Introduction
Human embryonic stem cells (hESCs) are derived from
inner cell masses of blastocyst-stage human embryos (1), and they have an almost unlimited
self-renewal ability, together with the potential to differentiate
into any cell type in the body. The self-renewal capacity of hESCs
is regulated by a set of transcription factors, including Oct-4,
Nanog and Sox-2 (2). The
differentiation of hESCs in vitro provides a model for
studying the cellular and molecular mechanisms of early
development, and hESCs may be utilized as tools for drug discovery
and modeling diseases (3,4). Traditionally, the maintenance and
propagation of hESCs require feeder cells, including mitotically
inactivated mouse embryonic fibroblasts (MEFs) (1) or human fibroblasts (5–7),
which secrete various factors that prevent hESCs from spontaneous
differentiation. Several studies have focused on secreted factors
released from MEF feeder layers that have the capacity to maintain
the self-renewal of hESCs, and have identified a number of factors
responsible for the maintenance of hESC pluripotency (8–10).
Basic fibroblast growth factor (bFGF) is the key growth factor in
the maintenance of undifferentiated hESC growth (11–13);
therefore, hESCs are commonly cultured in medium supplemented with
knockout serum-replacement (KSR) together with bFGF on inactivated
MEF feeder cells. In recent years, various protocols for culturing
embryonic stem cells have become available with newer trends moving
toward feeder-free or serum-free culture. However, for human and
mouse embryonic stem cells, fibroblast feeder layers are often used
at certain phases in the culturing procedure. The feeder cells,
often MEF, provide a substrate that increases plating efficiency,
helps maintain pluripotency, and facilitates the survival and
growth of stem cells (14).
As previously mentioned, KM3 cells display
fibroblast-like morphology, have characteristics such as rapid
growth and low nutritional requirements, are able to support the
growth of hESCs and are a novel type of feeder cell for the
long-term proliferation of hESCs in an undifferentiated and
pluripotent state (15). At
present, KM3 cells have been expanded for >300 passages and have
continued to maintain a fibroblast-like morphology. On this basis,
the purpose of the present study was to establish a type of feeder
cell that is cryopreservable and that may be directly used for hESC
culture, and to evaluate the effectiveness of the feeder cells as a
support for hESC subculture following recovery.
Materials and methods
Treatment with mitomycin C
KM3 cells were respectively treated with 10, 20 and
40 μg/ml concentrations of mitomycin C (Roche Diagnostics GmbH,
Mannheim, Germany) for 2 h at 37°C in 5% CO2 in air at
95% humidity. This treatment was initiated when the KM3 cells had
reached 80–90% confluence (2 days after passage). The cells were
washed with phosphate-buffered saline (PBS) five times, then
treated with 0.25% trypsin/ethylenediamine-N,N,N′,N′-tetraacetic
acid (EDTA; Invitrogen Life Technologies, Carlsbad, CA, USA) at
37°C for 3 min and collected by centrifugation (120 × g, 5 min).
The cells were then seeded in a 6-well cell cluster multidish
(Nunc, Copenhagen, Denmark) at a density of 4.0×105
cells/well. The culture medium contained 90% Dulbecco’s modified
Eagle’s medium (DMEM; Invitrogen Life Technologies) and 10% newborn
bovine serum (NBS; Sijiqing Biotechnology Co., Hangzhou, China).
The cells were cultivated for 7 days at 37°C in 5% CO2
in air at 95% humidity to identify the optimum concentration of
mitomycin C.
Cryopreservation and recovery of KM3
cells
Mitomycin C at a concentration of 10 μg/ml was
selected for the treatment of KM3 cells that had reached 80–90%
confluence (2 days after passage), by the process described above.
Freezing medium, which comprised 10% dimethyl sulfoxide [DMSO;
Aladdin Reagents (Shanghai) Co., Ltd., Shanghai, China] and 90%
fetal bovine serum (FBS; Invitrogen Life Technologies) was added
dropwise to the collected cells, which were then placed inside a
Nalgene Cryo 1°C Freezing Container (Corning Incorporated,
Tewksbury, MA, USA ). The freezing container was placed in a
freezer at −70°C or in liquid nitrogen for 15, 30 and 60 days
following gentle reduction of the temperature. At least five tubes
were subjected to each cryopreservation treatment. KM3 cells that
were treated with mitomycin C but which did not undergo
cryopreservation served as the control. Cells cryopreserved in
liquid nitrogen for 15 days following treatment with mitomycin C
were designated the liquid nitrogen 15 day group; the other groups
were named in an analogous manner. An exception is for the −70°C 60
day treatment; a group termed the −70°C 60 day complement group has
been added, and all experiment data for cryopreservation at −70°C
for 60 days were obtained from this group. The cryovials were
quickly thawed in a 37°C water bath following various
cryopreservation times. Fresh culture medium was added dropwise to
the vials to dilute the cryoprotectants. The cells were collected
by centrifugation (120 × g, 5 min) after washing in culture medium.
Culture medium was added dropwise to a total of 1 ml and Trypan
blue staining was then conducted. The cell suspension (90 μl) was
mixed with 10 μl 0.4% Trypan blue solution, and the number of blue
cells was counted within 3 min to obtain the rate of Trypan blue
exclusion. The cells were seeded in a 6-well cell culture cluster
at a density of 4.0×105 cells/well according to the
Trypan blue exclusion. The cells were cultivated for 4 days at 37°C
in 5% CO2 in air at 95% humidity prior to the morphology
of the KM3 cells being observed.
Hematoxylin and eosin (H&E) staining
and scanning electron microscopy
Cells were thawed following cryopreservation at
−70°C or in liquid nitrogen for 60 days. The cells were seeded in a
24-well cell culture cluster at a density of 0.8×105
cells/well according to the Trypan blue exclusion and cultivated
for 4 days at 37°C in 5% CO2 in air and 95% humidity.
The cells were fixed in 4% paraformaldehyde (PFA) for 20 min, and
then H&E staining using a kit (G1120; Solarbio, Beijing, China)
was conducted according to the manufacturer’s instructions. The
cell morphology was observed under a scanning electron microscope
(Hitachi Limited, Tokyo, Japan) (16).
Growth curve
Cells were seeded in a 96-well cell culture cluster
at a density of 0.2×105 cells/well according to the
Trypan blue exclusion in 200 μl medium after thawing at various
times following cryopreservation by the two methods. There were
five parallel wells per group. The plates were incubated at 37°C in
5% CO2 in air at 95% humidity for 7 days. The number of
living cells was determined by MTT assay (Amresco, Solon, OH, USA),
using a previously described method (17).
Western blot analysis
Cells cryopreserved at −70°C or in liquid nitrogen
for 15 or 60 days after treatment with mitomycin C were thawed,
then seeded in a 6-well cell culture cluster at a density of
4.0×105 cells/well according to the Trypan Blue
exclusion and cultivated for 4 days at 37°C in 5% CO2 in
air at 95% humidity. The total proteins of the cells were extracted
following a previously described method (18). The total proteins were separated by
SDS-PAGE and transferred to a polyvinylidene difluoride (PVDF)
membrane. The membranes were blotted with primary antibodies
against bFGF (BBI Antibody, Sangon Biotech, Shanghai, China) at a
concentration of 1:600, and β-actin (Proteintech Group, Inc.,
Chicago, IL, USA) at a concentration of 1:2,000, respectively,
overnight at 4°C. The membranes were then incubated with
species-specific horseradish peroxidase-conjugated secondary
antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at
a concentration of 1:3,000. Finally, the immunoblots were
visualized using ECL Western blotting detection reagents (GE
Healthcare, Buckinghamshire, UK).
hESC culture
A hESC line (SHhES2) was donated by Dr Jin Ying,
School of Medicine, Shanghai Jiao Tong University (19). Subculture of the hESCs was
conducted used a previously described procedure (15), as follows: i) KM3 cells after
thawing were seeded in a 6-well cell culture cluster at the density
of 4.0×105 cells/well according to the Trypan Blue
exclusion. There were a large number of dead cells and relatively
few adherent cells in the −70°C 60 day group. Thus, a group named
−70°C 60 days complement was created in order to observe whether
cells cryopreserved at −70°C for 60 days are able to support the
subculture of hESCs. The term complement indicates that additional
cells of the −70°C 60 day group were used to provide a confluence
similar to that of the other groups according to the degree of
adherence. ii) The following day, hESCs were implanted. iii) The
condition of the feeder cells was observed, the cell number was
counted and the differentiation of the hESCs colonies prior to
passaging was analyzed. Then, hESC colonies were seeded on fresh
feeder at the same rate. The same steps were repeated on passage.
Finally, the expansion folds of each generation were obtained. iv)
According to the growth of hESCs, they were continuously cultured
for five passages on the thawed feeder cells. v) When passaged to
the fifth passage, alkaline phosphatase (ALP) staining was applied
to the 6-well cell culture cluster. Prior to analysis, adherent
cell layers were washed twice with PBS and air dried. Staining was
performed using cytochemistry staining kits (Shanghai Sun Biotech
Co., Ltd., Shanghai, China) according to the manufacturer’s
recommendations, with the exception of staining with hematoxylin.
Differentiation of hESCs was defined as a proportion of
differentiated cells in the hESC clones of >30%.
Statistical analysis
All experimental points were performed in triplicate
or quadruplicate, and all assays were repeated a minimum of three
times. Normally distributed variables were expressed as means ±
standard deviation (SD). For multiple group comparisons, analysis
of variance (ANOVA) with Dunnett’s post test was used. All
statistical analyses were performed using the SPSS statistical
software package, version 17.0 (SPSS, Inc., Chicago, IL, USA).
P<0. 05 was considered to indicate a statistically significant
difference.
Results
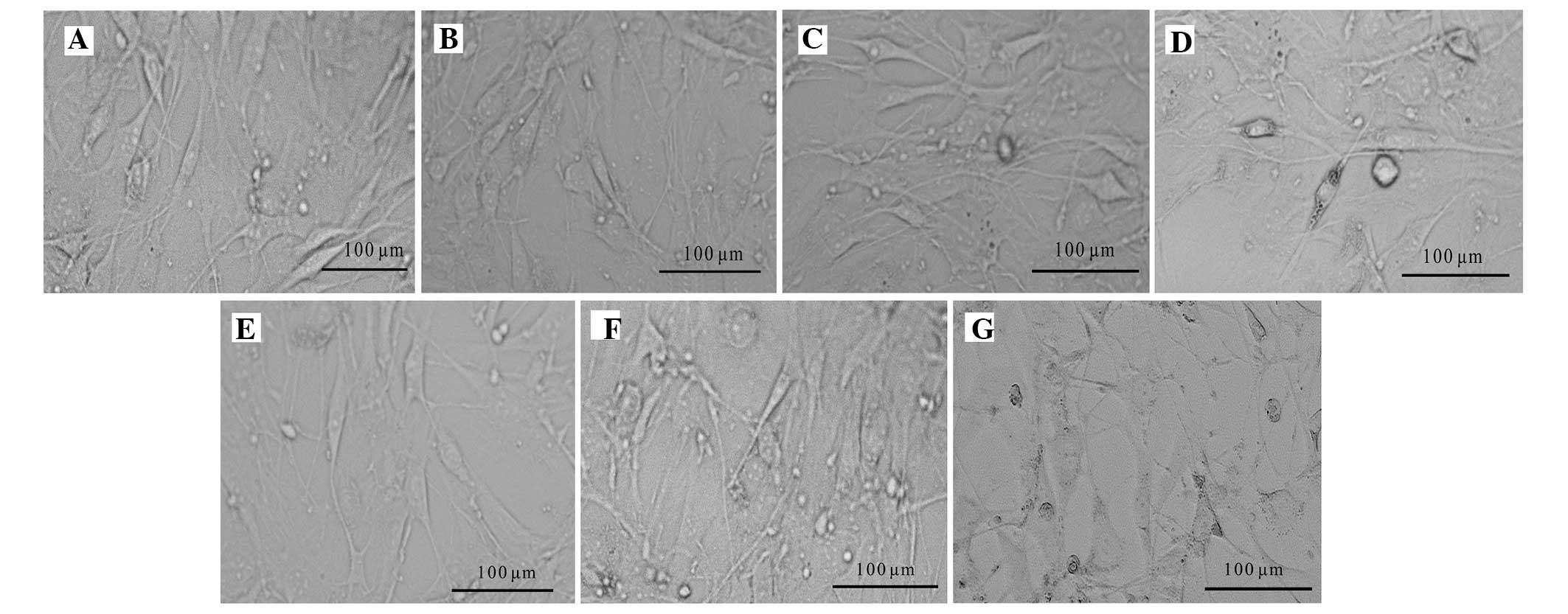
Optimum concentration of mitomycin C
KM3 cells that were not treated with mitomycin C
were highly proliferative and had reached 100% confluence on the
third day after treatment (Fig.
1A–C). When the cells were treated with mitomycin C at a
concentration of 10 μg/ml for 2 h, mitosis was inhibited without
cell death. The feeder cells could be maintained for 7 days
(Fig. 1D–G). Treatment of the
cells with 20 and 40 μg/ml mitomycin C for 2 h caused a large
number of cells to die after 24 h (Fig. 1H–O).
Survival rate of KM3 cells
There were six groups in this assay and each group
had five parallel wells. A summary of the cell survival rates is
presented in Table I. Whether in
liquid nitrogen for two months or −70°C for one month, the survival
rates of KM3 cells were >80%, while the rate was only 66.40%
when cryopreserved in −70°C for 60 days.
 | Table ISurvival rate of KM3 cells
cryopreserved by different methods for various times after
treatment with mitomycin C. |
Table I
Survival rate of KM3 cells
cryopreserved by different methods for various times after
treatment with mitomycin C.
| Time | Liquid nitrogen
(%) | −70°C (%) |
|---|
| 15 days | 92.60±0.89 | 91.00±2.00 |
| 30 days | 89.20±2.39 | 87.80±1.64 |
| 60 days | 84.60±1.14b | 66.40±2.88a,b |
Morphology of KM3 cells
The KM3 cells that had not been treated with
mitomycin C were fusiform and had few cytoplasmic granules. The
cell nucleus was generally oval and the karyotheca was clearly
visible. It was easy to observe the nucleoli (typically 3–5). A
large number of microvilli were visible under the scanning electron
microscope (Fig. 2A and a). The
cells in the liquid nitrogen 60 day group grew well, and were
essentially the same as those in the control group with respect to
morphology and growth characteristics. However, the number of
microvilli was reduced and their length was shorter. After
recovery, the cells adhered more slowly compared with those in the
control group (Fig. 2B, b, C and
c). A greater number of dead cells were observed in the −70°C
60 day group. The outwardly extending adherent cells were in a poor
condition, with no typical morphology and almost no microvilli. The
cells were readily detached in the rinsing process (Fig. 2D and d).
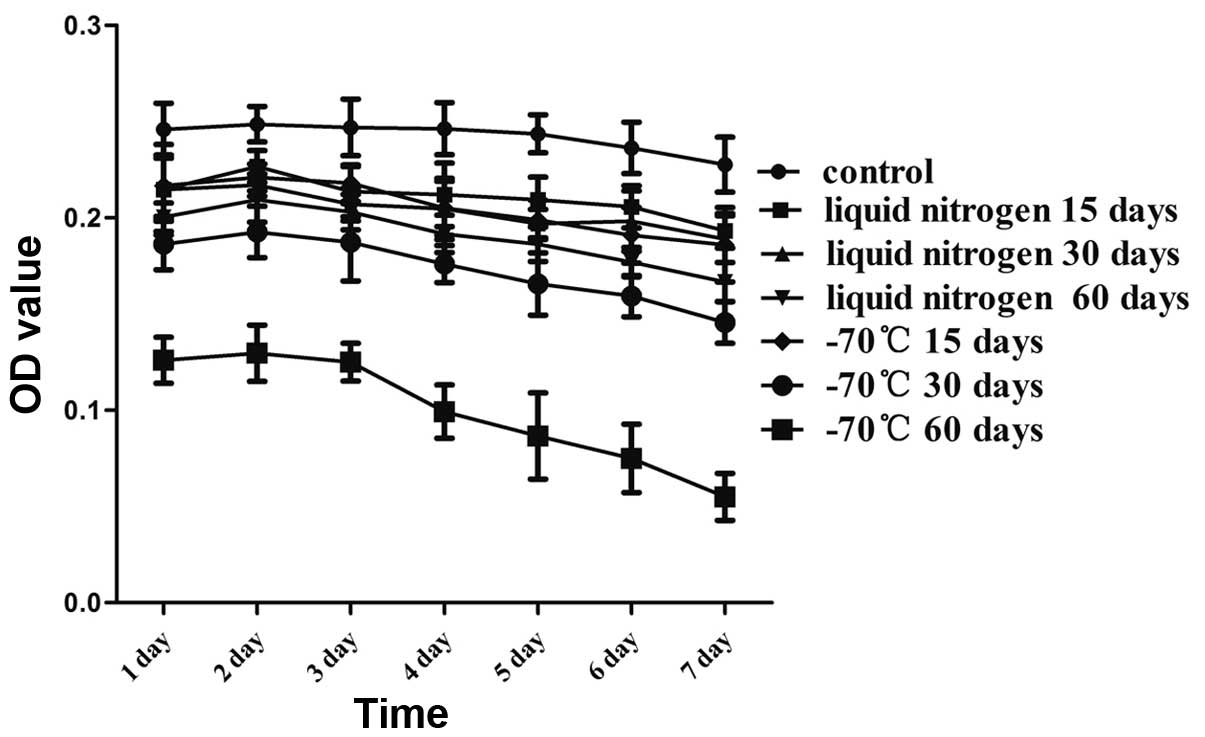
Growth curves
In order to investigate the biological activity of
the KM3 cells, the cells were incubated for 7 days following rapid
thawing. As shown in Fig. 3, the
number of adherent cells was significantly reduced in the −70°C 60
day group from the first day compared with that in the control
group. In addition, the number of adherent cells in the −70°C 60
day group clearly continued to decline after 3 days. There were
greater numbers of attached cells in the other thawed groups;
however, these numbers were reduced compared with those in the
control group. The cryopreserved KM3 cells remained stable for at
least 4 days and the cell growth curves decreased slowly.
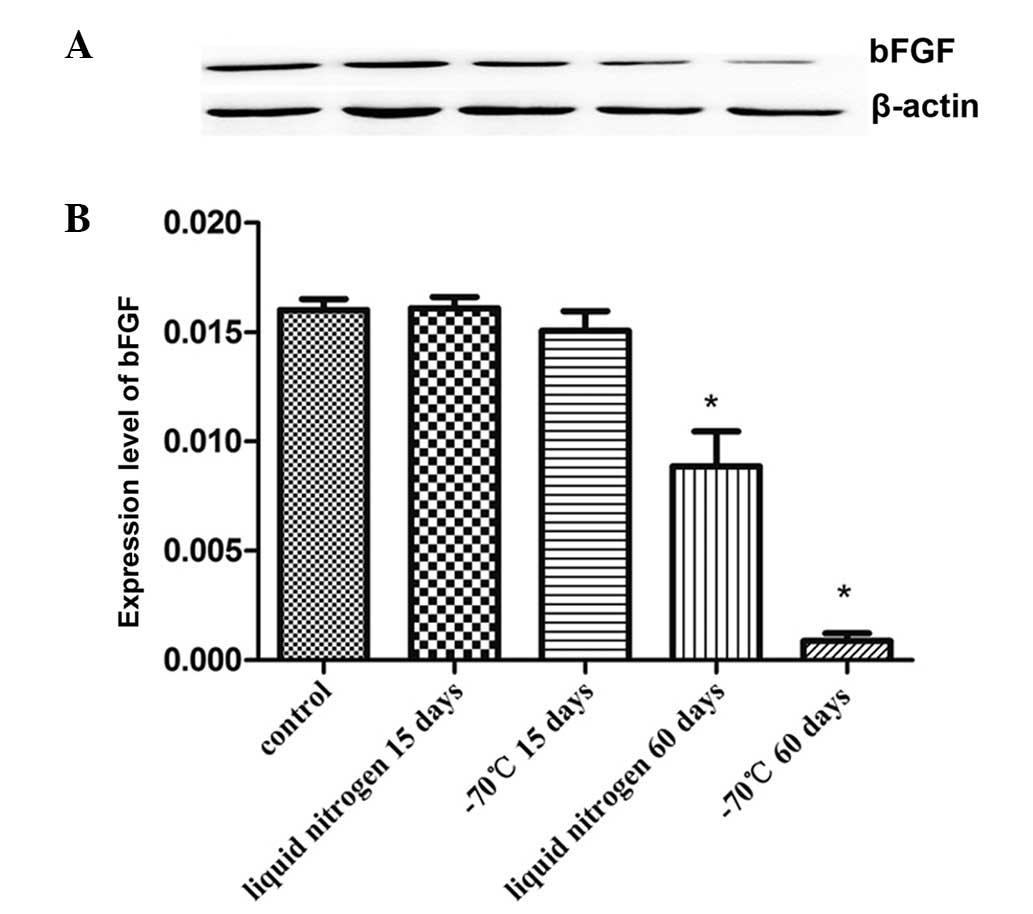
bFGF expression levels of KM3 cells
The proteins of KM3 cells were tested to evaluate
the expression levels bFGF. As shown in Fig. 4, there was no significant
difference in the expression level of bFGF between the short-term
cryopreservation groups (15 days) and the control group. However,
the bFGF expression level fell following 60 days of
cryopreservation, and the reduction in the −70°C 60 day group was
particularly evident.
Characteristics of hESCs cultured on
thawed KM3 cells
hESCs were cultured on mitotically inactive KM3
cells. These hESCs were continuously cultured and split once every
4 days. The hESCs was then transferred from KM3 cells to recovered
KM3 cells. Certain colonies continued to grow in KM3 as a control.
It was observed that hESC colonies grown on liquid nitrogen 60 day
feeder layers retained the typical undifferentiated morphology
(round with defined colony edges) and exhibited no significant
difference from the control group, as shown in Fig. 5A, a, D and d). The number of hESCs
colonies seeded on the −70°C 60 days feeder layers was distinctly
reduced, possibly due to the presence of dead cells resulting in a
low density. Whether cell numbers were supplemented (in the
complement group) or not, the majority of the hESC colonies were
clearly differentiated and loosely arranged, with no clear
boundary, thin clumps and morphological heterogeneity (Fig. 5G, g, H and h). A statistical
analysis of the differentiation rates of hESC colonies cultivated
on thawed KM3 cells was conducted and is shown in Table II. With the prolongation of frozen
time, the differentiation rate of hESCs increased, particularly
obvious with the rate reaching 37.67% of the −70°C 60-day group.
The proliferation times of hESCs cultured on different feeders is
shown in Fig. 6. The passaging
ability of the −70°C 60 days complement group was less effective
than that of the other cryopreservation groups.
 | Table IIDifferentiation rate of hESCs cultured
on the KM3 feeder cells cryopreserved by two different methods for
various times following treatment with mitomycin C. |
Table II
Differentiation rate of hESCs cultured
on the KM3 feeder cells cryopreserved by two different methods for
various times following treatment with mitomycin C.
| Time | Liquid nitrogen
(%) | −70°C (%) |
|---|
| 0 days | 5.33±2.08 | 5.33±2.08 |
| 15 days | 7.67±1.53 | 8.67±3.06 |
| 30 days | 10.33±2.51 | 12.33±4.04 |
| 60 days | 16.33±2.08b | 37.67±3.51a,b |
Feeding of hESCs
The KM3 feeder cells supported the growth of hESCs
when co-cultured with the hESCs in hESC medium for 4 days. Fusiform
cells became elongated. However, in the −70°C 60 day complement
group, the cell bodies were dark with a poor three-dimensional
shape and there was an increased number of dead cells. The feeder
cells of the liquid nitrogen 60 day group retained a better status
and exhibited no clear morphological changes, as shown in Fig. 7.
Discussion
The use of a feeder layer is one of the most
commonly used methods for the culture of hESCs. Studies have shown
that the proliferation of cells of the feeder layer may be reduced
when they are treated with mitomycin C or exposed to radiation, but
the cells remain able to survive, and are able to secrete certain
cytokines required by the hESCs, such as fibroblast growth factor,
insulin-like growth factor and leukemia inhibitory factor. Thus,
they are able to support the subculture of hESCs (8–10).
KM3 is an immortalized cell line. Early experimental
results showed that KM3 cells are able to function as feeder layers
for the expansion of hESCs in vitro; clones of hESCs remain
in the typical undifferentiated state, with maintenance of their
pluripotency (15). Treatment with
a mitomycin C at a concentration of 10 μg/ml for 2 h significantly
inhibits the proliferation of KM3 cells, without causing cell
death, and the KM3 cells are able to survive for 1–2 weeks.
However, mitomycin C causes KM3 cells to die when its concentration
is too high.
Cell cryopreservation is one of the main methods of
cell preservation, with the −70°C freezing method and liquid
nitrogen cryopreservation method being commonly used. The −70°C
freezing method is simple to conduct, and can be used to freeze
cells in batches; however, the activity of cells is likely to be
decreased following long-term cryopreservation. Due to its lower
temperature, the liquid nitrogen cryopreservation method may
temporarily cause the cells to enter a non-growing state in order
to preserve their cell characteristics. It also can be used to
freeze cells for the long-term; however, meeting the experimental
requirements is challenging due to a more complex method of
operation, and the quantities of cells that may be frozen by this
method are limited (20–22). hESCs are known to require precise
conditions for culture and are routinely cultured in the presence
of feeder cells, which provide a complex conditioning environment
(23).
The preliminary results of the present study showed
that a KM3 feeder layer can effectively maintain the long-term
subculture of hESCs and maintain the totipotency of hESCs. On this
basis, in the present study, a batch of KM3 cells treated with 10
μg/ml mitomycin C was cryopreserved by −70°C freezing and with
liquid nitrogen for different time periods to observe the
biological activity and ability to support a hESC subculture after
rapid thawing. Different methods of freezing and various freezing
times were selected for the cryopreservation of the treated KM3
cells. The recovery rate of the KM3 cells treated with mitomycin C
was not statistically significantly different between the −70°C
group and liquid nitrogen group within one month, and cells frozen
by both methods were able to support the growth of hESCs. However,
with the extension of time, the recovery rate of the −70°C 60 day
group was only 66.40±2.88% after cryopreservation for two months,
and the state of the cells was poor. In the −70°C 60 day group,
H&E staining and scanning electron microscopy showed that the
morphology of the cells was irregular, the boundaries of the nuclei
were unclear and almost no clear nucleoli and microvilli structures
were observed. Microvilli are associated with the ability to adhere
and exchange substances (24,25);
therefore, in the process of washing, the cells are readily
detached due to the reduced number of microvilli. The growth state
was unstable; on the first day after recovery, a large number of
dead cells appeared. Although certain cells did not undergo Trypan
blue staining, they were not able to adhere or adhered
ineffectively. Thus, the number of adherent cells was significantly
reduced when compared with the other groups when the same number of
cells were seeded. Taking into account that the density of the
feeder cells may influence the maintenance of hESCs (6,26–28),
cell numbers were increased in the −70°C 60 day group in order to
provide a number of adherent cells that was consistent with those
in the other groups. Even though the cell number was supplemented,
it was observed that the proliferation rate of the hESCs was slower
in the −70°C 60 day group than that of the other groups from the
beginning of the third generation; hESC colonies on the feeder were
evidently differentiated (loosely arranged with no clear boundary,
thin clumps and morphological heterogeneity). This indicates that
KM3 cells treated with mitomycin C should not be stored for a long
time in a freezer at −70°C. If mitomycin C-treated KM3 cells are
preserved in the long-term for use as a feeder layer, the number of
implanted cells should be increased. Although feeder cells
preserved by this method may normally maintain the hESC subculture
to some extent, the effect of long-term freezing is poor compared
with that of short-term cryopreservation in a −70°C freezer and
liquid nitrogen cryopreservation.
Notably, hESC colonies may partly or completely
differentiate due to changes in certain factors in the process of
passaging, but can be restored to the normal state by passaging
following removal of the factors. This may be explained by the
presence of numerous undifferentiated cells in the differentiated
hESC colonies, or the differentiated colonies having
retro-differentiation ability (29,30).
In the current study, it was confirmed that bFGF plays an important
role in maintaining the self-renewal and pluripotency of hESCs.
When the −70°C 60 day group was compared with the other groups, the
bFGF secretion level was markedly lower in the −70°C 60 day group,
which may be a reason for the hESC differentiation that was
observed. Further study of the cells from the −70°C 60 day group
should be conducted to investigate whether hESC differentiation is
inhibited by increasing the level of bFGF in the hESC culture
medium. For the liquid nitrogen 60 day group, the state of the
cells did not significantly change following cryopreservation and
the cells were able to support the hESC subculture. It was observed
that hESCs grown on this feeder formed typical nest-like structures
with a close arrangement and clear edge boundaries. Importantly,
the cells from this group were not observed to be significantly
different compared with those of the control group. The feeder
cells that were co-cultured with hESCs remained fusiform at the
fourth day, which highlighted their biological activity.
The provision of a steady supply of qualified,
homogeneous and ready-to-use feeder cells is one of the key factors
for hESC research and the progression of hESC subculture studies.
Cryopreserved KM3 cells that have been treated with 10 μg/ml
mitomycin C may be directly used as feeder layer for hESCs after
recovery, and the effect is relatively good with −70°C short-term
or liquid nitrogen cryopreservation. This study provides new
alternative materials and methods for the continuous stability of
hESC subculture techniques.
Acknowledgements
This study was funded by the Startup Foundation for
Advanced Talents, Jiangsu University (no. 09JDG037) and the
Student’s Scientific Research Foundation of Jiangsu University (no.
12A118, no. 12A142).
References
|
1
|
Thomson JA, Itskovitz-Eldor J, Shapiro SS,
et al: Embryonic stem cell lines derived from human blastocysts.
Science. 282:1145–1147. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Niwa H: How is pluripotency determined and
maintained? Development. 134:635–646. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Darabi R and Perlingeiro RC:
Lineage-specific reprogramming as a strategy for cell therapy. Cell
Cycle. 7:1732–1737. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reubinoff BE, Pera MF, Fong CY, et al:
Embryonic stem cell lines from human blastocysts: somatic
differentiation in vitro. Nat Biotechnol. 18:399–404. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hovatta O, Mikkola M, Gertow K, et al: A
culture system using human foreskin fibroblasts as feeder cells
allows production of human embryonic stem cells. Hum Reprod.
18:1404–1409. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng L, Hammond H, Ye Z, et al: Human
adult marrow cells support prolonged expansion of human embryonic
stem cells in culture. Stem Cells. 21:131–142. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Inzunza J, Gertow K, Strömberg MA, et al:
Derivation of human embryonic stem cell lines in serum replacement
medium using postnatal human fibroblasts as feeder cells. Stem
Cells. 23:544–549. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lim JW and Bodnar A: Proteome analysis of
conditioned medium from mouse embryonic fibroblast feeder layers
which support the growth of human embryonic stem cells. Proteomics.
2:1187–1203. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cai J, Chen J, Liu Y, et al: Assessing
self-renewal and differentiation in human embryonic stem cell
lines. Stem Cells. 24:516–530. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chin AC, Fong WJ, Goh LT, et al:
Identification of proteins from feeder conditioned medium that
support human embryonic stem cells. J Biotechnol. 130:320–328.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Levenstein ME, Ludwig TE, Xu RH, et al:
Basic fibroblast growth factor support of human embryonic stem cell
self-renewal. Stem Cells. 24:568–574. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang G, Zhang H, Zhao Y, et al: Noggin and
bFGF cooperate to maintain the pluripotency of human embryonic stem
cells in the absence of feeder layers. Biochem Biophys Res Commun.
330:934–942. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park Y, Kim JH, Lee SJ, et al: Human
feeder cells can support the undifferentiated growth of human and
mouse embryonic stem cells using their own basic fibroblast growth
factors. Stem Cells Dev. 20:1901–1910. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin S and Talbot P: Methods for culturing
mouse and human embryonic stem cells. Methods Mol Biol. 690:31–56.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu J, Hu S, Ma Q, et al: Immortalized
mouse fetal liver stromal cells support growth and maintenance of
human embryonic stem cells. Oncol Rep. 28:1385–1391.
2012.PubMed/NCBI
|
|
16
|
Talbot MJ and White RG: Cell surface and
cell outline imaging in plant tissues using the backscattered
electron detector in a variable pressure scanning electron
microscope. Plant Methods. 9:402013. View Article : Google Scholar
|
|
17
|
Lee DY, Lee MK, Kim GS, et al: Brazilin
inhibits growth and induces apoptosis in human glioblastoma cells.
Molecules. 18:2449–2457. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang N, Ren GD, Zhou Z, et al: Cooperation
of myocardin and Smad2 in inducing differentiation of mesenchymal
stem cells into smooth muscle cells. IUBMB Life. 64:331–339. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li C, Yang Y, Lu X, et al: Efficient
derivation of Chinese human embryonic stem cell lines from frozen
embryos. In Vitro Cell Dev Biol Anim. 46:186–191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brockbank KG, Carpenter JF and Dawson PE:
Effects of storage temperature on viable bioprosthetic heart
valves. Cryobiology. 29:537–542. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Galmes A, Besalduch J, Bargay J, et al:
Long-term storage at −80 degrees C of hematopoietic progenitor
cells with 5-percent dimethyl sulfoxide as the sole cryoprotectant.
Transfusion. 39:70–73. 1999.
|
|
22
|
Massie I, Selden C, Hodgson H and Fuller
B: Storage temperatures for cold-chain delivery in cell therapy: a
study of alginate-encapsulated liver cell spheroids stored at −80°C
or −170°C for up to 1 year. Tissue Eng Part C Methods. 19:189–195.
2013.PubMed/NCBI
|
|
23
|
Lu J, Hou R, Booth CJ, et al: Defined
culture conditions of human embryonic stem cells. Proc Natl Acad
Sci USA. 103:5688–5693. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Murai T, Sato M, Nishiyama H, et al:
Ultrastructural analysis of nanogold-labeled cell surface
microvilli in liquid by atmospheric scanning electron microscopy
and their relevance in cell adhesion. Int J Mol Sci.
14:20809–20819. 2013. View Article : Google Scholar
|
|
25
|
Ubelmann F, Chamaillard M, El-Marjou F, et
al: Enterocyte loss of polarity and gut wound healing rely upon the
F-actin-severing function of villin. Proc Natl Acad Sci USA.
110:E1380–E1389. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ozolek JA, Jane EP, Esplen JE, et al: In
vitro neural differentiation of human embryonic stem cells using a
low-density mouse embryonic fibroblast feeder protocol. Methods Mol
Biol. 584:71–95. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Heng BC, Liu H and Cao T: Feeder cell
density - a key parameter in human embryonic stem cell culture. In
Vitro Cell Dev Biol Anim. 40:255–257. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park SP, Lee YJ, Lee KS, et al:
Establishment of human embryonic stem cell lines from frozen-thawed
blastocysts using STO cell feeder layers. Hum Reprod. 19:676–684.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hou P, Li Y, Zhang X, et al: Pluripotent
stem cells induced from mouse somatic cells by small-molecule
compounds. Science. 341:651–654. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maherali N and Hochedlinger K: Guidelines
and techniques for the generation of induced pluripotent stem
cells. Cell Stem Cell. 3:595–605. 2008. View Article : Google Scholar : PubMed/NCBI
|