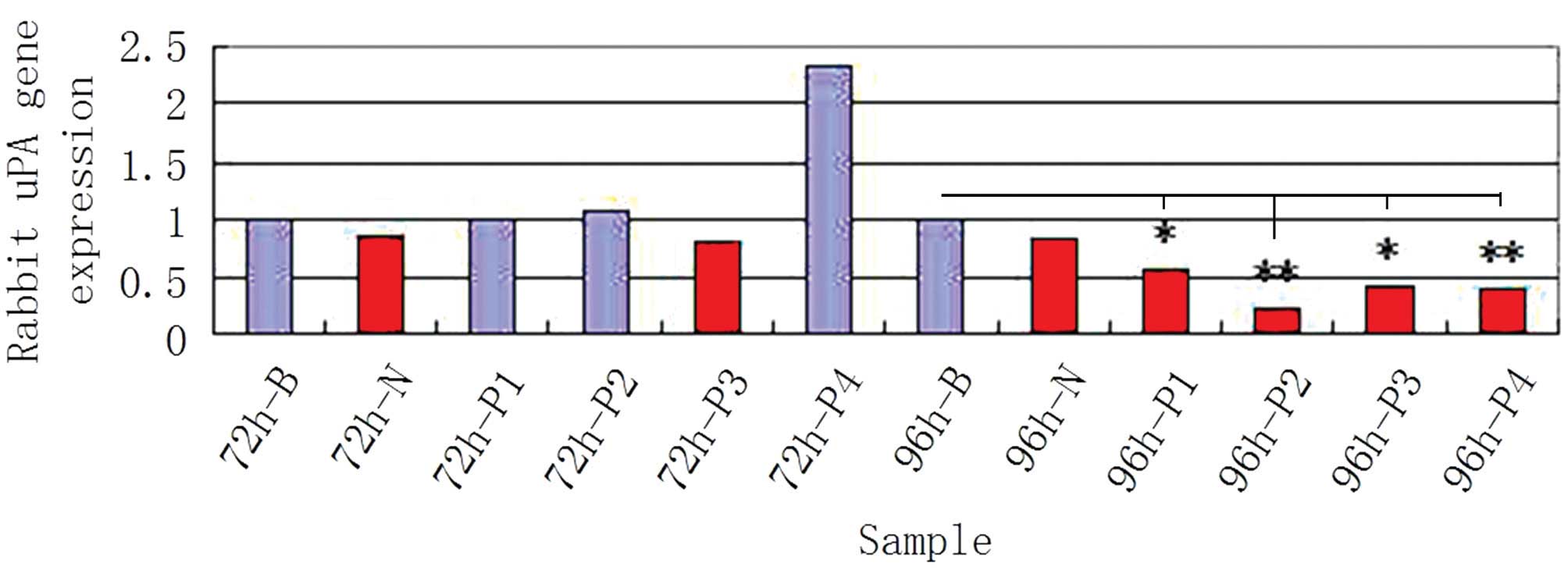
curve method. The results indicated that the mRNA expression level
of uPA decreased significantly following transfection of P1, P2, P3
or P4 plasmids into the cartilage cells (Fig. 5). In addition, the corresponding
rates for the empty plasmid and NC increased, further demonstrating
that these four plasmids exhibited a significant inhibiting
influence on uPA (Fig. 6).
Furthermore, the knockout effectiveness for the P2 RNAi plasmid was
better compared with the other three plasmids. This observation was
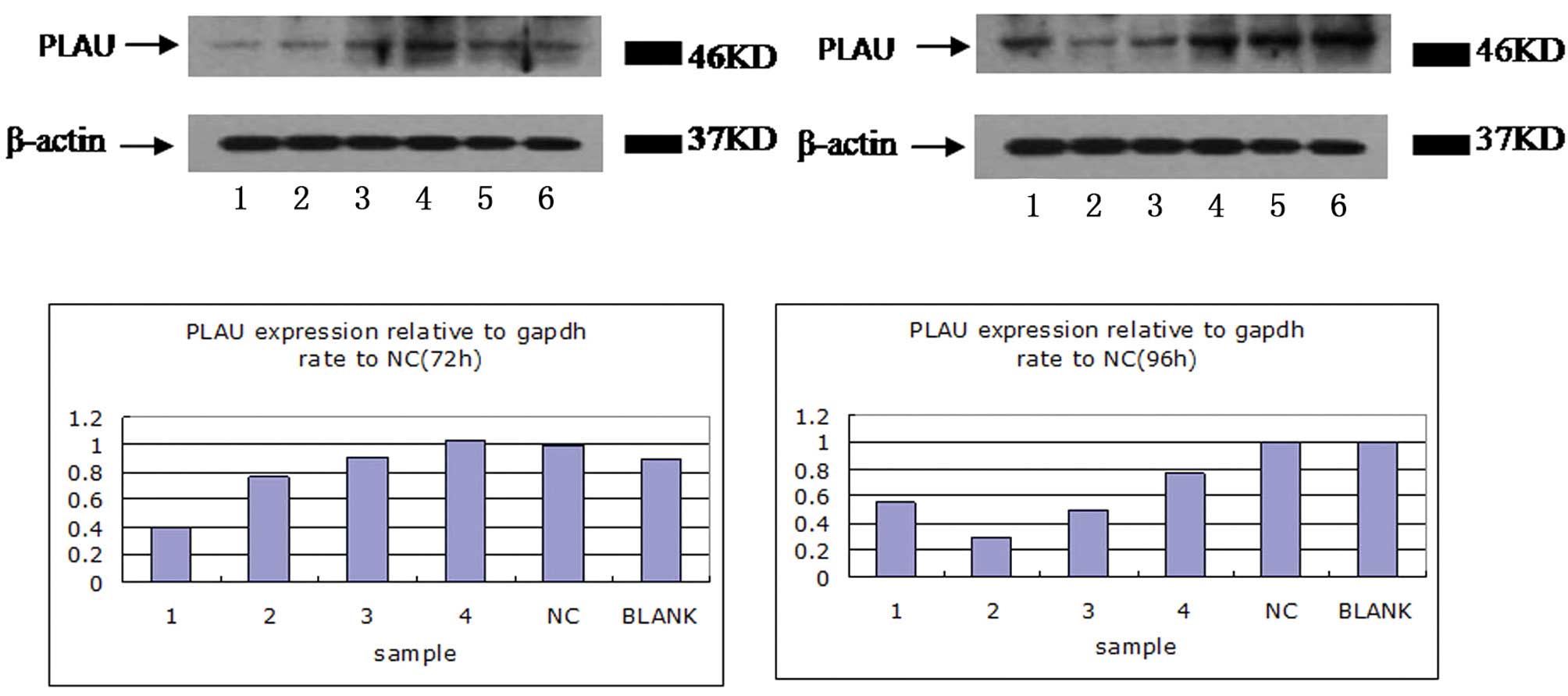
confirmed by western blot analysis. The four plasmids all inhibited
the protein expression of uPA, however, the knockout effectiveness
for P2 and P3 RNAi plasmids was better compared with the P1 and P4
RNAi plasmids (Fig. 7; P<0.05).
Therefore, the P2 plasmid may have the most efficient inhibiting
effect on uPA.
Discussion
OA is a progressive degenerative disease with the
main pathological feature of mechanical abnormalities in the
cartilage tissue (5). Although
there have been a number of comprehensive clinical and fundamental
studies, the definite pathomechanism underlying the pathological
changes remains unclear, thus, clinical treatment is becoming more
difficult (6–8). In general, OA is considered to be a
chronic degenerative disorder, involving all areas of the joint,
including cartilage tissue, subchondral bone and the synovium
(9). OA may be caused by a
combination of multiple factors, including tissue damage,
supersession, metabolization, genetics, heredity and immunization
(10,11). Based on a number of in-depth
studies that used molecular biological technology, it has been
identified that MMPs, uPA, A disintegrin and metalloproteinase with
thrombospondin motifs, among other proteolytic enzymes, are the
most fundamental factors contributing to the degradation process of
cartilage tissue (12–16). In addition, the uPA that is
secreted by macrophages in the synovium has been shown to play an
important role in the primary stages of OA, by causing the synovial
tissue to secrete large amounts of interleukin-1β, which in turn is
involved in increasing the synovial inflammatory reaction and the
degradation process. Furthermore, uPA can directly affect the
degradation of the extracellular matrix in cartilage cells and
activate zymogens of MMPs, transforming them into active protein
degradation enzymes that upgrade the degradation of the
extracellular matrix in cartilage cells. In addition, uPA is
involved in the regulation of mitogen-activated protein kinase and
other signaling pathways, thus, can influence the metabolic process
of cartilage cells (3,17,18).
Therefore, reducing the early degenerative metabolic process of
cartilage tissue can be achieved by inhibiting the expression of
uPA in the synovial and cartilage tissues.
RNAi is a biological process that causes specific
homologous mRNA degradation following internal or external double
stranded RNA entering the cells. The technique inhibits the
expression of the corresponding gene, which is followed by the
phenomenon of specific gene deletion. RNAi technology was developed
based on this model and has become a newly developed biotechnology
in recent years. The technique is a much simpler, more efficient
and powerful tool compared with gene knockout, and plays an
important role in gene functioning and gene treatment. Numerous
studies have shown that RNAi inhibits gene expression definitely,
and even applying traces of siRNA can decrease the coded pathogenic
gene production by 90% (19–21).
Furthermore, RNAi can knockout all the specific genes during an
experiment (22,23). Due to the cascading amplification
effect and the highly penetrative characteristic of RNAi, its usage
has a significant prospect in gene functional research and gene
treatment. However, previous biochemistry and adenoviral vector
methods resulted in inefficient and unstable transfection, thus,
successful transfection of the RNAi sequence into the original
targeted cells has become a barrier for the application of this
procedure. However, as a result of the recent identification of the
lentiviral vector, the transfection rate can reach 70% and gene
expression is stable for a long time in cells during cell
tranfection. In order to assess the effect of uPA in gene treatment
for the early pathological changes of OA, in the present study, a
specific targeted lentiviral vector was successfully constructed
against the uPA gene in a New Zealand rabbit. The targeted
sequence, which can highly silence the uPA gene, was screened out,
thus, this study established the foundation for further research on
the role of uPA in the pathogenesis of OA.
In conclusion, based on the uPA gene, the four
designed shRNA recombinant lentiviral vectors were all shown to
accomplish gene interference in the original cartilage cells of the
New Zealand rabbit. Expression levels of the targeted uPA gene
prior to and following silencing were compared using qPCR, and
differences in the silencing effectiveness were identified among
the four types of shRNA recombinant lentiviral vectors. Silencing
effectiveness was closely associated with the targeted sequence and
virus titer. The more concentrated the vectors during transfection,
the better the silencing effect. The combined results from qPCR and
western blot analysis revealed that P2 specific targeted-shRNA
sequence exhibited significant silencing efficacy. The results of
the present study have established the foundation for using this
targeted point to improve the research on the application of uPA in
OA development and gene treatment.
Acknowledgements
The study was supported by grants from the National
Science Foundation of China (nos. 30960387 and 81260453) and the
Xinjiang Bingtuan Special Program of Medical Science (nos.
2013BA020, 2012BC002 and 2011BC004).
References
|
1
|
Eden G, Archinti M, Furlan F, Murphy R and
Degryse B: The urokinase receptor interactome. Curr Pharm Des.
17:1874–1889. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kwaan HC and McMahon B: The role of
plasminogen-plasmin system in cancer. Cancer Treat Res. 148:43–66.
2009. View Article : Google Scholar
|
|
3
|
Kim KS, Lee YA, Choi HM, Yoo MC and Yang
HI: Implication of MMP-9 and urokinase plasminogen activator (uPA)
in the activation of pro-matrix metalloproteinase (MMP)-13.
Rheumatol Int. 32:3069–3075. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang JT and Gong SS: Effects of siRNA
specific to the protein kinase CK2α on apoptosis of laryngeal
carcinoma cells. Chin Med J (Engl). 125:1581–1585. 2012.
|
|
5
|
Zeng QY, Zang CH, Li XF, Dong HY, Zhang AL
and Lin L: Associated risk factors of knee osteoarthritis: a
population survey in Taiyuan, China. Chin Med J (Engl).
119:1522–1527. 2006.PubMed/NCBI
|
|
6
|
Racine J and Aaron RK: Pathogenesis and
epidemiology of osteoarthritis. R I Med J. 96:19–22.
2013.PubMed/NCBI
|
|
7
|
Maldonado M and Nam J: The role of changes
in extracellular matrix of cartilage in the presence of
inflammation on the pathology of osteoarthritis. Biomed Res Int.
Aug 28–2013.(Epub ahead of print). View Article : Google Scholar
|
|
8
|
Sengupta K, Krishnaraju AV, Vishal AA, et
al: Comparative efficacy and tolerability of 5-Loxin and Aflapin
against osteoarthritis of the knee: a double blind, randomized,
placebo controlled clinical study. Int J Med Sci. 7:366–377. 2010.
View Article : Google Scholar
|
|
9
|
Iagnocco A and Naredo E: Osteoarthritis:
research update and clinical applications. Rheumatology (Oxford).
51(Suppl 7): vii2–vii5. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Loeser RF: Aging processes and the
development of osteoarthritis. Curr Opin Rheumatol. 25:108–113.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Haseeb A and Haqqi TM: Immunopathogenesis
of osteoarthritis. Clin Immunol. 146:185–196. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kapoor M, Martel-Pelletier J, Lajeunesse
D, et al: Role of proinflammatory cytokines in the pathophysiology
of osteoarthritis. Nat Rev Rheumatol. 7:33–42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mobasheri A: Osteoarthritis year 2012 in
review: biomarkers. Osteoarthritis Cartilage. 20:1451–1464.
2012.PubMed/NCBI
|
|
14
|
Koskinen A, Vuolteenaho K, Nieminen R, et
al: Leptin enhances MMP-1, MMP-3 and MMP-13 production in human
osteoarthritic cartilage and correlates with MMP-1 and MMP-3 in
synovial fluid from OA patients. Clin Exp Rheumatol. 29:57–64.
2011.PubMed/NCBI
|
|
15
|
Lee AS, Ellman MB, Yan DA, et al: A
current review of molecular mechanisms regarding osteoarthritis and
pain. Gene. 527:440–447. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murab S, Chameettachal S, Bhattacharjee M,
Das S, Kaplan DL and Ghosh S: Matrix-embedded cytokines to simulate
osteoarthritis-like cartilage microenvironments. Tissue Eng Part A.
19:1733–1753. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yeh CC, Chang SF, Huang TY, et al: Shear
stress modulates macrophage-induced urokinase plasminogen activator
expression in human chondrocytes. Arthritis Res Ther. 15:R532013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yuasa T, Otani T, Koike T, Iwamoto M and
Enomoto-Iwamoto M: Wnt/beta-catenin signaling stimulates matrix
catabolic genes and activity in articular chondrocytes: its
possible role in joint degeneration. Lab Invest. 88:264–274. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kubowicz P, Żelaszczyk D and Pękala E:
RNAi in clinical studies. Curr Med Chem. 20:1801–1816. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shim MS and Kwon YJ: Efficient and
targeted delivery of siRNA in vivo. FEBS J. 277:4814–4827. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chu X, You H, Yuan X, et al: Protective
effect of lentivirus-mediated siRNA targeting ADAMTS-5 on cartilage
degradation in a rat model of osteoarthritis. Int J Mol Med.
31:1222–1228. 2013.PubMed/NCBI
|
|
22
|
Wilson RC and Doudna JA: Molecular
mechanisms of RNA interference. Annu Rev Biophys. 42:217–239. 2013.
View Article : Google Scholar
|
|
23
|
Mekkawy AH, Morris DL and Pourgholami MH:
Urokinase plasminogen activator system as a potential target for
cancer therapy. Future Oncol. 5:1487–1499. 2009. View Article : Google Scholar : PubMed/NCBI
|