Introduction
Glioblastoma is the most frequently identified type
of malignant brain tumor in adults and results in serious clinical
problems (1–3). Standard treatments for glioblastoma
include surgery, radiation and chemotherapy. However, traditional
surgery or radiotherapy alone is not fully effective and the median
survival period of patients with brain cancer is not satisfactory
(4,5). Radiotherapy in combination with
chemotherapy has evident advantages in curing brain cancer by
improving the three- to ten-year survival rates compared with those
in patients treated with radiotherapy alone (6). Previous studies have investigated
radiotherapy in combination with weekly nedaplatin and docetaxel
chemotherapy, and indicated that chemotherapy may significantly
increase the effect of radiotherapy on carcinomas, and reduce the
toxicity of chemotherapy (7–9).
Since chemotherapy, including nedaplatin, is toxic to normal cells,
novel therapeutic agents that specifically target tumor-related
cellular signaling molecules are necessary to improve treatment
(6).
Heat shock proteins (Hsps) are a group of proteins
that are classified according to their relative molecular masses
and include Hsp10, Hsp27, Hsp40, Hsp60, Hsp70, Hsp90 and Hsp110
(10). Hsp70 has been revealed to
be upregulated in certain types of cancer and may contribute to
resistance to chemotherapy (11).
Hsp90 is a type of chaperoning protein that is abundantly expressed
in cells and is required for the expression, conformation
maintenance and function of a large number of cellular proteins
(12,13). Certain Hsp90-affected proteins are
involved in the processes of tumor invasion, angiogenesis and
metastasis (14). They are also
important for the maturation and functioning of cellular signaling
proteins that induce mitogen-activated protein kinases (MAPK) and
nuclear factor-κB (NF-κB) pathways (15,16).
Certain cellular events, including tumorigenesis, lead to the
activation of the NF-κB pathway. Activation of the inhibitory κB
kinase (IKK) α and IKKβ leads to kinase phosphorylation and
subsequent ubiquitin-dependent degradation by the cellular
proteasomal pathway (17,18). Furthermore, Hsp90 stabilizes Raf-1,
Akt, and ErbB2 proteins (19–21),
which are involved in the processes of that counteract
radiation-induced cell death (22–24).
Inhibitors of Hsp90, including geldanamycin and its
derivatives, increase the radiosensitivity of tumor cell lines
derived from the glioma, prostate, pancreas and cervix. However,
poor solubility, formulation difficulties and the hepatotoxicity of
such compounds have limited their clinical application. Recently,
the isoxazole resorcinol derivative NVP-AUY922 revealed an
inhibitory effect on carcinoma cells by targeting the tumor
suppressor phosphatase and tensin homolog (25). Although clinically successful in
certain cancer types, one problem of Hsp90 therapy is that Hsp90
inhibitors often trigger the heat shock response, leading to an
increase in the expression level of Hsp70 (26). Hsp70 induction often results in
drug resistance and the advancement of the disease (27). Thus, the discovery of a method to
maintain the effect of Hsp90 inhibitors without increasing the
levels of Hsp70 is important.
NVP-BEP800 is a novel, fully synthetic, orally
available Hsp90 inhibitor of the 2-aminothieno[2,3-d]pyrimidine
class (28,29). The compound has favorable
pharmaceutical and pharmacological properties. It is reported to
demonstrate strong antiproliferative activity against various tumor
cell lines at tolerable doses (29,30).
However, the effect of NVP-BEP800 on glioblastoma remains
unknown.
In the present study, the effect of NVP-BEP800 in
combination with radiation on glioblastoma cells was determined.
The effect of the combined treatment on cell viability and
apoptosis was analyzed and the underlying mechanism was
investigated. The results were evaluated to determine whether a
combination of NVP-BEP800 and radiotherapy may be an effective
therapeutic strategy for the treatment of glioblastoma.
Materials and methods
Cell culture and reagents
The T98G human glioblastoma cell line was provided
by the Hangzhou Normal University (Hangzhou, China) and cultured at
37°C in a humidified 5% CO2 incubator. Cells were grown
in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA,
USA) supplemented with 10% (v/v) fetal bovine serum (Invitrogen)
and antibiotics (100 U/ml penicillin and 100 mg/ml streptomycin).
NVP-BEP800 (InvivoGen, San Diego, CA, USA) were dissolved in
dimethyl sulfoxide (DMSO).
X-ray irradiation
Cells were grown in flasks and irradiated using a
superficial radiotherapy system (SRT)-100 X-ray (Tema Sinergie
S.p.A, Faenza RA, Italy), with a locator diameter of 10 cm, 70 kV
and 10 mA, and a depth of 3 cm, at the Affiliated Hospital of
Hangzhou Normal University. The irradiation (10 Gy) was performed
at room temperature for 20 min. For combined treatments, cells were
irradiated 24 h following the addition of NVP-BEP800 into the
medium. The experiments were repeated at least three times.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Prior to treatments, the cells were placed into
6-well plates in medium at a density of 1×105
cells/well, three wells per treatment group and cultured for 24 h.
The cells were divided into treatment groups and treated
accordingly for 40 h: vehicle control (DMSO, 0.016%, v/v),
NVP-BEP800 (0.05, 0.1 or 0.2 μM), irradiation (10 Gy), or
NVP-BEP800 (0.05, 0.1 or 0.2 μM) in combination with irradiation
(10 Gy). Upon completion of the treatment, all cells were incubated
with 0.5 mg/ml MTT for 3 h according to the manufacturer’s
instructions (Sigma Chemical Co., St. Louis, MO, USA). The relative
viability of the treated cells to the untreated control cells was
measured. The absorbance was measured at 490 nm on a microplate
reader (Bio-Rad Laboratories, Hercules, CA, USA).
Apoptosis assay
Following treatment, the cells were harvested,
washed twice with phosphate-buffered saline (PBS) and fixed by
incubation in 4% paraformaldehyde for 30 min at room temperature.
The cells were washed again with PBS to remove the fixative. The
fixed cells were resuspended in PBS containing Hoechst 33258 (5
μg/ml) and incubated at room temperature for 15 min in the dark.
Cells were placed on glass slides and examined for cells with
apoptotic morphology (nuclear condensation and chromatin
fragmentation) using a fluorescence microscope (Olympus IX81,
Olympus, Tokyo, Japan) provided by Hangzhou Normal University. To
determine the apoptotic level, 300 nuclei from random microscopic
fields were analyzed from each sample. Data are presented as the
mean percentages of apoptotic cells.
Immunoblot analysis
The total proteins were harvested from the treated
cells, separated on 10% sodium dodecyl sulfate/polyacrylamide gel
electrophoresis (SDS/PAGE) gels, and then subjected to immunoblot
analyses. The primary antibodies against IKKβ, Hsp70 and β-actin
were purchased from Santa Cruz Biotechnology, Inc., (Santa Cruz,
CA, USA); anti-IKKβ, cat# sc-8014, 1:150; anti-Hsp70, cat#
sc-32239, 1:200; anti-β-actin, cat# sc-130301, 1:10,000. Secondary
antibodies used in this study were goat anti-mouse immunoglobulin G
(IgG)-horse radish peroxidase (HRP; cat# sc-2005, 1:5,000; Santa
Cruz Biotechnology, Inc.,). Bound antibodies were detected using an
enhanced chemiluminescence (ECL) system (Pierce Biotechnology,
Inc., Rockford, IL, USA). The mean normalized optical density of
IKKβ and Hsp70 protein bands relative to the optical density of
β-actin bands from the same condition was calculated. The
experiments were repeated at least three times.
Quantitative reverse
transcription-polymerase chain reaction (qPCR)
qPCR analyses of the mRNA levels of IKKβ and Hsp70
in cells were performed. The total RNAs were harvested from cells
using an RNeasy kit (Qiagen, Hilden, Germany) according to the
manufacturer’s instructions. The RT-PCR experiments were repeated
at least three times. RNA was reverse transcribed into cDNA using
random primers in an ImProm-II™ reverse transcription system
(Promega Corporation, Madison, WI, USA) according to the
manufacturer’s instructions. The expression levels of IKKβ and
Hsp70 mRNA were quantified by qPCR using an ABI Prism®
7900HT sequence detection system (Applied Biosystems, Foster City,
CA, USA). The primers used are shown in Table I. An assay reagent containing
premixed primers and a
4,7,2′-trichloro-7′-phenyl-6-carboxyfluorescein (VIC)-labeled probe
(Applied Biosystems; cat. no. 4310884E) was applied to detect the
expression levels of endogenous glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) mRNA. Template-negative and RT-negative
conditions were used as controls. Amplification of IKKβ and Hsp70
cDNAs and the endogenous GAPDH cDNA were monitored via changes in
the 6-carboxyfluorescein (FAM) and VIC fluorescent intensities,
respectively, with the ABI 7900HT software (Applied Biosystems).
The relative amounts of the IKKβ and Hsp70 transcripts were
normalized to the amount of cellular GAPDH mRNA.
 | Table IPrimers used in the quantitative
reverse transcription-polymerase chain reaction (qPCR). |
Table I
Primers used in the quantitative
reverse transcription-polymerase chain reaction (qPCR).
| Primers | Sequences
(5′-3′) |
|---|
| IKKβ_F |
5′-TGGCAATCGGCTTAGCGAT-3′ |
| IKKβ_R |
5′-GATCGGTATAGCCCGTTAA-3′ |
| Hsp70_F |
5′-CGGATTAGCCGTATGCATGC-3′ |
| Hsp70_R |
5′-GATCAATTACGGATTCGTAC-3′ |
Statistical analysis
Data are expressed as mean ± standard deviation.
SPSS software, version 10.0 (SPSS. Inc., Chicago, IL, USA) was used
to carry out independent sample Student’s t-tests. P<0.05 was
considered to indicate a statistically significant difference.
Results
NVP-BEP800 in combination with X-ray
irradiation inhibits the viability of glioblastoma cells
To determine whether NVP-BEP800 affects glioblastoma
cells, T98G human glioblastoma cells were treated with DMSO
(0.016%, v/v) only, NVP-BEP800 (0.05, 0.1 or 0.2 μM), NVP-BEP800
(0.05, 0.1 or 0.2 μM) in combination with X-ray irradiation (10 Gy,
20 min), or X-ray irradiation only (10 Gy), for 40 h. Cell
viability was measured using the MTT assay following the completion
of the treatments. The treatment with DMSO served as a non-drug
control.
As shown in Fig. 1,
the cell viabilities decreased by treatment with NVP-BEP800 (0.05,
0.1 or 0.2 μM) in a dose-dependent manner, when compared with the
cells treated with DMSO only. Furthermore, combination with X-ray
irradiation significantly enhanced the inhibitory effect of
NVP-BEP800 on T98G cells, although the X-ray treatment alone only
slightly reduced the cell viability. These results suggest that
irradiation enhances the inhibitory effects of NVP-BEP800 on the
proliferation of malignant glioblastoma cells.
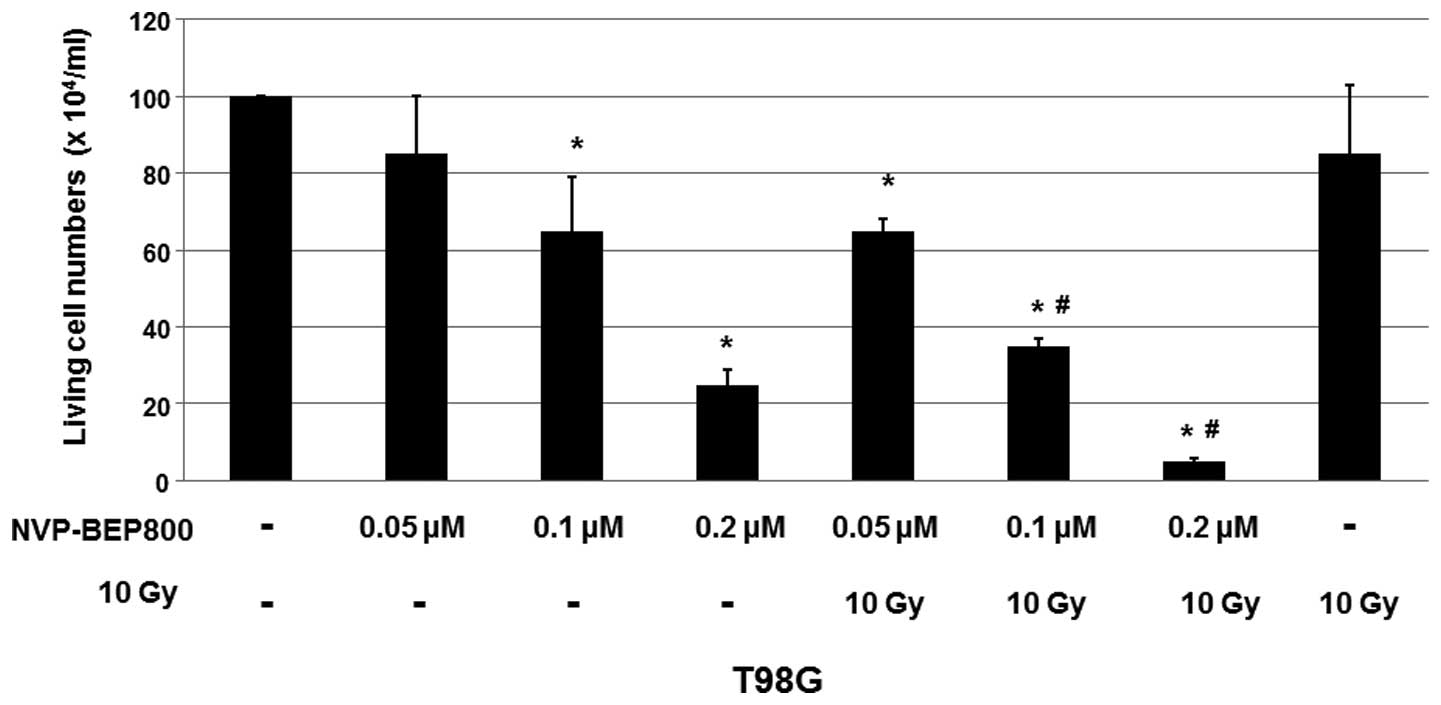 | Figure 1Cell viability of cells treated with
dimethyl sulfoxide (DMSO), NVP-BEP800, NVP-BEP800 with X-ray
irradiation, or X-ray irradiation alone. A human glioblastoma cell
line, T98G, was treated with the vehicle control only (DMSO),
NVP-BEP800 (0.05, 0.1 or 0.2 μM) with or without X-ray irradiation,
or X-ray irradiation alone (10 Gy). Cells irradiated with X-rays
were used as the irradiation control. Cell viability was measured
using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) assay 40 h following the addition of NVP-BEP800.
Values are expressed as means ± standard devations.
*P<0.05 vs. corresponding control with DMSO only;
#P<0.05 vs. the relative conditions in the absence of
X-ray irradiation. |
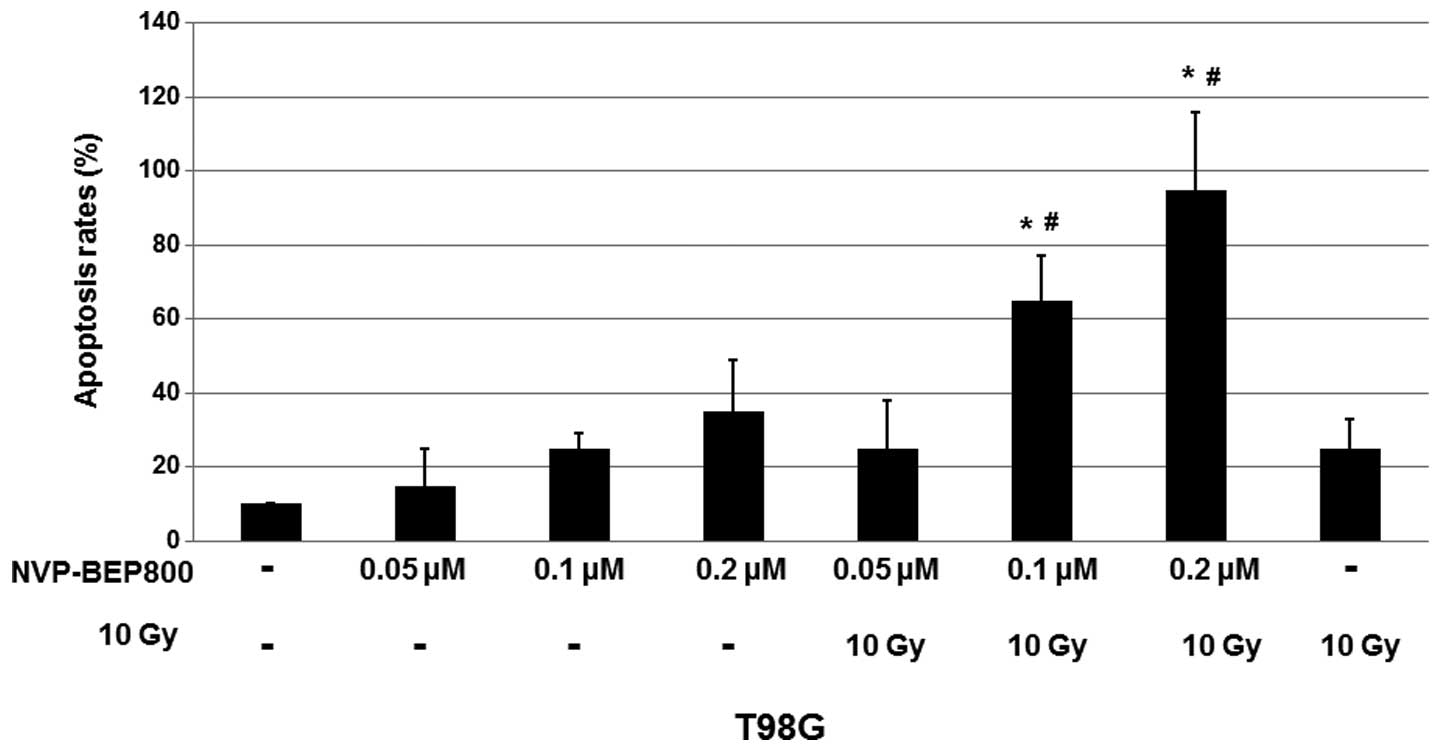
NVP-BEP800 induces apoptosis in the human
glioblastoma cells
Since NVP-BEP800 reduced the proliferation of
glioblastoma cells, it was investigated whether NVP-BEP800 was able
to induce the apoptosis of T98G cells. Cells were treated with DMSO
(0.016%, v/v) only, NVP-BEP800 (0.05, 0.1 or 0.2 μM) alone or in
combination with X-ray irradiation (10 Gy, 20 min), or X-ray
irradiation alone (10 Gy), for 40 h. To quantify the apoptosis,
fluorescence microscopy assays were conducted following staining of
the various treated cells with Hoechst 33258.
As shown in Fig. 2,
treatment with NVP-BEP800 resulted in an increase in cell
apoptosis. When compared with the untreated control, 0.2 μM
NVP-BEP800 in combination with X-ray irradiation caused apoptosis
of the T98G cells with a mean rate of ~95%. These results indicate
that NVP-BEP800 combined with X-ray irradiation significantly
increases the apoptosis rate of cells.
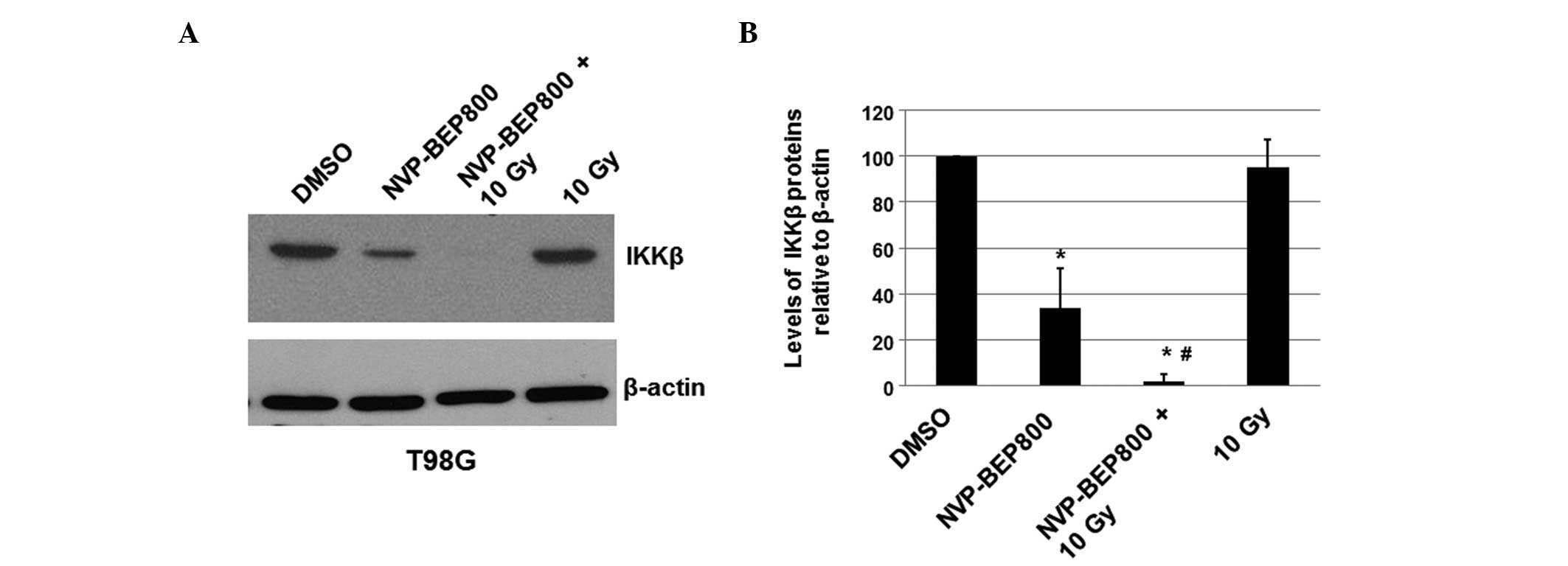
NVP-BEP800 inhibits the expression of
IKKβ protein
To determine whether NVP-BEP800 affects the
expression level of cellular IKKβ in T98G cells, the cells were
treated with vehicle control only (DMSO), NVP-BEP800 (0.2 μM) alone
or in combination with X-ray irradiation (10 Gy, 20 min), or with
X-ray irradiation alone (10 Gy). After 40 h, the total proteins
were isolated and the expression levels of IKKβ were determined by
immunoblot analysis. The cellular β-actin protein served as a
loading control. The mean normalized levels of the IKKβ protein
bands relative to the levels of the β-actin band under the same
conditions were calculated and subjected to statistical analyses.
The calculated ratios of the levels of the IKKβ proteins relative
to the β-actin levels are shown in Fig. 3, along with one of the blots. The
treatment of cells with NVP-BEP800 decreased the expression level
of IKKβ to 28% of that in the untreated sample. These results
indicate that NVP-BEP800 significantly decreases the expression
level of IKKβ in the treated glioblastoma cells. Compared with
cells treated with DMSO only and cells treated with X-ray
irradiation only, cells treated with NVP-BEP800 and X-ray
irradiation had significantly lower expression levels of IKKβ
protein NVP-BEP800 and X-ray irradiation may inhibit the
proliferation of glioblastoma cells through a mechanism associated
with the NF-κB signaling pathway.
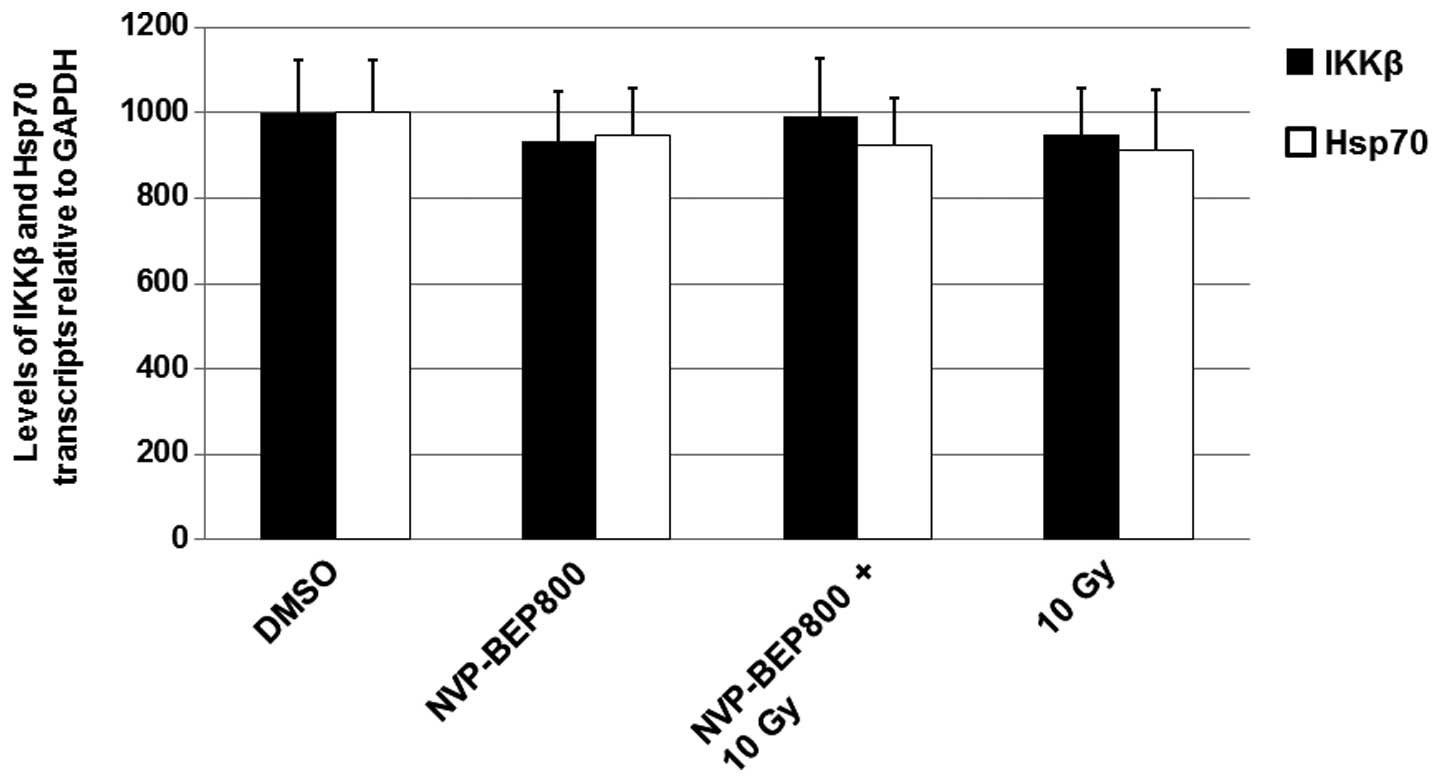
NVP-BEP800 and X-ray irradiation do not
affect the levels of IKKβ mRNA
Changes in the levels of protein expression are
often caused by altered gene transcription. Therefore, in the
current study, cells were treated with DMSO, NVP-BEP800 (0.2 μM)
alone or in combination with X-ray irradiation, or X-ray
irradiation alone (10 Gy), for 40 h. The cells were harvested and
the total RNAs were determined by qPCR. The level of mRNA
transcripts in the untreated cells (DMSO) were assigned a value of
1,000. As shown in Fig. 4, the
mean levels of IKKβ mRNA transcripts in cells treated with
NVP-BEP800, NVP-BEP800 + X-ray irradiation, or X-ray irradiation
alone were similar to those in cells treated with DMSO only. These
results suggest that NVP-BEP800 and X-ray irradiation do not affect
the levels of IKKβ mRNA.
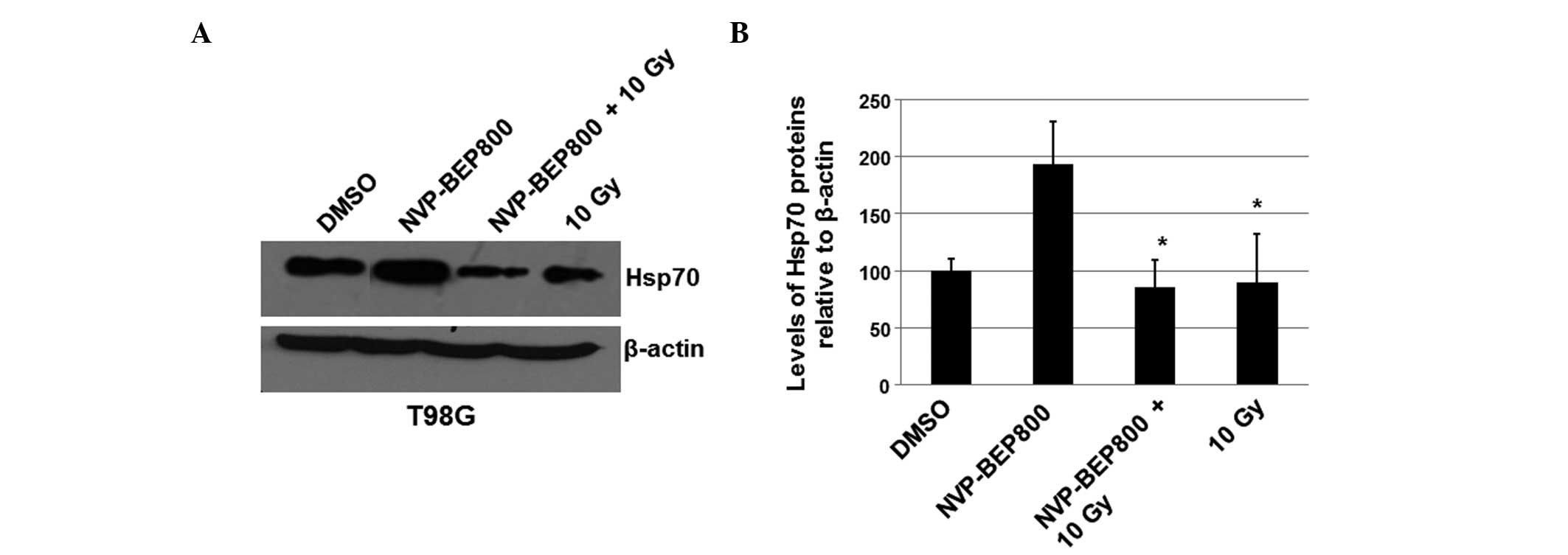
X-ray irradiation attenuates the
upregulation of Hsp70 levels by NVP-BEP800
To further investigate the molecular mechanisms
underlying the combined effects of NVP-BEP800 and X-ray
irradiation, the possible effect of NVP-BEP800 and X-ray
irradiation on the levels of Hsp70 was determined. The levels of
Hsp90 were not detected as they are not detectably affected by
Hsp90 inhibitors, possibly due to the high enrichment of Hsp90 in
cells. T98G cells were treated with vehicle control only (DMSO),
NVP-BEP800 (0.2 μM) alone or in combination with X-ray irradiation,
or X-ray irradiation alone (10 Gy), for 40 h. Whole-cell extracts
were isolated for the preparation of the total RNAs and proteins.
qPCR was performed to detect the levels of Hsp70 mRNA. The levels
of Hsp70 (Fig. 4) were not
markedly altered by the treatments.
An immunoblot assay was conducted to analyze the
expression level of Hsp70. It was revealed that the protein levels
of Hsp70 increased in cells treated with NVP-BEP800 alone (Fig. 5). However, the increase in the
levels of Hsp70 was attenuated by X-ray irradiation in the combined
treatment (Fig. 5). These results
suggest that X-ray irradiation may attenuate the drug resistance
associated with NVP-BEP800 since the higher level of Hsp70 is
associated with the drug resistance induced by Hsp90
inhibitors.
Discussion
Standard treatments for glioblastoma include
surgery, radiation and chemotherapy. Radiotherapy in combination
with chemotherapy has clear advantages in curing brain cancers by
improving the three to ten-year survival rate of patients compared
with that in patients treated with radiotherapy alone. In the
present study, the effects of NVP-BEP800 on the T98G human
glioblastoma cell line were determined. NVP-BEP800 is a novel
fully-synthetic, orally-available 2-amino-thieno[2,3-d]pyrimidine
derivative that acts as an Hsp90 inhibitor (28–30).
The current study demonstrated that combined treatment with
NVP-BEP800 and X-ray irradiation resulted in the synergistic
destruction of malignant cells. Furthermore, NVP-BEP800
significantly induced apoptosis in the human glioblastoma cells.
These results indicate that a combined treatment with NVP-BEP800
and X-ray irradiation may be an effective strategy for the
treatment of glioblastoma.
The mechanisms underlying the effects of NVP-BEP800
and X-ray irradiation were further investigated in the current
study. The immunoblot analysis data indicated that NVP-BEP800
markedly reduced the expression level of the IKKβ protein. The
inhibitory effect of NVP-BEP800 on the IKKβ protein may be the
mechanism responsible for the effect of NVP-BEP800 on the T98G
human glioblastoma cells. Since IKKβ is an important protein
involved in the NF-κB pathway (15,16),
it is hypothesized that the NF-κB pathway is associated with the
action of NVP-BEP800. A previous study reported that NVP-BEP800 may
exert a radiosensitization effect on A549 lung carcinoma and SNB19
glioblastoma cells, with a cell type-specific cytotoxicity
(31). This effect may be
associated with the destabilization and depletion of more than one
Hsp90-affected protein (32).
Multiple cellular processes may be altered through the combined use
of NVP-BEP800 and X-ray irradiation, resulting in the depletion of
the S phase and G2/M arrest, increased DNA damage, and the
induction of apoptosis (32). All
of these results suggest that NVP-BEP800 and X-ray irradiation may
have important implications for tumor therapy.
Previous studies have revealed that Hsp70 is
upregulated in certain types of cancer and mediates the drug
resistance of Hsp90 inhibitors to chemotherapy (11,26–27).
The present study demonstrated that X-ray irradiation is able to
significantly attenuate the increase in the levels of Hsp70 in
cells treated with NVP-BEP800. Since the higher levels of Hsp70 are
associated with drug resistance to Hsp90 inhibitors (11,26,27),
the effect of X-ray irradiation on Hsp70 levels may be another
mechanism, in addition to the effect of NVP-BEP800 on the NF-κB
signaling pathway, for the action of the combined treatment on
glioblastoma cells.
Acknowledgements
This study was supported by the Department of
Education of Zhejiang Province, China (Grant No. 201328763).
References
|
1
|
Thomas L, Di Stefano AL and Ducray F:
Predictive biomarkers in adult gliomas: the present and the future.
Curr Opin Oncol. 25:689–694. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thon N, Kreth S and Kreth FW: Personalized
treatment strategies in glioblastoma: MGMT promoter methylation
status. Onco Targets Ther. 6:1363–1372. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chowdhary S and Chamberlain M: Bevacizumab
for the treatment of glioblastoma. Expert Rev Neurother.
13:937–949. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tejada S, Aldave G, Marigil M, Gállego
Pérez-Larraya J, Domínguez PD and Díez-Valle R: Factors associated
with a higher rate of distant failure after primary treatment for
glioblastoma. J Neurooncol. 116:169–175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gaber M, Selim H and El-Nahas T:
Prospective study evaluating the radiosensitizing effect of reduced
doses of temozolomide in the treatment of Egyptian patients with
glioblastoma multiforme. Cancer Manag Res. 5:349–356. 2013.
|
|
6
|
Niyazi M, Schwarz SB, Suchorska B and
Belka C: Radiotherapy with and without temozolomide in elderly
patients with glioblastoma. Strahlenther Onkol. 188:154–159. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsumoto H, Hirabayashi Y, Kubota H, et
al: A combined therapy with docetaxel and nedaplatin for relapsed
and metastatic esophageal carcinoma. Anticancer Res. 32:1827–1831.
2012.PubMed/NCBI
|
|
8
|
Jingu K, Ariga H, Nemoto K, et al:
Long-term results of radiochemotherapy for solitary lymph node
metastasis after curative resection of esophageal cancer. Int J
Radiat Oncol Biol Phys. 83:172–177. 2012. View Article : Google Scholar
|
|
9
|
Zhu H, Huo X, Chen L, Wang H and Yu H:
Clinical experience with radio-, chemo- and hyperthermotherapy
combined trimodality on locally advanced esophageal cancer. Mol
Clin Oncol. 1:1009–1012. 2013.PubMed/NCBI
|
|
10
|
Jolly C and Morimoto RI: Role of the heat
shock response and molecular chaperones in oncogenesis and cell
death. J Natl Cancer Inst. 92:1564–1572. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rérole AL, Jego G and Garrido C: Hsp70:
anti-apoptotic and tumorigenic protein. Methods Mol Biol.
787:205–230. 2011.PubMed/NCBI
|
|
12
|
Picard D: Heat-shock protein 90, a
chaperone for folding and regulation. Cell Mol Life Sci.
59:1640–1648. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Whitesell L and Lindquist SL: HSP90 and
the chaperoning of cancer. Nat Rev Cancer. 5:761–772. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Neckers L and Neckers K: Heat-shock
protein 90 inhibitors as novel cancer chemotherapeutic agents.
Expert Opin Emerg Drugs. 7:277–288. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Defee MR, Qin Z, Dai L, Toole BP, Isaacs
JS and Parsons CH: Extracellular Hsp90 serves as a co-factor for
NF-κB activation and cellular pathogenesis induced by an oncogenic
herpesvirus. Am J Cancer Res. 1:687–700. 2011.PubMed/NCBI
|
|
16
|
Bandyopadhyay S, Chiang CY, Srivastava J,
et al: A human MAP kinase interactome. Nat Methods. 7:801–805.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang J, Lin Y, Guo Z, et al: The essential
role of MEKK3 in TNF-induced NFκB activation. Nat Immunol.
2:620–624. 2001.PubMed/NCBI
|
|
18
|
Wu MX, Ao Z, Prasad KV, Wu R and
Schlossman SF: IEX-1L, an apoptosis inhibitor involved in
NF-κB-mediated cell survival. Science. 281:998–1001. 1998.
|
|
19
|
Bull EE, Dote H, Brady KJ, et al: Enhanced
tumor cell radiosensitivity and abrogation of G2 and S phase arrest
by the Hsp90 inhibitor
17-(dimethylaminoethylamino)-17-demethoxygeldanamycin. Clin Cancer
Res. 10:8077–8084. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sato S, Fujita N and Tsuruo T: Modulation
of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci USA.
97:10832–10837. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schulte TW, Blagosklonny MV, Ingui C and
Neckers L: Disruption of the Raf-1-Hsp90 molecular complex results
in destabilization of Raf-1 and loss of Raf-1-Ras association. J
Biol Chem. 270:24585–24588. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ivanov VN and Hei TK: A role for
TRAIL/TRAIL-R2 in radiation-induced apoptosis and radiation-induced
bystander response of human neural stem cells. Apoptosis.
19:399–413. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carr SD, Green VL, Stafford ND and
Greenman J: Analysis of radiation-induced cell death in head and
neck squamous cell carcinoma and rat liver maintained in
microfluidic devices. Otolaryngol Head Neck Surg. 150:73–80. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ballarini F, Altieri S, Bortolussi S,
Giroletti E and Protti N: A model of radiation-induced cell
killing: insights into mechanisms and applications for hadron
therapy. Radiat Res. 180:307–315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bao XH, Takaoka M, Hao HF, et al:
Antiproliferative effect of the HSP90 inhibitor NVP-AUY922 is
determined by the expression of PTEN in esophageal cancer. Oncol
Rep. 29:45–50. 2013.PubMed/NCBI
|
|
26
|
Grem JL, Morrison G, Guo XD, et al: Phase
I and pharmacologic study of
17-(allylamino)-17-demethoxygeldanamycin in adult patients with
solid tumors. J Clin Oncol. 23:1885–1893. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo F, Rocha K, Bali P, et al: Abrogation
of heat shock protein 70 induction as a strategy to increase
antileukemia activity of heat shock protein 90 inhibitor
17-allylamino-demethoxy geldanamycin. Cancer Res. 65:10536–10544.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brough PA, Barril X, Borgognoni J, et al:
Combining hit identification strategies: fragment-based and in
silico approaches to orally active 2-aminothieno[2,3-d]pyrimidine
inhibitors of the Hsp90 molecular chaperone. J Med Chem.
52:4794–4809. 2009.PubMed/NCBI
|
|
29
|
Massey AJ, Schoepfer J, Brough PA, et al:
Preclinical antitumor activity of the orally available heat shock
protein 90 inhibitor NVP-BEP800. Mol Cancer Ther. 9:906–919. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stühmer T, Chatterjee M, Grella E, et al:
Anti-myeloma activity of the novel 2-aminothienopyrimidine Hsp90
inhibitor NVP-BEP800. Br J Haematol. 147:319–327. 2009.PubMed/NCBI
|
|
31
|
Niewidok N, Wack LJ, Schiessl S, et al:
Hsp90 inhibitors NVP-AUY922 and NVP-BEP800 may exert a significant
radiosensitization on tumor cells along with a cell type-specific
cytotoxicity. Transl Oncol. 5:356–369. 2012. View Article : Google Scholar
|
|
32
|
Stingl L, Stühmer T, Chatterjee M, Jensen
MR, Flentje M and Djuzenova CS: Novel HSP90 inhibitors, NVP-AUY922
and NVP-BEP800, radiosensitise tumour cells through cell-cycle
impairment, increased DNA damage and repair protraction. Br J
Cancer. 102:1578–1591. 2010. View Article : Google Scholar : PubMed/NCBI
|