Introduction
Platelets are central mediators of primary
homeostasis and mediate pathological thrombosis. Activated
platelets stimulate thrombus formation in response to the rupture
of an atherosclerotic plaque or endothelial cell erosion, thereby
promoting atherothrombotic disease (1). Antiplatelet treatment remains the
main therapy for patients with thrombosis and atherosclerosis
(2,3). Antiplatelet treatment for the
prevention of serious vascular events (including nonfatal
myocardial infarction and nonfatal stroke among a large number of
patients with a high risk for occlusive vascular events) and
vascular death is an important strategy, according to the results
of a collaborative meta-analysis of randomized trials (4). However, the number of studies
concerning the side-effects attributed to antiplatelet agents
(including aspirin, ticlopidine, clopidogrel, abciximab and
eptifibatide) is increasing. These negative effects include
allergic/hypersensitivity reactions and gastrointestinal disorders,
including ulceration of the gastric lining and hemorrhage, as well
as increased drug resistance in certain patients (5,6).Studies have shown that various
traditional Chinese medicines have antiplatelet activity (7,8).
Naringin, a type of flavonoid from Fructus Aurantiin, has numerous
biological activities, including anti-inflammatory (9,10)
and antioxidant (11,12) activities, regulation of glucose,
and lipid metabolism (13–15).
The present study explored the effect of naringin on
the aggregation and release of activated platelets in
hyperlipidemic rabbits.
Materials and methods
Experimental animals
Male New Zealand white rabbits (weight = 2.0–2.5 kg)
were provided by Beijing Animal Breeding Center (Beijing, China).
The animals were acclimated for at least one week under standard
conditions with free access to a standard diet and water. All
procedures were approved by the Animal Care and Use Committee of
Tianjin University of Traditional Chinese Medicine (approval
number: TCM-LAEC2013007; Tianjin, China) and conformed to the Guide
for the Care and Use of Laboratory Animals published by the U. S.
National Institutes of Health (NIH publication number 85–23,
revised 1996).
Drugs and reagents
Naringin was obtained from Shanghai Meilian
Biotechnology Co., Ltd. (Shanghai, China; CAS number: 10236-47-2).
Adenosine diphosphate (ADP), arachidonic acid (AA) and collagen
(COLL) were purchased from Chrono-Log Corp. (Havertown, PA, USA).
ELISA kits for P-selectin and platelet factor 4 (PF4) were obtained
from R&D Systems Inc. (Minneapolis, MN, USA). Fibrinogen (FIB),
activated partial thromboplastin time (APTT) and prothrombin time
(PT) kits were purchased from Stago Diagnosis Technology Co., Ltd.
(Paris, France). An intracytoplasmic Ca2+ testing kit
was obtained from Genmed Scientifics Inc. (Shanghai, China).
Establishment of hyperlipidemic rabbit
model
A total of 30 male rabbits were divided into two
groups (control group and group M) according to the total
cholesterol (TC) in their plasma. The rabbits in the control group
(six males) were fed with a basic diet during the experimental
period. The rabbits in group M (24 males) were fed a high
fat/cholesterol diet (1% cholesterol, 10% vegetable oil and 89%
base animal feeds) for four weeks. The amount of daily diet for
each animal was restricted to 50 g, and water was supplied ad
libitum. After four weeks, blood was collected from the ear
edge vein of the rabbits. The 24 hyperlipidemic rabbits in group M
were selected based on their significantly higher TC values
compared with those of the control group and then were divided into
four groups, namely, model group (model), high-dose naringin
treatment group (NH; 60 mg/kg/day), medium-dose naringin treatment
group (NM; 30 mg/kg/day), and low-dose naringin treatment group
(NL; 15 mg/kg/day).
Drug treatment
The rabbits in the treatment groups were orally
administered naringin once a day for 14 consecutive days. The
rabbits in the four groups, with the exception of the control
group, continued to be fed a high fat/cholesterol diet five times a
week to maintain the model.
Preparation of platelet-rich plasma (PRP)
and platelet-poor plasma (PPP)
The rabbits were locally anesthetized with 2%
lidocaine (1 ml), and then blood was collected from the common
carotid artery (CCA) 2 h after the final drug administration and
anticoagulated with citrate (3.8%; 1:9, v/v). PRP was obtained by
centrifugation at 800 rpm for 15 min, and the remaining blood was
further centrifuged at 3,500 rpm for 10 min to prepare the PPP. The
platelet concentration of the PRP was adjusted to
3–5×109 platelets/ml using the PPP.
Determination of platelet
aggregation
Platelet aggregation was measured using an
aggregometer (570-VS; Chrono-Log Corp.) according to the methods of
Born and Cross (16). In a typical
procedure, 0.25 ml PPP and PRP were placed in separate cuvettes and
stirred with a rotor at 37°C for 5 min. Platelet aggregation was
induced by the addition of ADP, AA or COLL (final concentrations of
13 μM, 500 μM and 10 mg/l, respectively). The results were recorded
as light transmission at maximum aggregation following the addition
of an aggregating agent. Data are expressed as the percentage
maximum aggregation.
Determination of the FIB levels, PT and
APTT
Blood was collected from the CCA and anticoagulated
with citrate (3.8%; 1:9, v/v). The plasma was separated by
centrifugation at 3,500 rpm for 10 min. The levels of FIB in the
plasma, and the PT and APTT were determined with an automatic blood
coagulation analyzer (Diagnostica Stago STart 4 hemostasis
analyzer; Stago Diagnosis Technology Co., Ltd.)
Determination of the levels of TC,
triglyceride (TG), high-density lipoprotein (HDL) and low-density
lipoprotein (LDL)
Blood was collected from the CCA. Following
placement in a water bath for 30 min at 37°C, the serum was
separated by centrifugation at 3,500 rpm for 15 min. The levels of
serum TC, TG, HDL and LDL were determined with an automatic
analyzer (7020; Hitachi, Tokyo, Japan).
Determination of the levels of PF4 and
P-selectin
Blood was collected from the CCA. Following
placement in a water bath for 30 min at 37°C, the serum was
separated by centrifugation at 3,500 rpm for 10 min. The levels of
serum P-selectin and PF4 were determined with the ELISA kits
according to manufacturer’s instructions.
Determination of the cytosolic free
calcium concentration ([Ca2+]i)
Following washing twice with Ca2+-free
Tyrode’s buffer (Beijing Reagan Biotechnology Co., Ltd., Beijing,
China), the platelets were suspended in Ca2+ Tyrode’s
buffer (containing 0.38% bovine serum albumin). The platelet
concentration was adjusted to 2×108 platelets/ml, and
then [Ca2+]i was determined using the
intracytoplasmic Ca2+ testing kit according to the
manufacturer’s instructions.
Statistical analysis
All data are expressed as the mean ± standard error
of the mean. Statistical analysis was performed using analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using SPSS software, version 11.5 (SPSS, Inc., Chicago, IL,
USA).
Results
Effects of naringin on the platelet
aggregation induced by ADP, AA and COLL
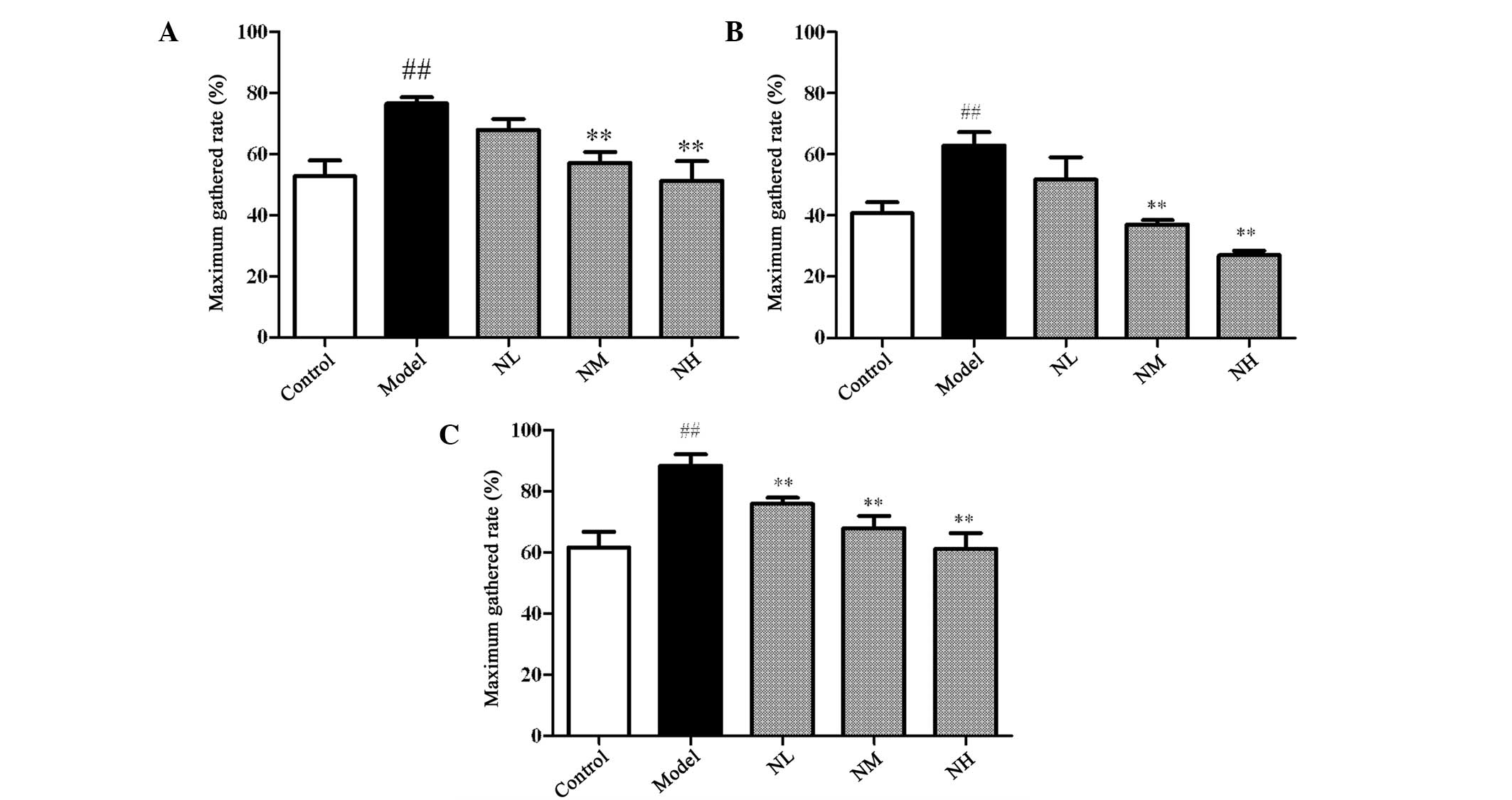
The maximum gathered rates induced by AA, ADP and
COLL in the model group were significantly increased compared with
those of the control group (P<0.01), as shown in Fig. 1. The maximum gathered rates induced
by AA and ADP were significantly inhibited by the medium and high
doses of naringin compared with those of the model group
(P<0.01). Each dose of naringin significantly inhibited the
maximum gathered rates induced by COLL compared with those of the
model group (P<0.01).
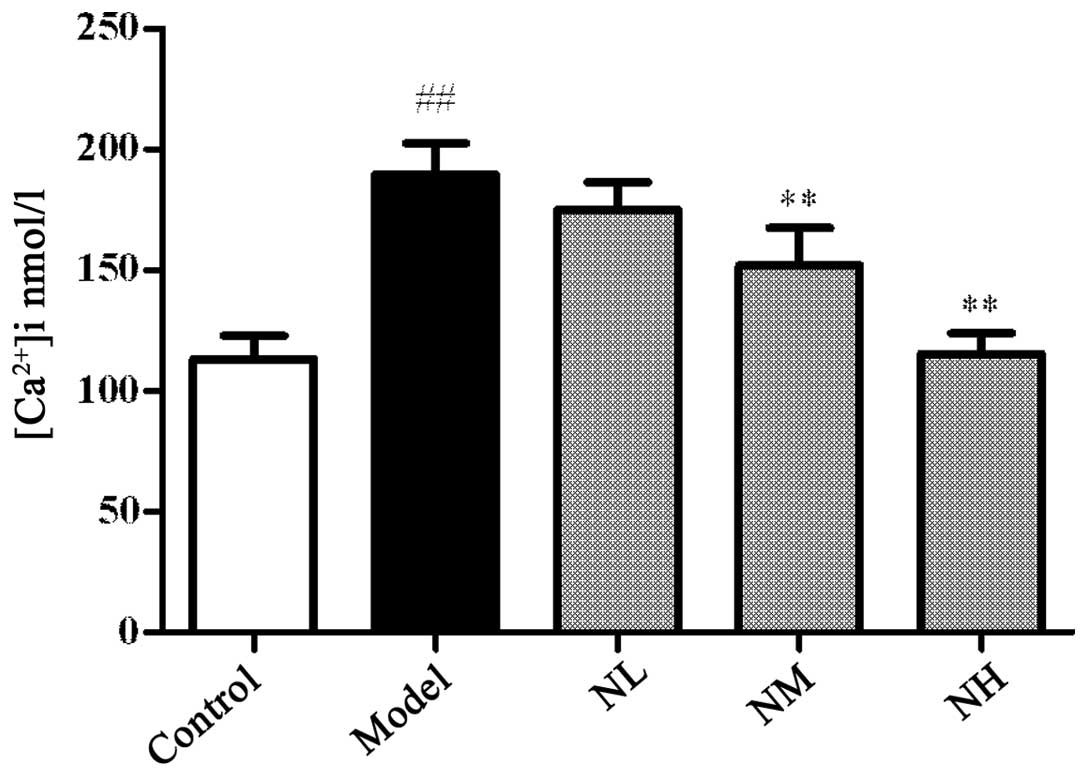
Effects of naringin on the platelet
[Ca2+]i
The platelet [Ca2+]i
significantly increased in the model group compared with that in
the control group (P<0.01), and the low dose of naringin had no
significant effect on the platelet [Ca2+]i
compared with that in the model group. However, the medium and high
doses of naringin significantly reduced the platelet
[Ca2+]i compared with that of the model group
(P<0.01; Fig. 2).
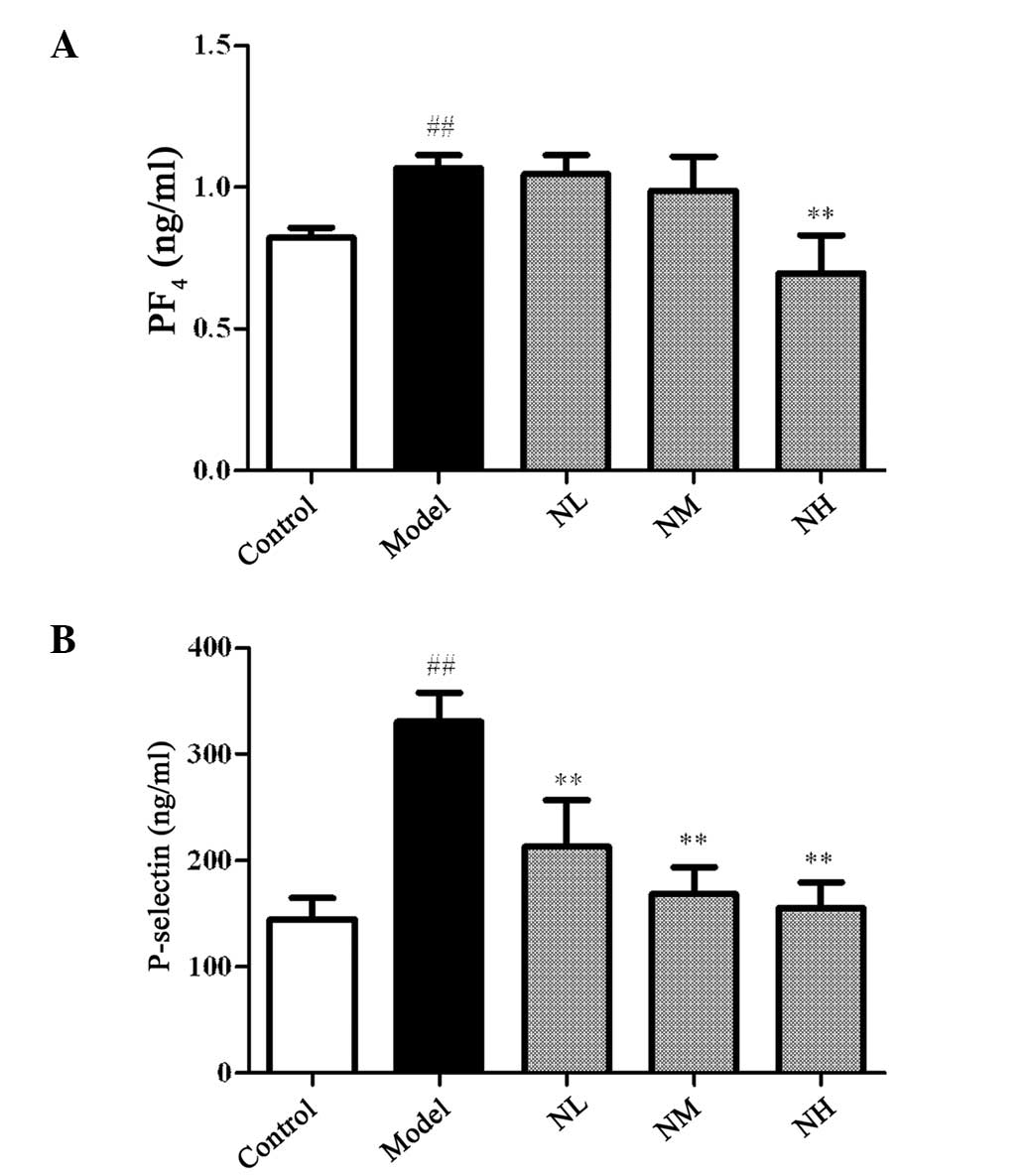
Effects of naringin on the levels of
P-selectin and PF4 in the hyperlipidemic rabbits
Fig. 3 shows that
the levels of PF4 and P-selectin in the model group significantly
increased compared with those in the control group (P<0.01). The
levels of PF4 were not significantly reduced by the low and medium
doses of naringin compared with those in the model group, but were
significantly reduced by the high dose (P<0.01). Each dose of
naringin significantly reduced the levels of P-selectin in the
hyperlipidemic rabbits compared with those in the model group
(P<0.01).
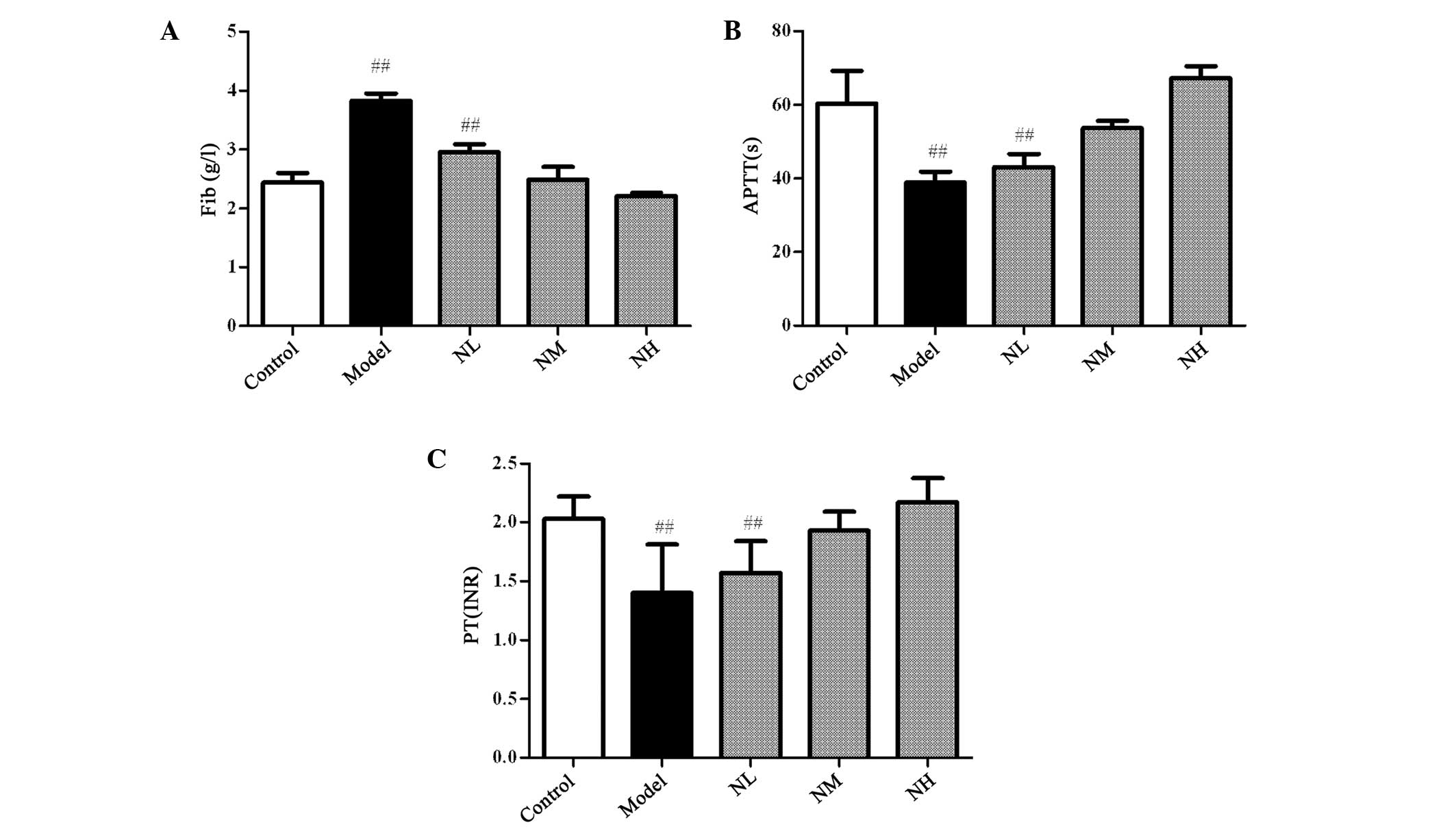
Effects of naringin on the APTT, PT, and
FIB levels in the hyperlipidemic rabbits
In the hyperlipidemic rabbits, the levels of FIB
significantly increased, whereas the PT and APTT significantly
decreased compared with those of the control group (P<0.01).
Naringin reduced the levels of FIB in the plasma and prolonged the
PT and APTT to improve the blood hypercoagulable state of the
hyperlipidemic rabbit; however, no significant difference was
identified in the results for the naringin groups compared with
those of the control group (Fig.
4). This indicated that naringin could not cause bleeding.
Effects of naringin on the levels of
blood lipids in the hyperlipidemic rabbits
After four weeks of high-fat feeding, the levels of
TC, HDL and LDL significantly increased compared with those in the
control group (Table I). However,
the ratio of HDL/TC decreased, whereas the ratio of LDL/TC
increased compared with those in the control group. The high and
medium doses of naringin significantly reduced the levels of TC,
HDL and LDL in the plasma, but the HDL/TC ratio significantly
increased and the LDL/TC ratio decreased compared with those in the
model group (P<0.05-0.01).
 | Table IEffects of naringin on the blood lipid
levels of the hyperlipidemic rabbits. |
Table I
Effects of naringin on the blood lipid
levels of the hyperlipidemic rabbits.
| Group | TC | TG | LDL | HDL | LDL/TC | HDL/TC |
|---|
| Control | 0.94±0.164 | 0.67±0.124 | 0.22±0.083 | 0.49±0.097 | 0.25±0.134 | 0.55±0.200 |
| Model | 11.26±1.283a | 0.68±0.381 | 5.50±0.585a | 2.36±0.317a | 0.50±0.098a | 0.21±0.033a |
| NL | 9.89±1.989 | 0.61±0.259 | 5.11±0.327 | 2.27±0.390 | 0.53±0.089 | 0.24±0.067 |
| NM | 6.57±0.594c | 0.58±0.321 | 3.46±0.820c | 1.82±0.472b | 0.54±0.188 | 0.27±0.061b |
| NH | 3.38±0.260c | 0.53±0.193 | 2.02±0.475c | 1.34±0.399c | 0.60±0.122 | 0.40±0.113c |
Discussion
Hyperlipidemia reportedly triggers platelet
activation, lipid peroxidation, platelet granulocyte aggregation
and platelet aggregation. Sener et al (17) demonstrated that the expression of
P-selectin on the surface of platelets in patients with
hyperlipidemia is highly associated with the levels of TG, LDL and
HDL-cholesterol. Thus, blood lipids and platelet activation play
critical roles in the pathological link of thrombotic diseases to
the incidences of other cardiovascular events (18). Naringin has pharmacological
activity in the cardiovascular system and plays a role in the
regulation of blood glucose and blood lipids. Naringin inhibits the
oxidative susceptibility of LDL, which has a certain inhibitory
effect on the formation of atherosclerosis (19). The present study focused on the
effects of naringin on platelet activation and the coagulation
function in the hyperlipidemic pathological state.
The present study demonstrated that naringin exerted
positive modulatory effects on the levels of blood lipids in
hyperlipemic rabbits. The medium and high doses of naringin
significantly decreased the TC levels and increased the proportion
of HDL in the TC. Naringin also reduces the sensitivity of
platelets by adjusting the levels of blood lipids, thereby
achieving the desired effect of antiplatelet aggregation.
As a second messenger, calcium is involved in
platelet deformation, aggregation and the release reaction to
stimuli. Increased platelet [Ca2+]i activates
myosin light chain kinase, which leads to platelet deformation and
shrinkage. Multiple Ca2+-dependent proteases play an
important role in platelet activation. Ca2+ activates
phospholipase C and phospholipase A2, leading to the release of
thromboxane A2 and platelets. The activation of protein kinase C,
which is involved in the release and aggregation of platelets and
AA metabolism, requires the participation of Ca2+. The
activation state of platelet [Ca2+]i in the
hyperlipemic animal model in the present study was significantly
higher than that in the control group. The results of the present
study confirm that naringin reduces the
[Ca2+]i, indicating that naringin inhibits
platelet contraction and release by decreasing the platelet
[Ca2+]i concentration and thereby inhibiting
myosin light chain phosphorylation.
Naringin inhibited platelet aggregation induced by
AA, ADP and COLL in the present study to appreciable degrees and
had a marked dose-dependent effect, indicating that naringin
inhibits platelet activation via multiple pathways. The present
study also confirmed that naringin had no marked influence on the
normal coagulation function in the experimental animals. Further
studies are required to elucidate the specific mechanisms of
naringin. In conclusion, the results of the present study suggest
that naringin inhibits platelet overactivity by regulating the
levels of blood lipids and the concentration of platelet
cytoplasmic calcium.
Acknowledgements
This study is supported by the ‘Program for
Innovative Research Team in University’ (no. IRT1276) from the
Ministry of Education of the People’s Republic of China for
research on traditional Chinese medicine for the prevention and
treatment of cardiovascular disease.
References
|
1
|
Jennings LK: Mechanisms of platelet
activation: need for new strategies to protect against
platelet-mediated atherothrombosis. Thromb Haemost. 102:248–257.
2009.PubMed/NCBI
|
|
2
|
De Meyer SF, Vanhoorelbeke K, Broos K,
Salles II and Deckmyn H: Antiplatelet drugs. Br J Haematol.
142:515–528. 2008.
|
|
3
|
Siller-Matula JM, Krumphuber J and Jilma
B: Pharmacokinetic, pharmacodynamic and clinical profile of novel
antiplatelet drugs targeting vascular diseases. Br J Pharmacol.
159:502–517. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Antithrombotic Trialists’ Collaboration.
Collaborative meta-analysis of randomised trials of antiplatelet
therapy for prevention of death, myocardial infarction, and stroke
in high risk patients. BMJ. 324:71–86. 2002. View Article : Google Scholar
|
|
5
|
Speich HE, Earhart AD, Hill SN, et al:
Variability of platelet aggregate dispersal with glycoprotein
IIb-IIIa antagonists eptifibatide and abciximab. J Thromb Haemost.
7:983–991. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cannon CP, Rhee KE, Califf RM, et al:
REACH Registry Investigators: Current use of aspirin and
antithrombotic agents in the United States among outpatients with
atherothrombotic disease (from the REduction of Atherothrombosis
for Continued Health [REACH] Registry). Am J Cardiol. 105:445–452.
2010.PubMed/NCBI
|
|
7
|
Wang Y, Wang J, Guo L and Gao X:
Antiplatelet effects of Qishen Yiqi Dropping Pill in platelets
aggregation in hyperlipidemic rabbits. Evid Based Complement
Alternat Med. 2012:2054512012.PubMed/NCBI
|
|
8
|
Zhang Y, Tang Y, Guo J, et al: Dose-effect
relationship of traditional Chinese medicine formula for promoting
blood circulation to remove stasis on ADP-induced platelet
aggregation and rabbit plasma thrombin time. Zhongguo Zhong Yao Za
Zhi. 34:2821–2826. 2009.(In Chinese).
|
|
9
|
Kang SR, Park KI, Park HS, et al:
Anti-inflammatory effect of flavonoids isolated from Korea
Citrus aurantim L. on lipopolysaccharide-induced mouse
macrophage RAW 264.7 cells by blocking of nuclear factor-kappa B
(NF-κB) and mitogen-activated protein kinase (MAPK) signalling
pathways. Food Chem. 129:1721–1728. 2011.
|
|
10
|
Jagetia GC and Reddy TK: Alleviation of
iron induced oxidative stress by the grape fruit flavanone naringin
in vitro. Chem Biol Interact. 190:121–128. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kanno S, Shouji A, Tomizawa A, et al:
Inhibitory effect of naringin on lipopolysaccharide (LPS)-induced
endotoxin shock in mice and nitric oxide production in RAW 264.7
macrophages. Life Sci. 78:673–681. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Balestrieri ML, Castaldo D, Balestrieri C,
et al: Modulation by flavonoids of PAF and related phospholipids in
endothelial cells during oxidative stress. J Lipid Res. 44:380–387.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gorinstein S, Leontowicz H and Leontowicz
M: Effect of hesperidin and naringin on the plasma lipid profile
and plasma antioxidant activity in rats fed a
cholesterol-containing diet. J Sci Food Agric. 87:1257–1262. 2007.
View Article : Google Scholar
|
|
14
|
Jeon SM, Bok SH, Jang MK, et al:
Antioxidative activity of naringin and lovastatin in high
cholesterol-fed rabbits. Life Sci. 69:2855–2866. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singh D, Chander V and Chopra K:
Protective effect of naringin, a bioflavonoid on glycerol-induced
acute renal failure in rat kidney. Toxicology. 201:143–151. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Born GV and Cross MJ: The aggregation of
blood platelets. J Physiol. 168:178–195. 1963. View Article : Google Scholar
|
|
17
|
Sener A, Ozasvci D, Oba R, et al: Do
platelet apoptosis, activation, aggregation, lipid peroxidation and
platelet-leukocyte aggregate formation occur simultaneously in
hyperlipidemia? Clin Biochem. 38:1081–1087. 2005. View Article : Google Scholar
|
|
18
|
Pei W, Baron H, Müller-Myhsok B, et al:
Support for linkage of familial combined hyperlipidemia to
chromosome 1q21–q23 in Chinese and German families. Clin Genet.
57:29–34. 2000.
|
|
19
|
Ghaffari MA and Ghiasvand T: In vitro
effect of Naringin and Quercetin on LDL oxidation via their
influence in copper binding to LDL. Iranian Journal of Diabetes and
Lipid Disorders. 7:23–33. 2007.(In Persian).
|