Introduction
At present, the nomenclature of skin vascular tissue
tumors remains inconsistent, particularly in vascular tissue
tumors. Different classifications have also been used
unsatisfactorily. In 1996, the International Society of Vascular
Anomalies divided the vascular birthmarks into two categories:
Vascular tumors and vascular malformations; infantile hemangioma,
for example, is classified as a vascular tumor (1,2).
Infantile hemangioma is one of the most common benign tumors in
infants and young children; these tumors are superficially located
in the head and neck area (3). A
few cases have also been observed in the mucosa, muscle, bone
tissue and internal organs, which are affected in terms of
appearance and function. In extreme cases, these superficial tumors
threaten the life of the patient. Different theories, including the
heredity and gene mutation theory, the placental chorionic cell
ectopia theory, the endothelial progenitor cell or stem cell theory
and the angiogenesis imbalance theory (4–14),
have been proposed regarding the pathogenesis of hemangioma;
however, no decisive conclusion has been obtained to date.
Propranolol is a nonselective β-adrenergic receptor agonist. In
2008, a study proposed that propranolol elicits significant adverse
effects on hemangioma (15); thus,
propranolol has been increasingly used as the first-line treatment
against hemangioma. Therefore, the functions of the β-adrenergic
receptor in the pathogenesis of vascular tumors have attracted
increased attention. Isoproterenol is a β-adrenergic receptor
antagonist. A previous study demonstrated that isoproterenol
promoted angiogenesis in vitro, indicating that the
β-adrenergic receptor is important in the occurrence and
development of vascular tumors (16).
In the present study, specific concentrations of
propranolol and isoproterenol were used to compare the effects on
infantile hemangioma endothelial cells (IHECs) in an in
vitro cultivation environment of IHECs. This experiment was
conducted to further investigate the functions of the β-adrenergic
receptor in the development and progression of vascular tumors.
Subjects and methods
Subjects
A hemangioma specimen was resected clinically from a
proliferating hemangioma on the forehead of a nine-month-old female
patient. This study was conducted in accordance with the
Declaration of Helsinki and with approval from the Ethics Committee
of The First Affiliated Hospital of Xinjiang Medical University
(Urumqi, China). Written informed consent was obtained from the
guardian of the infant.
Primary cultivation of IHECs
The resected hemangioma specimen was immediately
placed and stored in RPMI-1640 serum-free medium and promptly
transferred to a laboratory laminar flow cabinet. Following the
removal of the supernatant, the specimen was placed in a sterile
Petri dish and rinsed with double-antibody phosphate-buffered
saline (PBS; HyClone Laboratories, Inc., South Logan, UT, USA)
twice. The dish was replaced and washed three time with PBS.
Specimen trimming was performed in the Petri dish to remove the
epidermis and the connective tissues, prior to the specimen being
cut into 1×1 mm sections and washed twice with PBS. A 0.25% trypsin
(HyClone Laboratories, Inc.) solution was used to digest the cells
for 3–4 h at 37°C with constant agitation. The RPMI-1640 medium,
containing 20% fetal bovine serum (Gibco®-BRL, Grand
Island, NY, USA), was then added to terminate digestion. The
mixture was filtered and centrifuged (179 × g for 5 min) and the
supernatant was subsequently discarded. Another portion of the
RPMI-1640 medium was used to resuspend the precipitate. The
supernatant was centrifuged (179 × g, 5 min) and discarded.
Endothelial cell growth medium 2 (EGM-2), containing vascular
endothelial growth factor (VEGF), hydrocortisone, ascorbic acid,
human alkaline fibroblast growth factor B, human insulin-like
growth factor and epidermal growth factor (Lonza Ltd., Basel,
Switzerland), was added to the mixture, which was then transferred
into a 25-cm2 flask and placed in an incubator with
CO2 at 37°C. After 24 h, the cells adhered to the flask
walls. The medium was changed at intervals of 2–3 days. Generation
passage was performed when the cells covered 70–80% of the flask
bottom. All of the procedures were completed under sterile
conditions in the laminar flow cabinet.
Identification of IHECs
The primary cells were obtained and digested with
0.25% trypsin solution. The digested cells were then centrifuged
(179 × g, 5 min) and EGM-2 was used to resuspend the precipitate.
The resulting suspension was transferred to the slide of a 10-cm
dish and developed at 37°C and with CO2. After 24 h, the
supernatant was discarded. The slide was washed three times with
PBS, fixed with 4% paraformaldehyde for 20 min and washed a further
three times with PBS; blocking solution was subsequently added to
the slide, which was kept at room temperature for 30 min. The slide
was then washed with PBS, and primary rabbit anti-human von
Willebrand factor (vwf) polyclonal antibody (Wuhan Boster
Biological Technology, Ltd., Wuhan, China) was added to the slide.
The cells were subsequently cultivated overnight at 4°C. In the
blank control group, PBS was used as the primary antibody.
Following overnight cultivation, the slide was washed three times
with PBS. The secondary antibody, polymerized horseradish
peroxidase (HRP)-labeled anti-rabbit immunoglobulin G (IgG) (Wuhan
Boster Biological Technology, Ltd.), was added to the slide, and
the cells were cultivated for 30 min at 37°C. The specimen was
stained using a Diaminobenzidine Chromogenic Staining kit (Beijing
Sequoia Jinqiao Biological Technology Co., Ltd., Beijing, China)
for 2 min. Staining was terminated with distilled water, and the
specimen was observed under a microscope.
A second experimental set was prepared using the
same procedure as above; however, rabbit anti-human VEGF receptor 2
(VEGFR-2) antibody (Wuhan Boster Biological Technology, Ltd.) was
instead used as the primary antibody, and polymerized HRP-labeled
anti-rabbit IgG antibody was used as the secondary antibody. In the
blank control group, PBS was used as the primary antibody.
Determination of cell growth curves using
the MTT assay
The second-generation cells were spread evenly on a
96-well plate at a concentration 2×104 cells per well.
EGM-2 (~200 μl) was added and the cells were incubated for 24 h.
The medium was subsequently changed and eight cells from each row
of wells were observed daily. Approximately 20 μl MTT solution (5
mg/ml; Sigma, St. Louis, MO, USA) was added daily into each well
and cultivation was continued for 4 h, prior to the supernatant
being discarded. Approximately 150 μl dimethyl sulfoxide (DMSO;
Sigma) was then added, and the system was agitated for 10 min. A
microplate reader (Thermo Fisher Scientific, Inc., Rockford, IL,
USA) was used to determine the absorbance of each well at a
wavelength of 490 nm for 10 consecutive days. In the cell growth
curve, time was set as the abscissa and the average absorbance was
set as the ordinate.
Effect of propranolol on cell growth
curves
Propranolol (Tianjin Lisheng Pharmaceutical Co.,
Ltd., Tianjin, China) was dissolved in DMSO and then diluted with
EGM-2 to prepare working solutions at three concentrations: 10, 15
and 20 μg/ml. The final DMSO concentration was 0.16%. Following the
preparation, the solutions were filtered with a 0.22-μm filter and
sub-packaged.
The second-generation cells were obtained for
digestion, centrifugation and counting. These cells were evenly
spread on a 96-well plate at a cell concentration of
2×104 cells per well; 200 μl EGM-2 was added and the
cells were incubated for 24 h. Following adhesion of the cells to
the walls, the medium was replaced with the 10, 15 and 20 μg/ml
working solutions, respectively. Blank EGM-2 (without propranolol)
and 0.16% DMSO-containing EGM-2 were used as the controls, with
each treatment replicated four times. The cells were cultured in a
CO2 incubator, and the absorbance was determined using
the MTT assay at 24, 48, 72 and 96 h; simultaneously, the
morphological changes were observed and recorded. The
non-parametric test of the multiple-sample-related measurements was
performed to compare the difference in absorbance between each
concentration and the control group. The average absorbance curve
of the cells at each concentration was plotted, with time as the
abscissa and average absorbance as the ordinate.
Effects of isoproterenol on cell growth
curves
The effects of isoproterenol were observed following
the procedures used to determine the effects of propranolol;
however, isoproterenol was used instead of propranolol.
Statistical analysis
The non-parametric test of the
multiple-sample-related measurements used in the data analysis was
performed using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA).
Results
Cultivation of IHECs
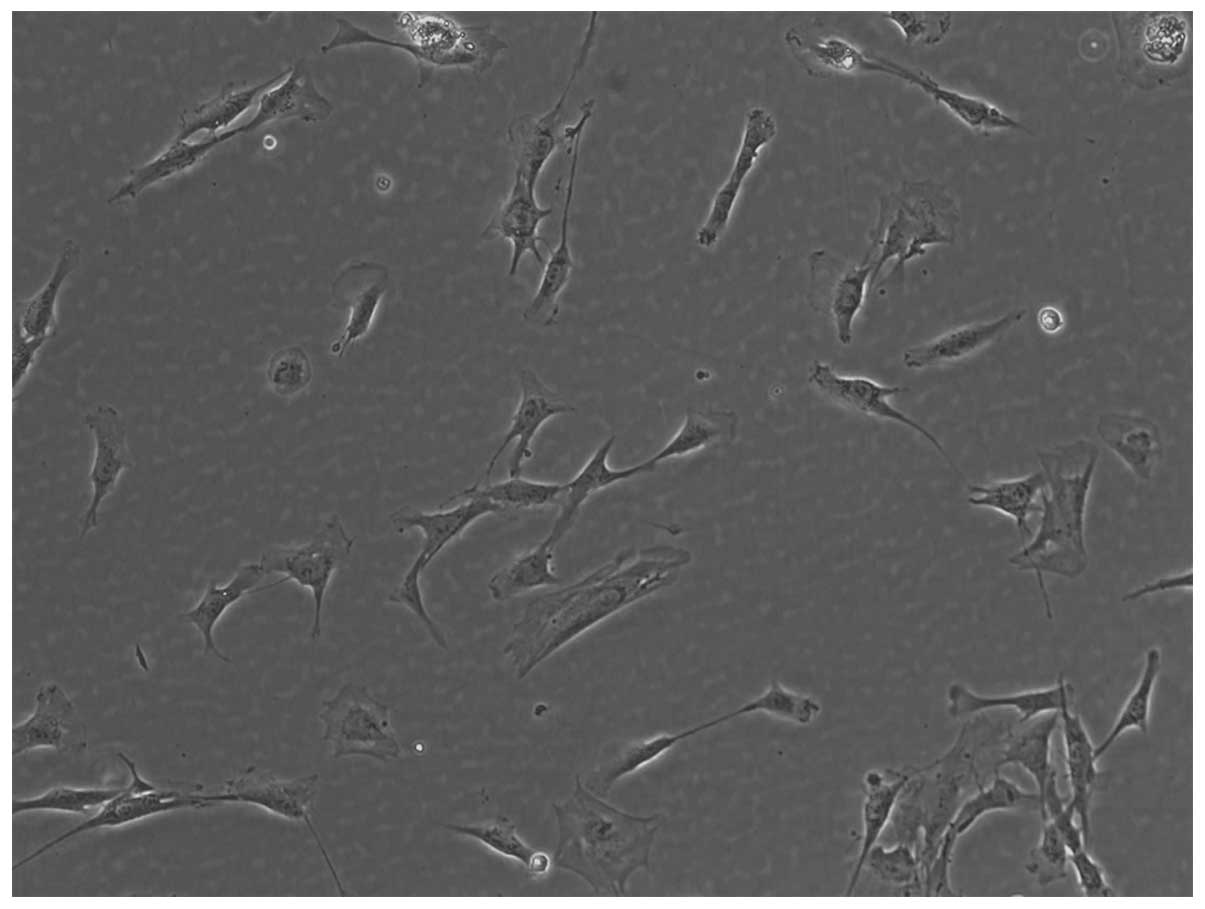
After the 24-h primary cultivation of IHECs, the
cells gradually adhered to the walls, appearing round to polygonal
in shape. Between days 2 and 3, the cell number gradually
increased, and between days 4 and 5 the cells, half of which
appeared polygonal, gradually fused. Between days 8 and 9, the
cells gradually became fusiform and funicular, and blood
vessel-like structures were visible in certain regions. Generation
passage was performed when the cells occupied 80% of the bottom of
the container (Fig. 1).
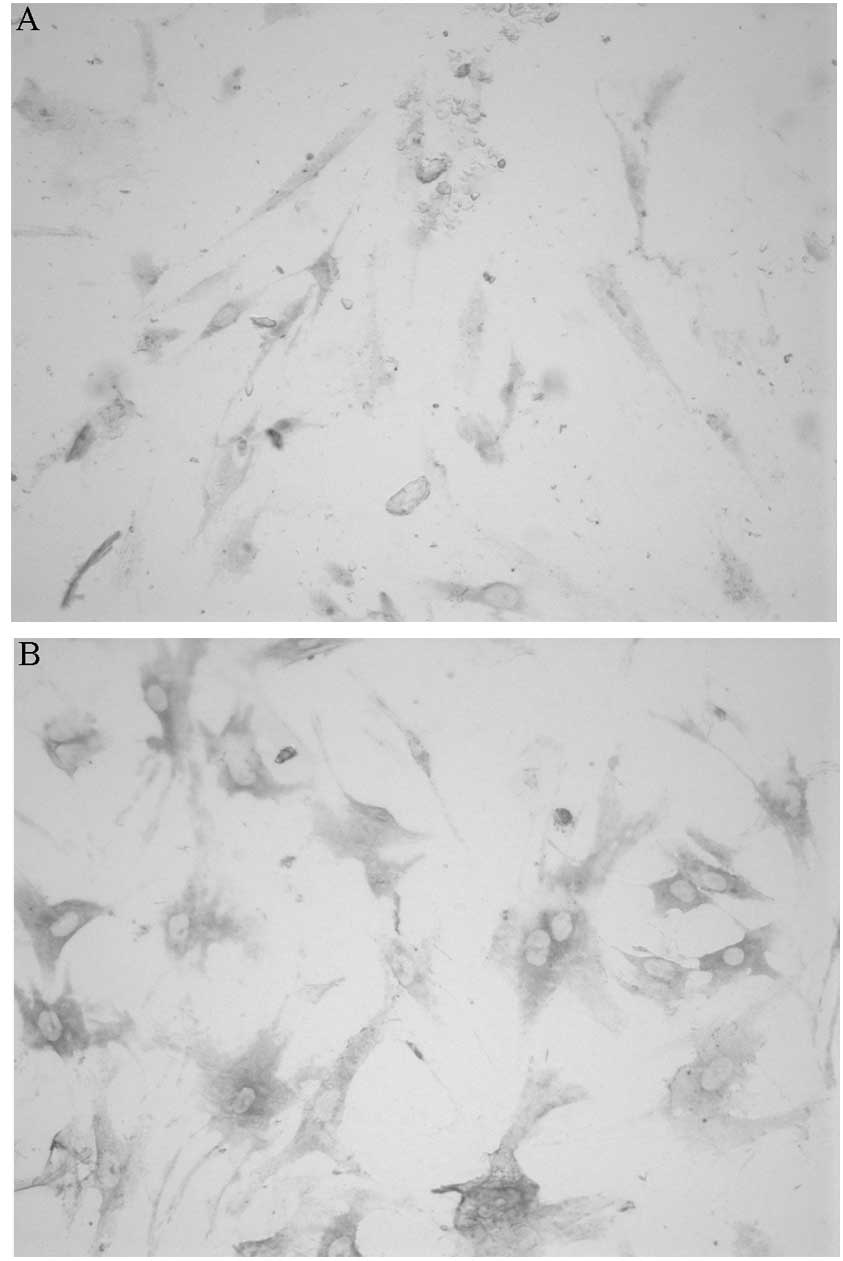
Identification of IHECs
Upon conducting immunohistochemistry, it was
observed that the cells were polygonal and spindle-shaped with
brownish-yellow-stained cytoplasm; the nucleus remained unstained,
indicating a positive result. These results were consistent with
those in previous studies (17,18),
in which vwf was present in the cytoplasm; therefore, it was
confirmed that these cells were IHECs (Fig. 2A). A similar staining method was
used to determine the presence of VEGFR-2 in the cytoplasm,
yielding the same positive results as the previous experiment and
confirming that the cells were IHECs (Fig. 2B).
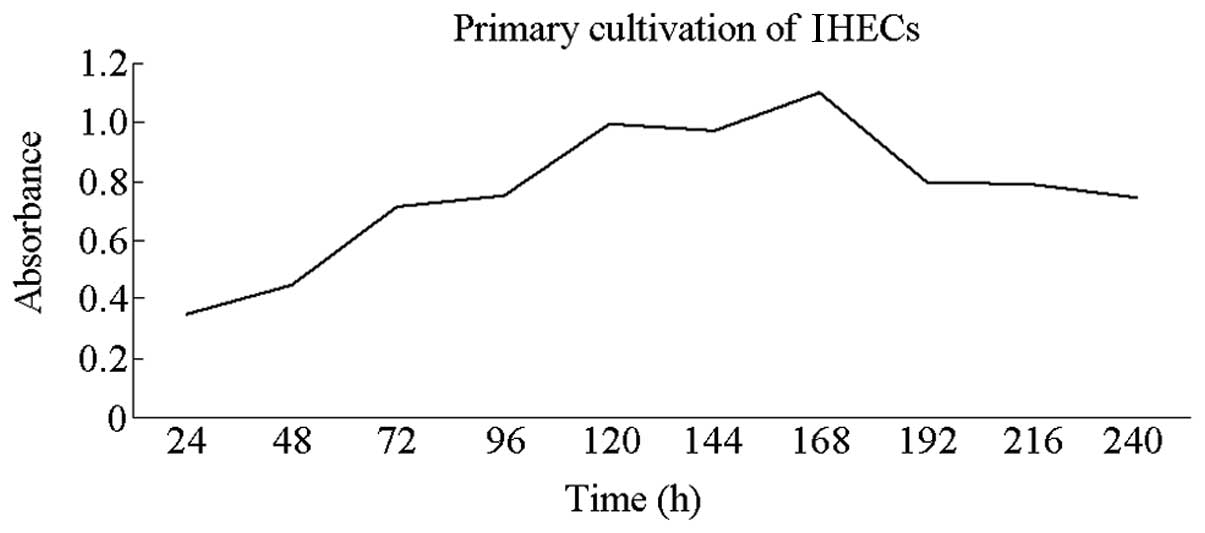
Determination of the growth curve of the
IHECs
The MTT assay was used to determine the IHEC growth
curves. The results indicated a slow increase in the average
absorbance of the cells on days 1 and 2. This parameter rapidly
increased between days 3 and 5 but slightly decreased at day 6. On
days 7 and 8, absorbance increased, prior to decreasing and then
reached a plateau. Absorbance decreased gradually between days 9
and 10 (Fig. 3). This result was
consistent with those from other studies (17,18).
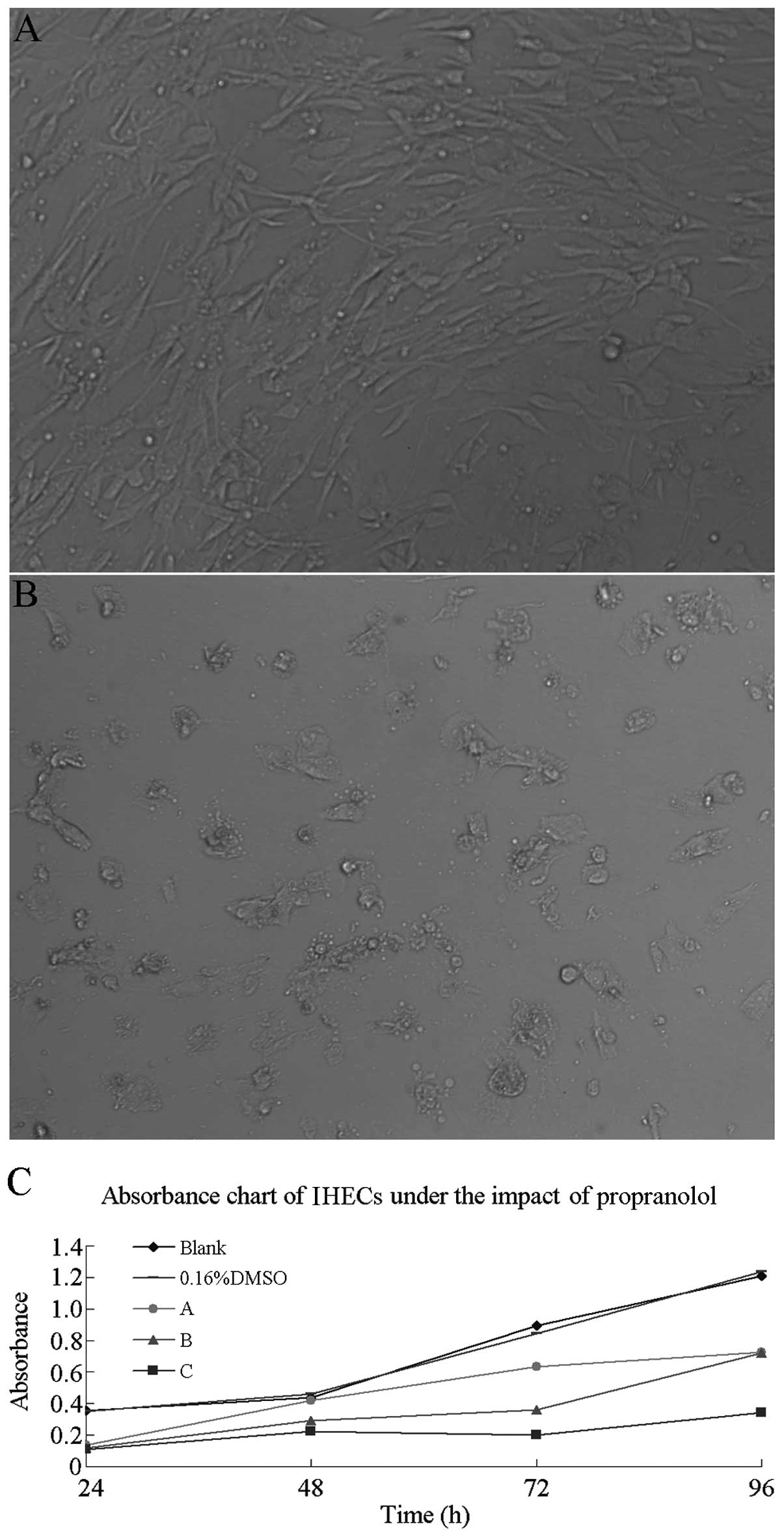
Effect of propranolol on IHECs
In the in vitro culture environment, the
absorbance of IHECs was measured using the MTT assay following
cultivation in 10, 15 and 20 μg/ml propanol working solution,
respectively, for 24, 48, 72 and 96 h. SPSS 17.0 (SPSS, Inc.)
software was used for the statistical analysis of the absorbance at
each concentration. The results showed no significant difference in
the average absorbance of the IHECs among the blank, the DMSO and
the three propranolol concentration groups in the 24- to 48-h
time-frame (P>0.05). However, a difference in absorbance was
observed in the 72-to 96-h time-frame. The inter-group comparison
revealed that while no significant differences were observed among
the control, the DMSO and the 10 and 15 μg/ml propranolol groups, a
significant difference was identified between the 20 μg/ml group
and the blank group (P<0.05). A change was also observed in the
cell morphology: The IHECs in each well initially proliferated and
then adhered to the walls 24 h before the medium was replaced
(Fig. 4A). Comparing this result
with the conditions in the well after 96 h and the replaced medium,
it was found that the morphological characteristics of IHECs had
changed (Fig. 4B). In particular,
the IHECs appeared round or almost round, the cellular space
increased and the cell number was low. The stacked line chart of
IHEC absorbance under different concentrations of propranolol is
shown in Fig. 4C.
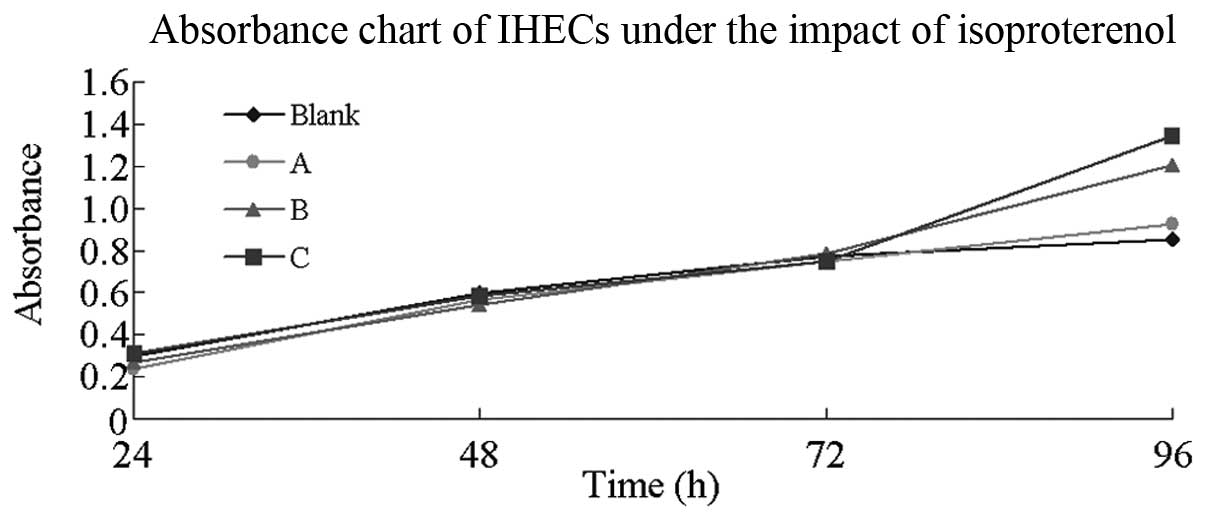
Effect of isoproterenol on IHECs
In the in vitro culture environment, the
average absorbance of the IHECs was measured using the MTT assay
following cultivation in 5, 10 and 20 μg/ml isoproterenol working
fluid, respectively, for 24, 48, 72 and 96 h. Isoproterenol-free
medium was used as the blank control group. No significant
differences were found in the average absorbance of the IHECs among
the groups in the 24-to 72-h time-frame (P>0.05); however, an
absorbance difference was observed at 96 h among the blank group
and the isoproterenol-containing groups. The inter-group comparison
revealed that a high isoproterenol concentration corresponded to a
high absorbance (P<0.05). The stacked line chart of IHEC
absorbance under different concentrations of isoproterenol is shown
in Fig. 5.
Discussion
Vascular tumors, including infantile hemangioma,
delayed pyogenic granuloma and other rare vascular tumors occurring
during infancy and early childhood, are characterized by a
proliferation of endothelial cells, while vascular malformations
are abnormalities of vascular morphogenesis. Vascular tumors can
readily occur in the neonatal period; however, unlike infantile
hemangioma, vascular malformations do not grow rapidly nor regress
spontaneously in the first year following birth (19).
Infantile hemangioma can be divided into the
proliferation period (three to 12 months old), the regression
period (one to three years old) and the regression completion
period (three to seven years old) (20). Although infantile hemangioma in
certain patients undergoes self-regression, hemangioma in the
proliferation period reproduces rapidly. This condition can affect
the function of important surface organs and internal vital organs
and can potentially be life-threatening (19).
The present study targeted infantile hemangioma in
the proliferation period (nine months old), and a surgically
resected specimen was obtained for the primary cultivation of the
IHECs in EGM-2. A previous study on the pathogenesis of hemangioma
indicated that growth factors, including VEGFs, fibroblast growth
factors and human insulin-like growth factors, have an important
function in the formation of vascular tumors (21). The medium used in this study
contained various growth factors and selectively promoted the
growth of IHECs. In the primary cultivation of IHECs at the
proliferation period, normal medium is not able to satisfy the
requirements of cell growth, and the medium containing specific
cytokines is more suitable. The growth curve of the IHECs shows
that the cells grew slowly between days 1 and 2, and that growth
increased rapidly between days 3 and 5, decreased slightly at day 6
and increased again between days 7 and 8. A plateau was ultimately
reached and growth decreased between days 9 and 10.
Since the discovery of propranolol for the treatment
of hemangioma, propranolol has been widely used on affected
children and achieved favorable results (22,23).
Although the therapeutic mechanism remains unclear, the involvement
of the β-adrenergic receptor has been considered. In the present
study, two drugs, mutually antagonistic towards each other, were
used to investigate the functions of the β-adrenergic receptor in
the development of vascular tumors.
Following the intervention with different
propranolol-concentration mediums, the results revealed that the
low-concentration groups (10 and 15 μg/ml) had no significant
adverse effects on the IHECs. In the high-concentration group (20
μg/ml), absorbance decreased between the 72- and 96-h time-points
compared with the other groups, indicating that cell growth was
inhibited. The morphological observations also showed that cell
growth was significantly limited following propranolol action. The
growth curves showed that the average absorbance in the
high-concentration group was decreased compared with that in the
blank control group, indicating that cell growth was suppressed by
propranolol. The degree of suppression corresponded to the drug
concentration.
Isoproterenol is a non-selective β-adrenergic
receptor agonist that exhibits a mutually antagonistic effect with
propranolol. The present study identified that, following culture
for 24–72 h with a certain concentration of
isoproterenol-containing medium, negligible effects were observed
in the IHECs of each group. In addition, no significant differences
were observed in the absorbance among the groups. After 96 h, a
high concentration of isoproterenol corresponded to a high
absorbance in each group, indicating that isoproterenol elicited
stimulating effects on cell growth. In contrast to the effects of
propranolol on the IHECs, isoproterenol elicited a stimulating
effect on the IHECs, with an activity degree that was proportional
to the concentration.
The present study revealed that propranolol elicits
a positive therapeutic effect on infantile hemangioma; however, the
mechanism remains unclear. Considering different theories,
including the cytokine, signal transduction and receptor theories,
the mechanism involved in such effects has yet to be determined.
This study used two mutually antagonistic drugs to act on the
β-adrenergic receptor. The results showed the contrasting effects
of these drugs on IHECs. The results further indicated that the
β-adrenergic receptor plays a role in infantile hemangiomas and
that the therapeutic effect of propranolol was largely induced via
the β-adrenergic receptor. However, further investigation is
required to determine the exact mechanism.
References
|
1
|
Enjolras O and Mulliken JB: Vascular
tumors and vascular malformation (new issuses). Adv Dermatol.
13:375–423. 1997.PubMed/NCBI
|
|
2
|
Mulliken J and Enjolras O: Congenital
hemangiomas and infantile hemangioma: missing links. J Am Acad
Dermatol. 50:875–882. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cahill AM and Nijs EL: Pediatric vascular
malformations: pathophysiology, diagnosis, and the role of
interventional radiology. Cardiovasc Intervent Radiol. 34:691–704.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Itinteang T, Tan ST, Guthrie S, Tan CE,
McIntyre BC, Brasch HD and Day DJ: A placental chorionic villous
mesenchymal core cellular origin for infantile haemangioma. J Clin
Pathol. 64:870–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Régnier S, Dupin N, Le Danff C, Wassef M,
Enjolras O and Aractingi S: Endothelial cells in infantile
haemangiomas originate from the child and not from the mother (a
fluorescence in situ hybridization-based study). Br J Dermatol.
157:158–160. 2007.PubMed/NCBI
|
|
6
|
Zhang GY, Yi CG, Li X, Liang ZQ, Wang RX,
Liu DE, Zhang LM, Meng CY and Guo SZ: Proliferation hemangiomas
formation through dual mechanism of vascular endothelial growth
factor mediated endothelial progenitor cells proliferation and
mobilization through matrix metalloproteinases 9. Med Hypotheses.
70:815–818. 2008. View Article : Google Scholar
|
|
7
|
Takahashi K, Mulliken JB, Kozakewich HP,
Rogers RA, Folkman J and Ezekowitz RA: Cellular markers that
distinguish the phases of hemangioma during infancy and childhood.
J Clin Invest. 93:2357–2364. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng Q, Matsuda T and Hirst SJ: Signaling
pathways regulating interleukin-13-stimulated chemokine release
from airway smooth muscle. Am J Respir Crit Care Med. 169:596–603.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Queto T, Vasconcelos ZF, Luz RA, et al:
G-CSF suppresses allergic pulmonary inflammation, downmodulating
cytokine, chemokine and eosinophil production. Life Sci.
88:830–838. 2011. View Article : Google Scholar
|
|
10
|
Gordillo GM, Onat D, Stockinger M, Roy S,
Atalay M, Beck FM and Sen CK: A key angiogenic role of monocyte
chemoattractant protein-l in hemangioendothelioma proliferation. Am
J Physiol Cell Physiol. 287:C866–C873. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eum SY, Maghni K, Hamid Q, Eidelman DH,
Campbell H, Isogai S and Martin JG: Inhibition of allergic airways
inflammation and airway hyperresponsiveness in mice by
dexamethasone: role of eosinophils, IL-5, eotaxin, and IL-13. J
Allergy Clin Immunol. 111:1049–1061. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
North PE, Waner M, Mizeracki A and Mihm MC
Jr: GLUT-1: a newly discovered immunohistochemical marker for
juvenile hemangiomas. Hum Pathol. 31:11–22. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bauland CG, Smit JM, Bartelink LR,
Zondervan HA and Spauwen PH: Hemangioma in the newborn: increased
incidence after chorionic villus sampling. Prenat Diagn.
30:913–917. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boye E, Yu Y, Paranya G, Mulliken JB,
Olsen BR and Bischoff J: Clonality and altered behavior of
endothelial cells from hemangiomas. J Clin Invest. 107:745–752.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Léauté-Labrèze C, Dumas de la Roque E,
Hubiche T, Boralevi F, Thambo JB and Taïeb A: Propanolol for severe
hemangiomas of infancy. N Engl J Med. 358:2649–2651. 2008.
|
|
16
|
Ji Y, Chen S, Li K, Xiao X, Zheng S and Xu
T: The role of β-adrenergic receptor signaling in the proliferation
of hemangioma-derived endothelial cells. Cell Div. 8:12013.
|
|
17
|
Ma G, Lin XX, Jin YB, et al: Isolation,
culture and identification of endothelial cells in infantile
hemangioma. Zhonghua Zheng Xing Wai Ke Za Zhi. 24:144–147. 2008.(In
Chinese).
|
|
18
|
Ma G, Lin XX, Jin YB, et al: Culture and
cryopreservation of endothelial cells in vitro in infantile
hemangioma. Zhonghua Zheng Xing Wai Ke Za Zhi. 24:197–200. 2008.(In
Chinese).
|
|
19
|
Bolognia JL, Jorizzo JL and Rapini RP:
Dermatology. 2nd Edition. Saunders (W.B) Co. Ltd; Philadelphia, PA,
USA: pp. 1905–1937. 2012
|
|
20
|
Léauté-Labrèze C, Prey S and Ezzedine K:
Infantile haemangioma: part I. Pathophysiology, epidemiology,
clinical features, life cycle and associated structural
abnormalities. J Eur Acad Dermatol Venereol. 25:1245–1253.
2011.PubMed/NCBI
|
|
21
|
Takahashi K, Mulliken JB, Kozakewich HP,
Rogers RA, Folkman J and Ezekowitz RA: Cellular markers that
distinguish the phases of hemangioma during infancy and childhood.
J Clin Invest. 93:2357–2364. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sans V, de la Roque ED, Berge J, Grenier
N, et al: Propranolol for severe infantile hemangiomas: follow-up
report. Pediatrics. 124:e423–e431. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sommers Smith SK and Smith DM: Beta
blockade induces apoptosis in cultured capillary endothelial cells.
In Vitro Cell Dev Biol Anim. 38:298–304. 2002.PubMed/NCBI
|