Introduction
Bile duct epithelial cells are considered to be the
most important cells involved in acute rejection reaction (ARR)
following orthotopic liver transplantation (1). However, recent studies have
demonstrated that the interaction and time overlap between
inflammatory cell infiltration and angiogenesis in the portal area
are the primary causes of ARR following orthotopic liver
transplantation (2). It has been
reported that macrophages and lymphocytes stimulate angiogenesis
via the release of angiogenic factors, including vascular
endothelial growth factor (VEGF) and basic fibroblast growth factor
(bFGF) (3,4). With respect to mechanism of action,
VEGF is endothelial cell-specific; it stimulates endothelial
proliferation, increases vascular permeability and changes the gene
expression of endothelial cells. It is the most potent known
vascular permeability agent (5,6).
bFGF stimulates the proliferation of endothelial cells, smooth
muscle cells and fibroblasts, as well as the formation of small
arteries; however, bFGF does not increase vascular permeability
(7).
The roles of VEGF and bFGF in mediating the
interactions between and concurrent timing of inflammatory cell
infiltration and angiogenesis in the portal area have yet to be
elucidated. Therefore, this was investigated in the present study
in order to further the understanding of ARR following orthotopic
liver transplantation.
Materials and methods
Subjects and groups
The inbred line DA (RT1a) to LEW (RT11) rat
orthotopic liver transplantation ARR model was established using
the classic two-cuff technique as previously described by Kamada
and Calne (8). The rats were
purchased from the SLAC Laboratory Animal (Shanghai, China), and
were housed in filter-capped polycarbonate cages and maintained
under constant environmental conditions (average 22°C, humidity
50%). The rats were kept on a 12h/12h light-dark cycle and had
unrestricted access to purified bottled drinking water and standard
chow. A total of 48 rats were equally divided into a DA (RT1a)-LEW
(RT11) ARR VEGF group and a DA (RT1a)-LEW (RT11) ARR bFGF group.
The two groups were further divided into three subgroups by the
number of days following transplantation (days 1, 3 and 7). A total
of eight rats served as a control group without receiving any
treatment. Histopathological classification of acute allograft
reaction was performed according to the ‘Banff’ international
criteria (9). Immunohistochemistry
(IHC) studies were performed using the standard
streptavidin-biotin-peroxidase complex method. In brief, tissue
slides were deparaffinized and rehydrated. The slides were scanned
using Motic Med 6.0 CMIAS (Motic China Group, Co., Ltd., Xiamen,
China). The rejection activity index (RAI) was obtained by
comprehensive analysis of VEGF and bFGF in liver acute rejection
reaction. The study protocol was approved by Ethics Committee of
the Second Military Medical University.
Determination of VEGF and bFGF levels by
ELISA
Serum VEGF and bFGF levels were detected by ELISA
(double antibody sandwich ABC-ELISA method) using a VEGF-C and
FGF-basic human ELISA kit (Invitrogen Corporation, Camarillo, CA,
USA), by drawing 2 ml venous blood from the inferior vena cava at
days 1, 3 and 7 following liver transplantation. The results were
determined as follows: i) the absorbance (A) value at 450 nm was
calculated by correcting for the blank value; ii) using the A value
of the standard product, a standard curve was drawn on
semi-logarithmic paper; iii) the VEGF and bFGF levels were then
determined according to the A value of the sample using the
standard curve.
Detection of tissue VEGF and bFGF levels
using immunohistochemistry
The expression levels of VEGF and bFGF in the
transplanted liver and spleen tissues were assayed using
immunohistochemistry. The rabbit anti-rat VEGF and bFGF
immunoglobulin G1 monoclonal antibodies (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, US) were used at ratios of 1:80 and 1:120,
respectively. EnVision reagent (horseradish peroxidase/rabbit) was
obtained from Dako (Glostrup, Denmark). The immunohistochemical
results were analyzed quantitatively using a true color medical
image analysis system (Motic Med 6.0 CMIAS; Motic China Group, Co.,
Ltd.). A positive result for VEGF was defined as the presence of
yellow brown or dark brown particles in cytoplasm. First, the 10
most concentrated areas of positive cells were randomly selected
(magnification, ×100), and then transferred to the CMIAS
(magnification, ×400) to calculate the number of positive cells on
the screen per mm2 by amplifying by a factor of 1.6.
Known positive SNU-1 gastric cancer tissue (Shanghai Institutes for
Biological Sciences, Shanghai, China) was used as the positive
control, and phosphate-buffered solution was used instead of the
primary antibody as the negative control.
Determination of VEGF and bFGF mRNA
expression in the liver tissue by quantitative polymerase chain
reaction (qPCR)
VEGF and bFGF expression levels in the liver tissue
were detected using a TRIzol kit (Shanghai Biological Engineering
Technology Service Co., Ltd., Shanghai, China); reverse
transcription (RT)-PCR (Takara, Shiga, Japan); two-step RT-PCR kit;
and DL 1,000 DNA marker (Takara Biotechnology Co., Ltd., Dalian,
China). The primers used for qPCR were as follows: VEGF forward,
5′-ACCTCACCAAAGCCAGCACA-3′ and reverse, 5′-GGC ATGGTGGTGACATGGTT-3′
(amplification product, 536 bp); bFGF forward,
5′-ACACGTCAAACTACAACT CCA-3′ and reverse,
5′-TCAGCTCTTAGCAGACATTGG-3′ (amplification product, 243 bp). qPCR
was performed using the Mx3000P qPCR System (Stratagene, La Jolla,
CA, USA). The cDNA was then used for qPCR in 20 μl SYBR Premix Ex
Taq (Takara Biotechnology Co., Ltd). qPCR was performed under the
following conditions: 5 min at 95°C, 40 cycles of 30 sec at 95°C,
30 sec at 60°C, and 1 min at 72°C. All results were normalized
against β-actin amplification. CT values for triplicate reactions
were averaged and relative expression was determined using the
comparative CT method.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analysis was performed using SPSS software,
version 17.0 (SPSS, Inc., Chicago, IL, USA). Comparison of mean
values between multiple groups was performed using a t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Serum VEGF and bFGF levels in ARR
following rat orthotopic liver transplantation
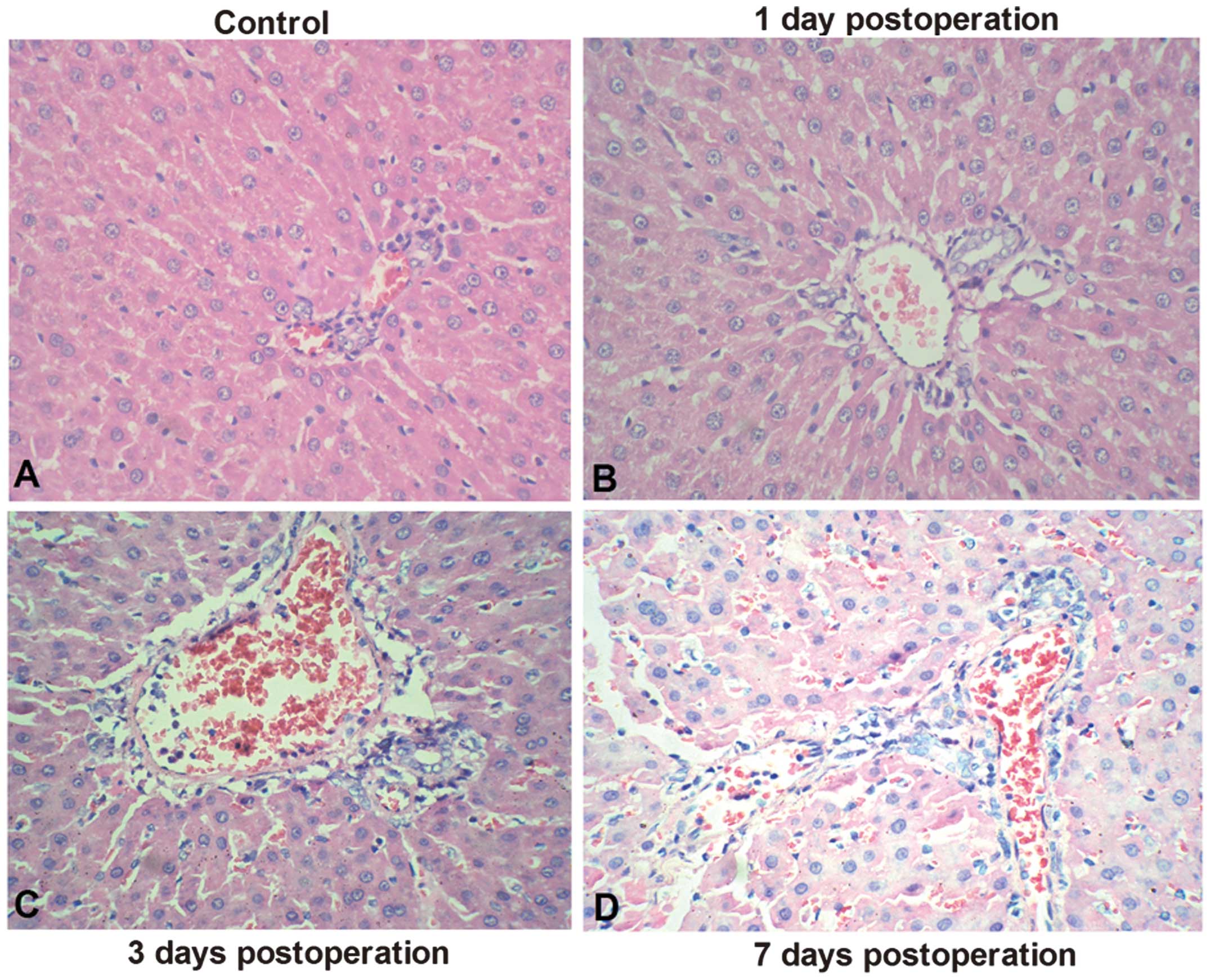
The rat orthotopic liver transplantation ARR model
was established using the classic two-cuff technique. Acute
allograft rejection was determined 1, 3 and 7 days following
transplantation (Fig. 1). To
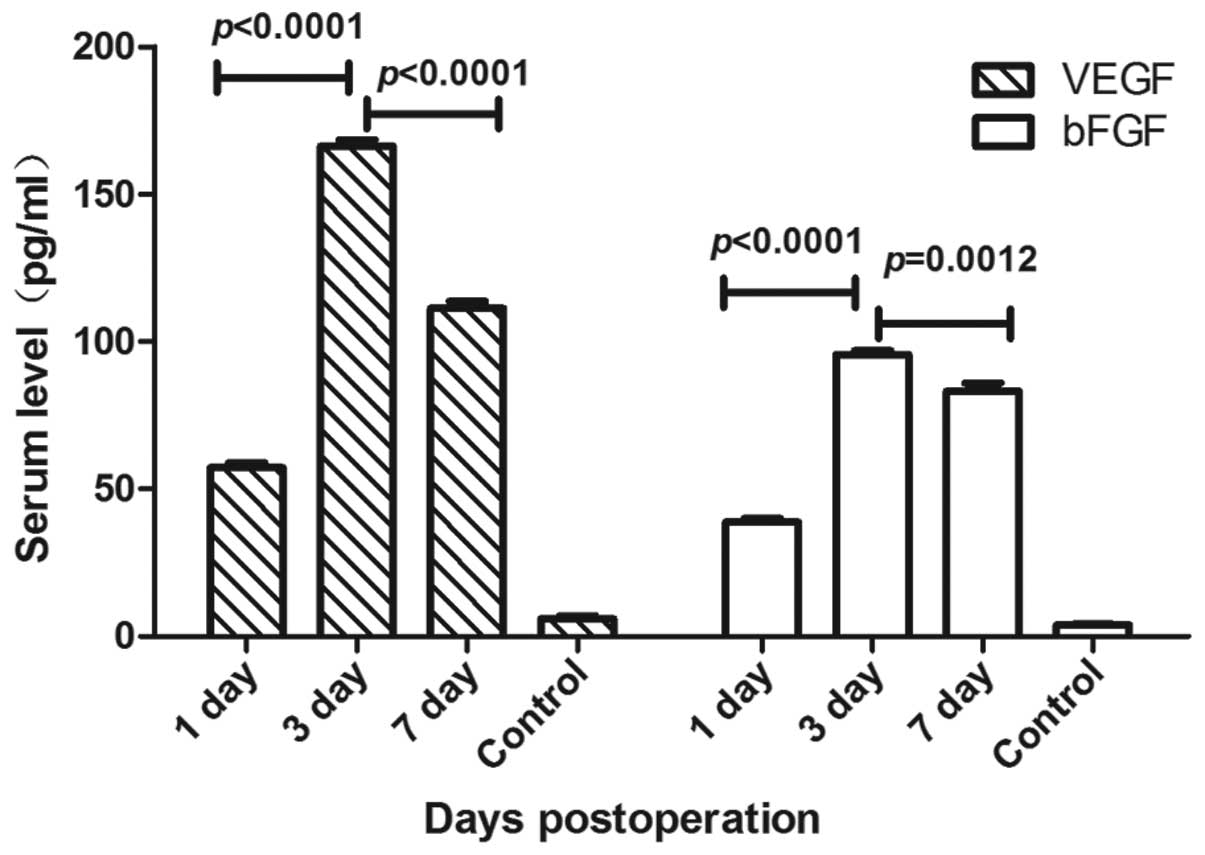
accurately quantify serum VEGF and bFGF expression levels in ARR
following rat orthotopic liver transplantation, ELISAs were
performed 1, 3, and 7 days following transplantation. The serum
VEGF and bFGF expression levels are shown in Fig. 2. Significant differences were
observed in the VEGF level at day 3 compared with those on the
other days (P<0.0001). It was found that the serum VEGF levels
at day 3 were higher (mean, 166.30±2.16 pg/ml) than those on days 1
and 7 (mean, 57.16±1.61 and 111.0±2.43 pg/ml, respectively).
Furthermore, serum bFGF levels were elevated at day 3 (mean,
95.64±1.26 pg/ml) compared with those on day 1 (mean, 38.74±1.35
pg/ml), and then decreased by day 7 (mean, 83.21±2.79 pg/ml). These
results suggest that VEGF and bFGF levels may be critical for the
development of ARR following rat orthotopic liver
transplantation.
Detection of VEGF and bFGF expression
levels in the liver tissue using immunohistochemistry
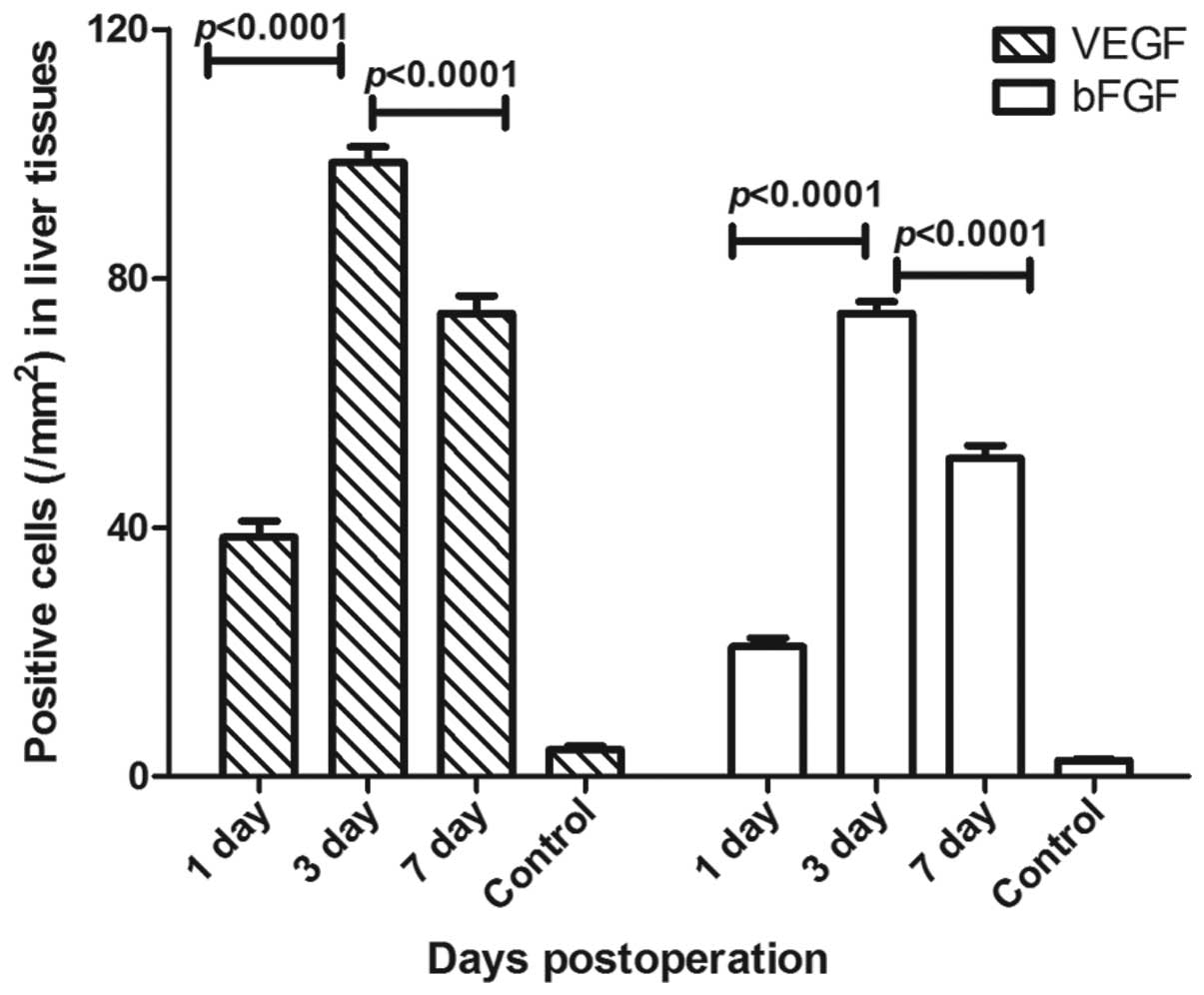
Cell infiltration with large amounts of VEGF and
bFGF expression was detected in the rats following transplantation,
and there were significant differences in VEGF and bFGF expression
between the three time points (P<0.0001).
The number of VEGF positive cells after 3 days was
found to be higher (mean, 98.6±2.5/mm2) compared with
the numbers on days 1 and 7 (mean, 38.4±2.6 and
74.3±2.8/mm2, respectively). Furthermore, the number of
bFGF positive cells 3 days following transplantation was higher
(mean, 74.4±1.9/mm2) compared with the numbers on days 1
and 7 (mean, 18.7±2.9 and 51.1±2.0/mm2, respectively;
Fig. 3). However, only very low
levels of VEGF expression in a small number of hepatocytes were
observed between central vein endothelial cells and infiltrating
cells, whereas bFGF expression was detected in this area.
Detection of VEGF and bFGF expression in
the spleen tissue using immunohistochemistry
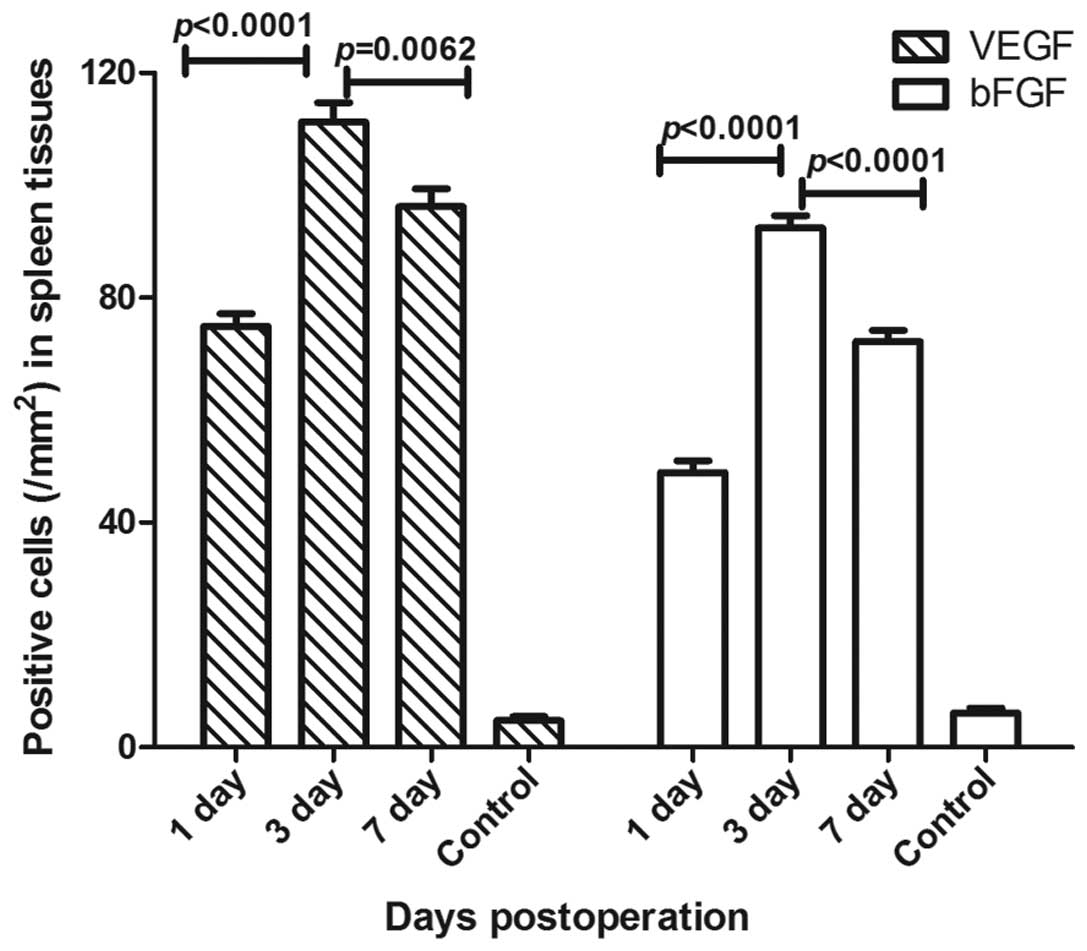
VEGF and bFGF expression levels were detected by
immunohistochemistry in the spleen tissue, and there were
significant differences between the three groups (P<0.01). It
was found that the number of VEGF positive cells after 3 days was
higher (mean, 111.3±3.4/mm2) compared with the numbers
on days 1 and 7 (mean, 74.9±2.3 and 96.2±3.2/mm2,
respectively). Furthermore, the number of bFGF positive cells after
3 days was also higher (mean, 92.4±2.2/mm2) compared
with the numbers on days 1 and 7 (mean, 48.9±2.2 and
72.2±2.0/mm2, respectively; Fig. 4). VEGF was primarily expressed in
the red pulp, and a small amount was expressed in the lymphatic
sheath around the artery and the marginal area, with lymphocytes
predominating and a small amount of macrophages. There was also a
small amount of VEGF expression in the endothelial cells of the
trabecular veins. bFGF expression was detected in the red pulp, and
a small amount in the lymphatic sheath around the artery and the
marginal area.
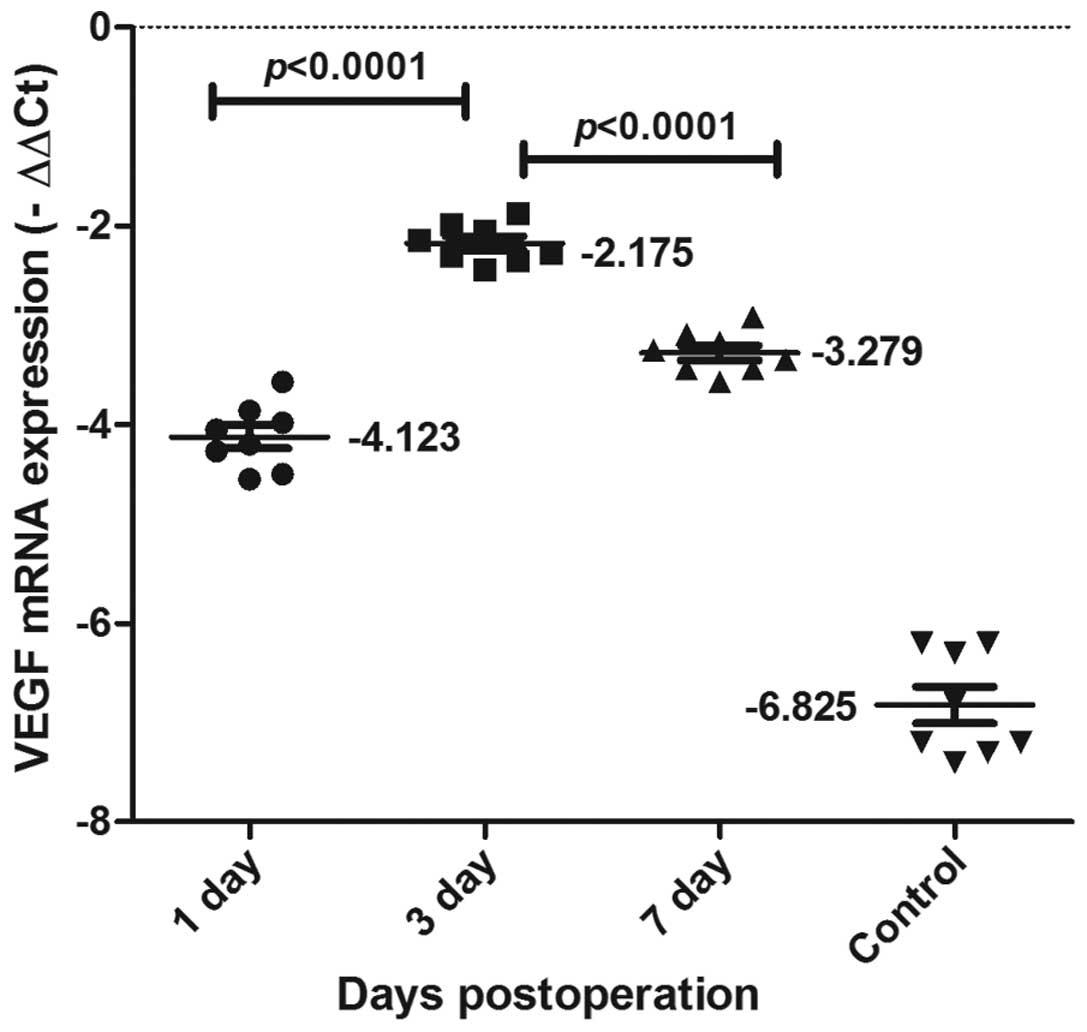
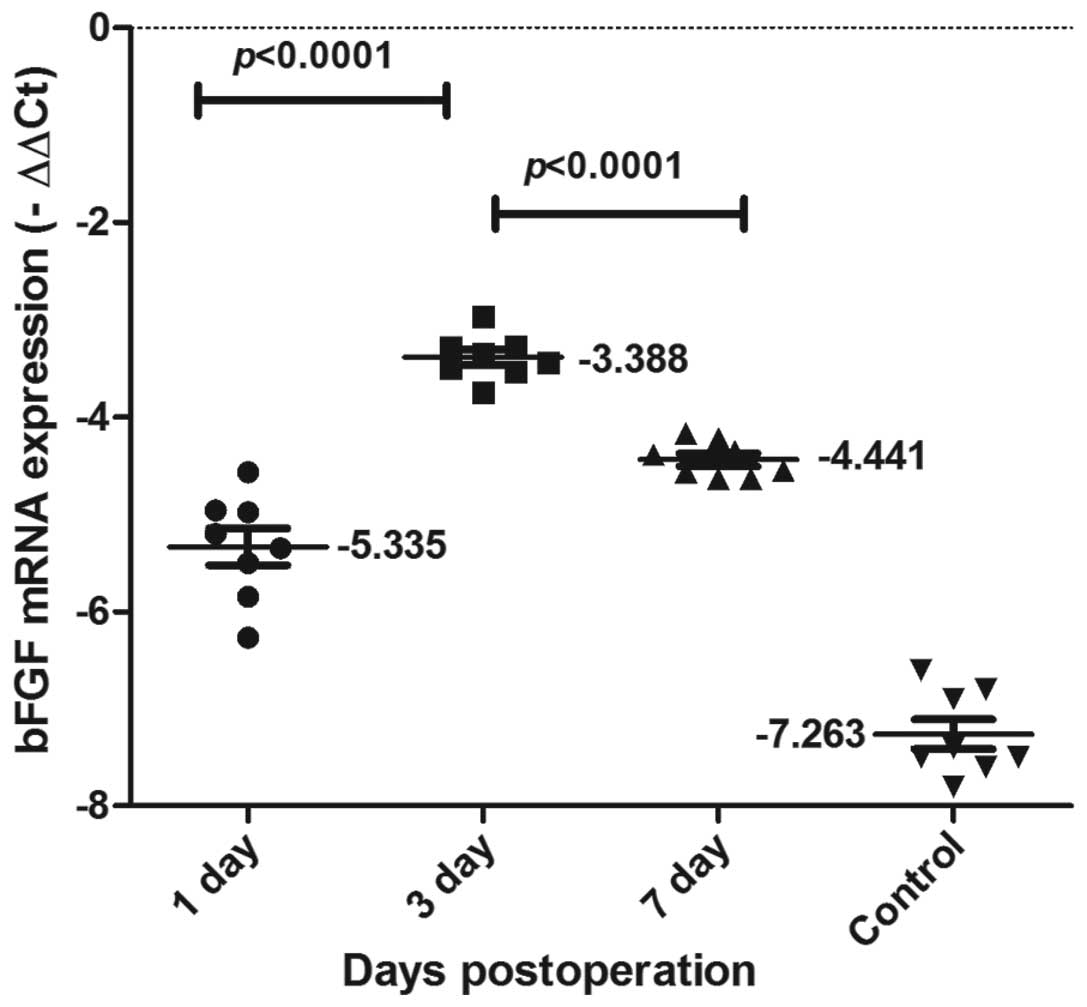
VEGF and bFGF mRNA expression in the
liver tissue
As shown in Figs. 5
and 6, VEGF and bFGF mRNA
expression was detected using qPCR in each of the groups. VEGF and
bFGF mRNA expression levels increased from 1 day following the
surgery, reached a peak at day 3, and then declined gradually,
although remaining at a relatively high level. VEGF and bFGF mRNA
expression levels changed dynamically, peaking and then declining.
Significant differences were observed between the three time-points
(P<0.0001).
Discussion
Bile duct epithelial cells are the most important
cells involved in ARR following orthotopic liver transplantation.
However, according to the three pathological changes summarized by
Berman et al (10), the
presentation of bile duct epithelial cell only reflects one aspect
of ARR and does not explain the whole pathogenesis of ARR. This is
supported by the fact that management of the bile duct alone does
not solve the problem of ARR following orthotopic liver
transplantation in clinical practice. Furthermore, bile duct
epithelial cells also require normal expression of vascular
endothelial cells so that they are able to obtain nutrients via
microvessels and function normally (2).
Previous studies have shown that VEGF and bFGF are
the primary growth factors that directly induce the division,
proliferation and migration of endothelial cells and angiogenesis
(11). In addition,
immunoreactivity for VEGF has been found in the extracellular
matrix of the portal tracts in normal and non-tumorous parts of
liver, but not in the hepatocytes and bile duct epithelium
(12). The results from the
present study demonstrate that although the expression levels of
VEGF and bFGF increased during the rejection process of liver
transplantation, the bFGF expression level was lower; its
expression was weaker, the scope of expression was wider, and the
peak time was delayed compared with that of VEGF (Figs 5 and 6). This suggests that although VEGF and
bFGF may mediate immunoinflammatory responses and angiogenesis in
orthotopic liver transplantation, VEGF has a more important and
specific role.
The exact role of VEGF in alloimmunity remains to be
elucidated. Tambur et al (13) observed that VEGF was expressed in
human allografted heart tissue, and that this expression was
associated with ARR and chronic rejection. Furthermore, Conti et
al (14) demonstrated that the
inflammation-promoting effect of VEGF occurs mainly in the initial
stages of the inflammatory cascade rather secondary to the T
lymphocyte-mediated activation reaction, suggesting that VEGF is
locally produced immediately following transplantation. This is
consistent with the findings from the present study, that VEGF
expression was enhanced on the first day following liver
transplantation rejection. In addition, trauma, including the entry
of platelets and white blood cell (WBC) supplements into the graft,
further facilitates the expression of VEGF, and cytokines and
chemokines that have important roles in the process of rejection.
Early VEGF expression has been found to promote the repair of T
lymphocytes and mononuclear cells (15). Furthermore, Fallsehr et al
(16) observed that the action of
VEGF on endothelial cells and macrophages activated nuclear factor
κB, which subsequently induced the synthesis of inflammatory
cytokines and chemokines.
A previous study demonstrated that intercellular
adhesion molecule-1, vascular cell adhesion protein-1 and
endothelial cell protein increased the adhesion of WBCs to the
endothelial tissue and migration to the inflammatory area (17). In combination with the results from
the present study, this suggests that VEGF production in liver
tissues may be induced by anoxia or hypoxia, which is inevitable
during transplantation, as well as via infiltration of neutrophils
and macrophages into the graft.
Histologically, the spleen is made of white pulp, a
marginal zone and red pulp. The marginal zone contains a greater
number of T lymphocytes and macrophages and, therefore, the spleen
is the first site at which antigens are captured and identified and
an immunoresponse is triggered. The periarterial lymphatic sheath
in the T-cell area of the spleen contains large amounts of T cells.
In addition, the plenic cord of the red pulp contains large amounts
of macrophages and T cells. It was found in the present study that
VEGF was primarily expressed in the splenic marginal area
containing T cells and macrophages, and rarely in the B-cell area.
Microscopy demonstrated that the periarterial lymphatic sheath of
the white pulp was thickened and the edge was widened, indicating
that the cellular immunoresponse there was enhanced. Since the
marginal area of the spleen is the first place where an
immunoresponse is induced in the spleen, this suggests that VEGF
expression in this area may promote immature dendritic cells to
aggregate in the marginal area, where dendritic cells take up and
process antigens that enter the spleen, and then directly submit
them to T cells in the marginal area and activate them. It was also
found in the present study that VEGF expression peaked 3 days
following transplantation, which is consistent with the peak time
of inflammatory cell infiltration during ARR, providing further
support for the hypothesis that VEGF activates T cells during ARR
following liver transplantation.
In conclusion, VEGF may be an important intermediary
link between damage caused by physical, chemical and biological
factors and subsequent immune injury due to the aggregation,
activation and identification of lymphocytes and their effector
cells in ARR following orthotopic liver transplantation. However,
the exact mechanism underlying the action of VEGF and its
applications require further investigation.
Acknowledgements
This study was supported by the China Postdoctoral
Science Foundation Specific funded project (201003380), the Natural
Science Foundation of China (81372212), the Natural Science
Foundation of Jiangsu (BK2011251), Jiangsu Provincial Special
Program of Medical Science (BL2013012) and the Health Talents
Project for Jiangsu, China (LJ201157; RC2011038; BRA2011038) and
the Natural Science Foundation of Ningbo (2011A610057).
References
|
1
|
Renna-Molajoni E, Cinti P, Elia L, et al:
Mechanism of liver allograft rejection: indirect allorecognition.
Transplant Proc. 31:409–410. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou TB and Yang GS: Roles of vascular
endothelial growth factor in acute rejection reaction following
liver transplantation. Transpl Immunol. 25:207–209. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oh JY, Kim MK, Shin MS, et al: The
anti-inflammatory and anti-angiogenic role of mesenchymal stem
cells in corneal wound healing following chemical injury. Stem
Cells. 26:1047–1055. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reinders ME, Fang JC, Wong W, Ganz P and
Briscoe DM: Expression patterns of vascular endothelial growth
factor in human cardiac allografts: association with rejection.
Transplantation. 76:224–230. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kraft A, Weindel K, Ochs A, et al:
Vascular endothelial growth factor in the sera and effusions of
patients with malignant and nonmalignant disease. Cancer.
85:178–187. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pilmore HL, Eris JM, Painter DM, Bishop GA
and McCaughan GW: Vascular endothelial growth factor expression in
human chronic renal allograft rejection. Transplantation.
67:929–933. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuninaka T, Senga Y, Senga H and Weiner M:
Nature of enhanced mitochondrial oxidative metabolism by a calf
blood extract. J Cell Physiol. 146:148–155. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kamada N and Calne RY: A surgical
experience with five hundred thirty liver transplants in the rat.
Surgery. 93:64–69. 1983.PubMed/NCBI
|
|
9
|
Solez K and Racusen LC: The Banff
classification revisited. Kidney Int. 83:201–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
No authors listed. An international panel.
Banff schema for grading liver allograft rejection: an
international consensus document. Hepatology. 25:658–663. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bayliss J, Maguire JA, Bailey M, et al:
Increased vascular endothelial growth factor mRNA in endomyocardial
biopsies from allografts demonstrating severe acute rejection: a
longitudinal study. Transpl Immunol. 18:264–274. 2008. View Article : Google Scholar
|
|
12
|
Chow NH, Hsu PI, Lin XZ, et al: Expression
of vascular endothelial growth factor in normal liver and
hepatocellular carcinoma: an immunohistochemical study. Hum Pathol.
28:698–703. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tambur AR, Pamboukian S, Costanzo MR and
Heroux A: Genetic polymorphism in platelet-derived growth factor
and vascular endothelial growth factor are significantly associated
with cardiac allograft vasculopathy. J Heart Lung Transplant.
25:690–698. 2006. View Article : Google Scholar
|
|
14
|
Conti A, Scala S, D’Agostino P, et al:
Wide gene expression profiling of ischemia-reperfusion injury in
human liver transplantation. Liver Transpl. 13:99–113. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Borozan I, Chen L, Sun J, et al: Gene
expression profiling of acute liver stress during living donor
liver transplantation. Am J Transplant. 6:806–824. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fallsehr C, Zapletal C, Kremer M, Demir R,
von Knebel Doeberitz M and Klar E: Identification of differentially
expressed genes after partial rat liver ischemia/reperfusion by
suppression subtractive hybridization. World J Gastroenterol.
11:1303–1316. 2005. View Article : Google Scholar
|
|
17
|
Winn R, Vedder N, Ramamoorthy C, Sharar S
and Harlan J: Endothelial and leukocyte adhesion molecules in
inflammation and disease. Blood Coagul Fibrinolysis. 9(Suppl 2):
S17–S23. 1998.PubMed/NCBI
|