Introduction
Growth hormone deficiency (GHD), caused by problems
arising in the pituitary gland, is a medical condition in which the
body does not produce sufficient growth hormone (GH). GH is a
polypeptide hormone that stimulates growth and cell reproduction.
Idiopathic short stature (ISS) may be one of the causes of short
stature (1). This condition refers
to short children without an identifiable disorder of the
GH/insulin-like growth factor axis or other endocrine, genetic or
organ system disorders (2). GHD is
associated with a marked variety of neuroanatomical abnormalities,
including a hypoplastic pituitary gland, as identified by magnetic
resonance imaging (MRI). Neuroimaging has become an essential part
of the diagnostic process for children with GHD in measuring gland
size due to the excellent contrast and high spatial resolution
(3,4). Currently, the majority of pituitary
gland measurements are focused on height, which is considered to be
the standard indicator for pituitary gland size (5,6).
However, the size and shape of the normal pituitary gland vary
considerably and are also affected by age, gender and the hormonal
environment (5–10). The variation in shape of the
pituitary gland between individuals means that any assessment of
size is likely to be subject to a high degree of imprecision unless
a true volume is measured (11).
Previously, studies directly measured and indirectly
calculated pituitary gland volumes using three-dimensional (3D)
volumetry (11–14) and two-dimensional (2D) thin-slice
MRI, respectively, for more precise assessments (15,16).
Fink et al recommended that one-dimensional (height) and
indirect (2D) estimations of pituitary gland size and volume should
be replaced by direct volumetric analysis (11). However, only a few studies
(12) have focused on adolescents
or children with short stature.
In the present retrospective study, high-field
strength, high-resolution and thin-section 3D MRI sequences were
applied to directly measure the volume and height of the pituitary
gland in healthy children and children with GHD or ISS. The
volumetry values in the assessment of pituitary gland size and in
the diagnosis of pituitary gland lesions were investigated.
Materials and methods
MRI acquisition and volumetric
measurements
MRI was performed using a 3.0-T system with an
eight-channel quadrature head coil (MAGNETOM Verio; Siemens,
Munich, Germany). Thin-section volumetric studies were conducted
with a sequence of magnetization-prepared rapid gradient echo
imaging. The following parameters were used: Repetition
time/excitation, 1,900 msec/2.45 msec/1; section thickness, 1 mm;
inversion time, 900 msec; flip angle, 9°; field of view, 250 mm;
and matrix, 256 × 246. The total imaging time was 4 min 18 sec.
MRI scans were processed with a Syngo MAGNETOM Verio
system (Siemens). In all the cases, the volume was measured on the
sagittal image as the boundary is simple to define in this
orientation. The regions of interest (ROI) were determined
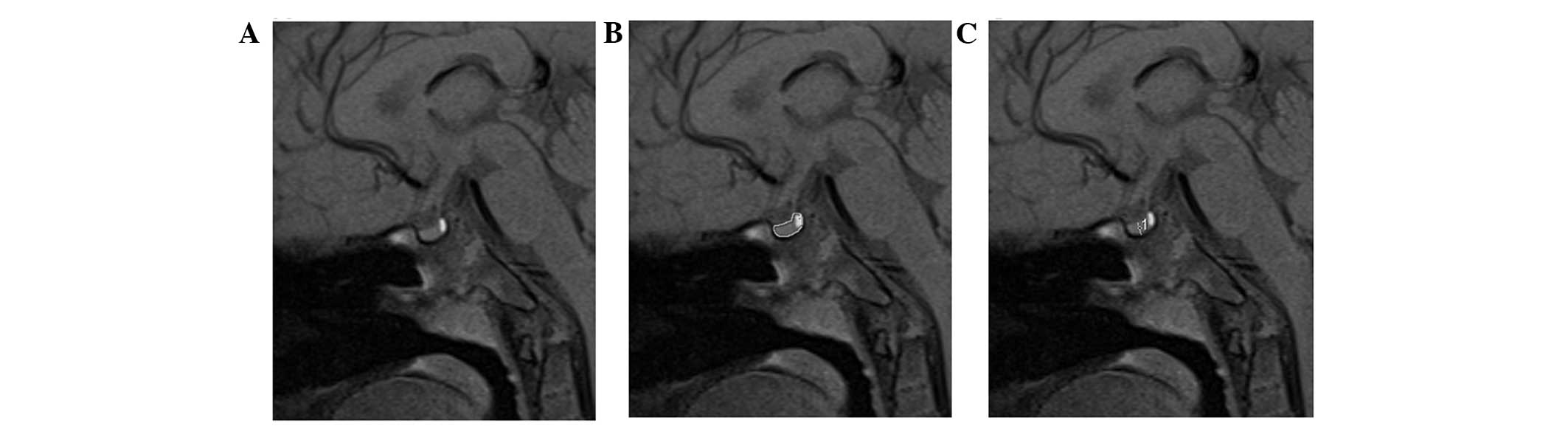
layer-by-layer with manual tracing using a mouse-guided cursor
(Fig. 1A and B). The regions did
not include the pituitary stalk, but included the neurohypophysis.
The volume of the pituitary gland was then calculated using the
section thickness and the ROI of every layer. The midsagittal
height was obtained from the straight-line distance from the
adenohypophysis midpoint of the upper edge to the edge of the gland
in the sella turcica bottom (Fig.
1C), according to the traditional method described by Fujisawa
(17). A comparison was performed
between the short stature children and normal children. Pituitary
gland volumes below the minimum value of the corresponding normal
range were regarded as dysplastic.
Volumetric measurements of the pituitary gland were
performed independently by two neuroradiologists and each
neuroradiologist measured the pituitary gland volume twice based on
the aforementioned method.
Subjects
A total of 75 Chinese children aged between 1 and 19
years (mean age, 9.39 years; Table
I) were recruited. The individuals had no clinical evidence of
pituitary gland lesions (intracranial lesions or endocrinological
abnormalities) (18), a history of
asphyxia or short-term delivery within 35 weeks. No abnormal
observations were identified on routine MRI examination.
 | Table IAge and gender distribution of healthy
children. |
Table I
Age and gender distribution of healthy
children.
| Age (years) | |
|---|
|
| |
|---|
| Participants | 1–4 | 5–9 | 10–14 | 15–19 | Total |
|---|
| Males (n) | 12 | 8 | 16 | 9 | 45 |
| Females (n) | 8 | 8 | 7 | 7 | 30 |
| Total (n) | 20 | 16 | 23 | 16 | 75 |
A total of 55 Chinese children with short stature
were included in the study, with ages ranging between 0 and 14
years (mean age, 8.6 years; Table
II). These children were further divided into two groups. Group
1 included 32 children with GHD, while group 2 consisted of 23
children with ISS. The inclusion criteria for children with GHD
were as follows: i) Height was below the third percentile among
children of the same age and gender; ii) growth rate was <4
cm/year; iii) bone age lagged behind the actual age by two years
(Greulich and Pyle standards); iv) serum GH peak was <10 μg/l
when stimulated with drugs (clonidine and levodopa) in the GH
secretion test; v) levels of serum thyroxine, triiodothyronine and
thyroid stimulating hormone were normal; and vi) patients were not
affected by genetic metabolic diseases, chromosomal aberrations or
any other diseases. The inclusion criteria for children with ISS
were the same as the aforementioned standards, with the exception
of a serum GH peak of >10 μg/l when stimulated with clonidine
and levodopain in the GH secretion test.
 | Table IIAge and gender distribution of
children with GHD or ISS. |
Table II
Age and gender distribution of
children with GHD or ISS.
| Age (years) | |
|---|
|
| |
|---|
| 1–4 | 5–9 | 10–14 | |
|---|
|
|
|
| |
|---|
| Condition | Male | Female | Male | Female | Male | Female | Total |
|---|
| GHD (n) | 2 | 4 | 8 | 6 | 9 | 3 | 32 |
| ISS (n) | 1 | 2 | 7 | 3 | 10 | 0 | 23 |
Written informed consent was obtained from all the
parents or guardians of the children, and all the experimental
procedures in the study were approved by the Ethical Committee of
Shandong Provincial Hospital of Shandong University (Jinan, China).
All the children underwent brain MRI for sellar evaluation between
August 2011 and June 2012.
Statistical analysis
Statistical analysis was performed using SPSS
software version 17.0 (SPPS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference. The
normal range of the pituitary gland volumes was expressed as the
mean ± standard deviation. The Student’s t-test was used to
evaluate the repetition test, while Pearson’s correlation
coefficient and regression analyses were performed to evaluate the
correlations between the volume and height of the pituitary
glands.
Results
Effect of age on pituitary gland height
and size
To examine the association between pituitary gland
volume and height with age, MRI was performed on 75 healthy
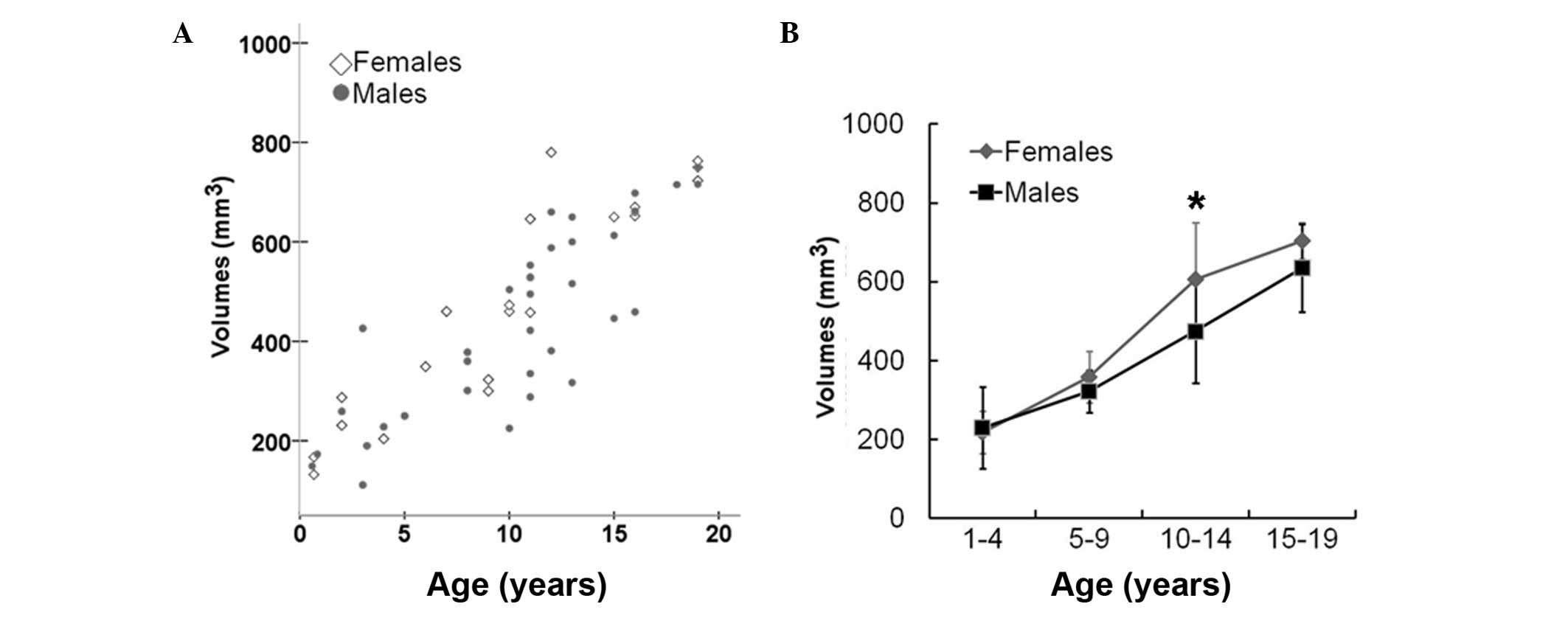
children. The pituitary gland exhibited an increasing growth trend
in volume over age (Fig. 2A and
Table III). A growth spurt in
the volume of the pituitary gland was observed in children aged
between 10 and 14 years-old, and this trend was more prominent in
females (P<0.05; Fig. 2B). By
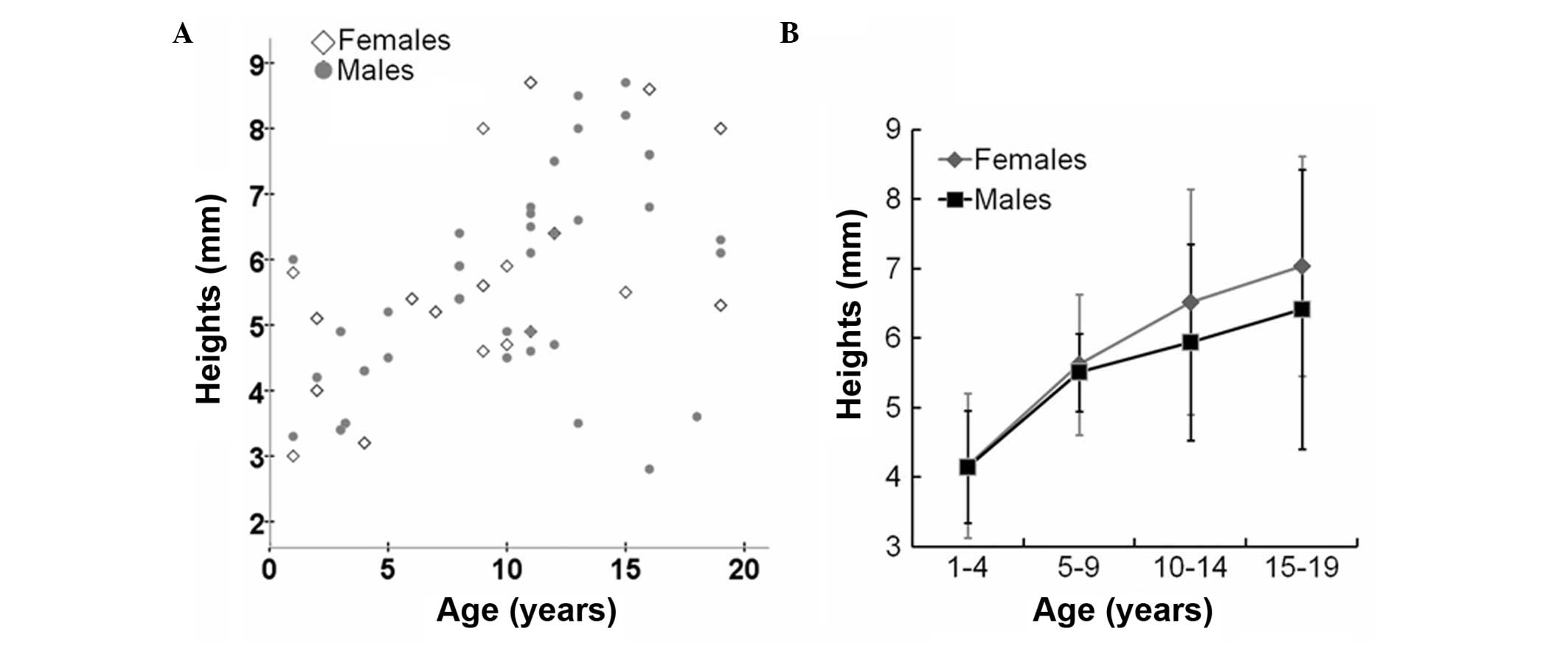
contrast, the height of the pituitary gland exhibited a gradual
increase without a growth spurt (Fig.
3 and Table III).
 | Table IIIVolumes and heights of the pituitary
gland in healthy children (mean ± standard deviation). |
Table III
Volumes and heights of the pituitary
gland in healthy children (mean ± standard deviation).
| Age (years) |
|---|
|
|
|---|
| Male | Female |
|---|
|
|
|
|---|
| Parameter | 1–4 | 5–9 | 10–14 | 15–19 | 1–4 | 5–9 | 10–14 | 15–19 |
|---|
| Volume
(mm3) | 229.2±104.4 | 322.3±54.0 | 474.4±132.0 | 635.3±111.0 | 217.9±53.8 | 358.0±65.6 | 606.1±144.1 | 704.4±46.7 |
| Height (mm) | 4.15±0.81 | 5.51±0.56 | 5.94±1.41 | 6.41±2.01 | 4.17±1.04 | 5.62±1.01 | 6.52±1.62 | 7.04±1.58 |
The correlation coefficient (r) and adjusted
determination coefficient (R2) were 0.661 and 0.437,
respectively, between the pituitary gland volume and height, as
determined by correlation and regression analysis. In the
repetition tests, no statistically significant difference was
observed between the two measurements of any observer (paired
t-test; P=0.164; power of test, 1−β>0.8). Similarly, no
statistically significant difference was observed between the
measurements of the two observers (P=0.182; power of test,
1−β>0.8).
These observations indicated that the volume of the
pituitary gland in normal children increased with age, with a
growth spurt between 10 and 14 years of age, whereas the height of
the pituitary gland increased gradually without a growth spurt.
Pituitary gland volume is an improved
indicator for GHD and ISS
To investigate the effectiveness of pituitary gland
volume and height in detecting GHD or ISS, MRI was conducted on 32
children with GHD and 23 children with ISS. In the 32 children with
GHD, 21 individuals had pituitary gland volumes below the minimum
value of the corresponding normal range, and the rate of
hypoplastic pituitary gland volume was 65.6% (Table IV). In the 23 children with ISS,
eight individuals had pituitary gland volumes below the minimum
value of the corresponding normal range, and the rate of
hypoplastic pituitary gland volume was 34.8% (Table IV). The rate of hypoplastic
pituitary gland height was 37.5% for children with GHD and 26.1%
for those with ISS (Table IV).
These observations demonstrated that the rates of hypoplastic
pituitary gland volume and height in children with GHD was higher
compared with those in the children with ISS, indicating that
pituitary gland volume was a superior indicator for the detection
of GHD and ISS.
 | Table IVComparison between pituitary volumes
and heights in children with GHD or ISS. |
Table IV
Comparison between pituitary volumes
and heights in children with GHD or ISS.
| GHD (n) | ISS (n) |
|---|
|
|
|
|---|
| Participants | Normal volume | Abnormal volume | Normal height | Abnormal height | Normal volume | Abnormal volume | Normal height | Abnormal height |
|---|
| Age (years) |
| 1–4 | 4 | 2 | 3 | 3 | 2 | 1 | 2 | 1 |
| 5–9 | 5 | 9 | 9 | 5 | 7 | 3 | 8 | 2 |
| 10–14 | 2 | 10 | 8 | 4 | 6 | 4 | 7 | 3 |
| Percentage | 34.4 | 65.6 | 62.5 | 37.5 | 65.2 | 34.8 | 73.9 | 26.1 |
Discussion
The size and shape of a normal pituitary gland
varies considerably and is affected by age, gender and the hormonal
environment (5–10). The pituitary gland size reflects
the level of associated hormones in the human body and is important
in the diagnosis of pituitary diseases (19). The development of the human body is
accompanied by changes to the pituitary gland (20). However, minor changes in pituitary
gland height are often difficult to detect as the morphology of the
pituitary gland and sella turcica can interfere with accurate
measurements. Variations in pituitary gland shape between
individuals means that any assessment of pituitary gland size is
likely to be subject to a high degree of imprecision unless a true
volume is measured (11).
Therefore, an increasing number of studies have measured the
pituitary gland volume in an attempt to have a more precise
assessment of the pituitary gland (12,13,20–22).
Currently, MRI measurements of the pituitary gland
volume include 2D geometric methods, voxel-based morphometry and
manual surveying and mapping of ROIs. Roldan-Valadez et al
(21) hypothesized that the
traditional geometric method should be replaced by 3D volumetric
measurement that had higher accuracy and smaller discrepancy.
Voxel-based morphometry is only used to measure the anatomical
structure with unclear demarcation, as this technique is poor for
the measurement of fine structure, but has the advantages of being
simple, saving time and labor. The ROI method may be used for more
precise positioning measurements based on anatomical and
histological boundaries, and is therefore the in vivo
measurement closest to the true size of the pituitary gland. Cui
et al (23) analyzed the
pituitary gland volumes in healthy Chinese individuals over the age
of 18 years, and their results indicated that 3D MRI clearly
demonstrated the morphology and precisely measured the volume of
the pituitary gland. However, relatively low MRI field strength was
used in their studies, as well as a scanning section thicker than 2
mm and a 2-mm scanning interval. In addition, the authors did not
investigate the pituitary gland volume in healthy people aged
<18 years-old. Takano et al (12) identified that there was a growth
spurt in children in the early teenage years, and this spurt was
more prominent in females, in a study of 199 healthy Japanese
adolescents below the age of 20 years. Similarly, the study by
Takano et al was also performed with a low MRI field
strength. Fink et al (11)
also demonstrated that assessments using sagittal or coronal data
reconstructions produced almost identical results. In the present
study, 3D MRI volumetry was used to estimate the pituitary gland
volume in healthy children and children with short stature.
Firstly, the pituitary gland surface area on each outlined region
was determined using the layer-by-layer method on sagittal imaging,
from which the volume was calculated by multiplying the surface
areas by the thickness of the layers (24).
To the best of our knowledge, there are currently no
useful reference data for the normal range of pituitary gland
volumes in Chinese children; thus, the present study investigated
the pituitary gland volume in healthy children. Only two studies
have reported a normal pituitary gland volume in children relative
to age. The first study analyzed age-associated pituitary gland
volumes in children up to the age of 10, but did not take into
account gender differences (11).
The other study included prepubertal and postpubertal children, but
was limited to the Japanese population (12).
The results obtained from healthy children
demonstrated a gradual linear increase in pituitary gland volume
over the first ten years of life, which was consistent with the
study by Fink et al (11).
The volume of the pituitary gland exhibited a growth trend with age
prior to the age of 20, and there was evidence of a growth spurt in
children in the early teenage years (10–14 years old), which was
more prominent in females compared with males. These results
indicated that the growth of the pituitary gland was more prominent
in adolescents, particularly in females. The largest difference in
pituitary gland volume was observed between the females and males
at the ages of 10–14 years, which was consistent with the studies
by Takano et al (12).
However, the volume of the pituitary gland appeared to differ
between Chinese and Japanese adolescents at the ages of 10–14 and
15–19 years. The reasons for the difference may be due to sample
sizes or ethnic differences.
The detection rate of hypoplastic pituitary gland
volume in children with GHD (65.6%) was higher compared with those
with ISS (34.8%). Deficiency of GH secreted by the anterior
pituitary gland affects the growth and development of children. MRI
volumetry measurements may aid clinicians to diagnose short
stature. However, there were also 34.4% of children with GHD
demonstrating a normal pituitary gland volume. Therefore, the
investigation and evaluation of MRI requires associations with
anatomical and functional abnormalities of the pituitary gland.
Hypoplastic pituitary gland volume was detected in 34.8% of the
children with ISS, indicating that the pituitary gland volume was
small in a subgroup of ISS children, although the secretion of GH
was normal. However, the mechanisms underlying these observation
require further investigation.
In addition, the results of the present study
demonstrated that the pituitary gland height exhibited an
increasing trend with age in healthy children. The increase in
pituitary gland height was moderate in adolescent females, but was
slower in males. The growth tendency was different between the
pituitary gland height and volume, and the pituitary gland volume
performed significantly better than height with regard to the
detection rate. In addition, the correlation and regression
analyses revealed that the r (0.661) and R2 (0.437)
values were low between the pituitary gland volume and height.
Therefore, the measurement of pituitary gland height should not
replace volumetry in the assessment of pituitary gland size due to
the imprecision.
In conclusion, 3D MRI volumetry was used in the
present study to elucidate the developmental characteristics of the
pituitary gland in healthy children. The results indicated that the
measurement of pituitary gland height was not able to replace
volumetry in the assessment of pituitary gland size. Reference data
provided by 3D MRI were valuable in the diagnosis of short stature,
however, the evaluation required an association with neuroimaging
and clinical functional abnormalities of the pituitary gland. The
main limitation of the present study was the small sample size;
thus, future, large scale studies are required to determine the
clinical utility of these results.
Acknowledgements
The study was supported by grants from the Shandong
Province Science and Technology Development Plan (nos. 2012GSF11820
and 2012YD18053).
References
|
1
|
Rakover Y, Silbergeld A, Lavi I, Masalha R
and Shlomo IB: Can exaggerated response to a GH provocative test
identify patients with partial GH insensitivity syndrome? Eur J
Endocrinol. 146:319–323. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pedicelli S, Peschiaroli E, Violi E and
Cianfarani S: Controversies in the definition and treatment of
idiopathic short stature (ISS). J Clin Res Pediatr Endocrinol.
1:105–115. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shah S, Waldman AD and Mehta A: Advances
in pituitary imaging technology and future prospects. Best Pract
Res Clin Endocrinol Metab. 26:35–46. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maghnie M, Lindberg A, Koltowska-Häggström
M and Ranke MB: Magnetic resonance imaging of CNS in 15,043
children with GH deficiency in KIGS (Pfizer International Growth
Database). Eur J Endocrinol. 168:211–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsunoda A, Okuda O and Sato K: MR height
of the pituitary gland as a function of age and sex: especially
physiological hypertrophy in adolescence and in climacterium. AJNR
Am J Neuroradiol. 18:551–554. 1997.PubMed/NCBI
|
|
6
|
Denk CC, Onderoğlu S, Ilgi S and Gürcan F:
Height of normal pituitary gland on MRI: differences between age
groups and sexes. Okajimas Folia Anat Jpn. 76:81–87. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Elster AD, Chen MY, Williams DW III and
Key LL: Pituitary gland: MR imaging of physiologic hypertrophy in
adolescence. Radiology. 174:681–685. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Elster AD, Sanders TG, Vines FS and Chen
MY: Size and shape of the pituitary gland during pregnancy and post
partum: measurement with MR imaging. Radiology. 181:531–535. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Doraiswamy PM, Potts JM, Axelson DA, et
al: MR assessment of pituitary gland morphology in healthy
volunteers: age and gender-related differences. AJNR Am J
Neuroradiol. 13:1295–1299. 1992.PubMed/NCBI
|
|
10
|
Elster AD: Modern imaging of the
pituitary. Radiology. 187:1–14. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fink AM, Vidmar S, Kumbla S, et al:
Age-related pituitary volumes in prepubertal children with normal
endocrine function: volumetric magnetic resonance data. J Clin
Endocrinol Metab. 90:3274–3278. 2005.PubMed/NCBI
|
|
12
|
Takano K, Utsunomiya H, Ono H, Ohfu M and
Okazaki M: Normal development of the pituitary gland: assessment
with three-dimensional MR volumetry. Am J Neuroradiol. 20:312–315.
1999.PubMed/NCBI
|
|
13
|
Zipursky AR, Whittle S, Yücel M, et al:
Pituitary volume prospectively predicts internalizing symptoms in
adolescence. J Child Psychol Psychiatry. 52:315–323.
2011.PubMed/NCBI
|
|
14
|
Egger J, Kapur T, Nimsky C and Kikinis R:
Pituitary adenoma volumetry with 3D slicer. PLoS One.
7:e517882012.PubMed/NCBI
|
|
15
|
Axelson DA, Doraiswamy PM, Boyko OB, et
al: In vivo assessment of pituitary volume with magnetic resonance
imaging and systematic stereology: relationship to dexamethasone
suppression test results in patients. Psychiatry Res. 44:63–70.
1992.
|
|
16
|
Teoh SK, Mendelson JH, Woods BT, et al:
Pituitary volume in men with concurrent heroin and cocaine
dependence. J Clin Endocrinol Metab. 76:1529–1532. 1993.PubMed/NCBI
|
|
17
|
Fujisawa I, Asato R, Nishimura K, et al:
Anterior and posterior lobes of the pituitary gland: assessment by
1.5 T MR imaging. J Comput Assist Tomogr. 11:214–220. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen N, Li KC and Wang X: Establishment of
the database of normal brain structure reference values based on
Chinese Han nationality adults. Zhonghua Fang She Xian Yi Xue Za
Zhi. 44:568–570. 2010.(In Chinese).
|
|
19
|
Greulich WW and Pyle SI: Radiographic
Atlas of Skeletal Development of the Hand and Wrist. 2nd edition.
Stanford University Press; Stanford, CA: 1959
|
|
20
|
Kato K, Saeki N and Yamaura A:
Morphological changes on MR imaging of the normal pituitary gland
related to age and sex: main emphasis on pubescent females. J Clin
Neurosci. 9:53–56. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wood JC, Noetzl L, Hyderi A, et al:
Predicting pituitary iron and endocrine dysfunction. Ann NY Acad
Sci. 1202:123–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roldan-Valadez E, Garcia-Ulloa AC,
Gonzalez-Gutierrez O and Martinez-Lopez M: 3D volumetry comparison
using 3T magnetic resonance imaging between normal and
adenoma-containing pituitary glands. Neurol India. 59:696–699.
2011.
|
|
23
|
Renz DM, Hahn HK, Schmidt P, et al:
Accuracy and reproducibility of a novel semi-automatic segmentation
technique for MR volumetry of the pituitary gland. Neuroradiology.
53:233–244. 2011.PubMed/NCBI
|
|
24
|
Cui B, Chen N, Wang X, et al:
High-resolution MRI study of pituitary glands in healthy adult of
the Han nationality. Zhonghua Fang She Xian Yi Xue Za Zhi.
44:579–584. 2010.(In Chinese).
|
|
25
|
Chen SC, Simon EM, Haselgrove JC, et al:
Fetal posterior fossa volume: assessment with MR imaging.
Radiology. 238:997–1003. 2006.PubMed/NCBI
|