Introduction
Traumatic brain injury (TBI) is the main cause of
mortality and disability in young individuals, and can directly
cause pathophysiological changes in the blood-brain barrier (BBB).
The BBB is primarily comprised of brain microvascular endothelial
cells, the basement membrane and glial cells surrounding the
capillaries. The endothelial cells come into contact with each
other at what are known as tight junctions (TJs). TJs consist of
the transmembrane proteins, occludins and claudins, that interact
on adjacent endothelial cells to form a physical barrier against
paracellular diffusion (1–3), and the accessory proteins, zonula
occludens (ZO) family (ZO-1 and ZO-2), that anchor the
transmembrane proteins to the cytoskeleton (4–6).
Netrin-1, one of three members in the mammalian
netrin family, stimulates angiogenesis and augments the response to
vascular endothelial growth factor (7). In addition, netrin-1 has been found
to be superior to vascular endothelial growth factor in restoring
nerve conduction velocity, possibly due to the potent effects on
vascular and neural biology (8).
Notably, netrin-1 may also play a role in the restoration of the
BBB. The angiogenic effect of netrin-1 offers unique therapeutic
potentials in restoring the BBB under pathological conditions,
including TBI.
Although the disruption of the BBB has been
previously investigated in several TBI models (9,10),
there is limited information with regard to the association between
netrin-1 and TJs. Therefore, the aim of the present study was to
analyze the correlation between netrin-1 and TJs in a TBI
model.
Materials and methods
Animal models
In total, 20 male Sprague-Dawley rats (weight,
250–280 g) were used in the study (10 rats in the TBI group and 10
rats in the sham-operated group). All animal procedures were
approved by the Nanchang University Medical School Animal Care and
Use Committee (Nanchang, China). The experimental TBI model was
established as previously described by Feeney et al
(11). Briefly, the animals were
anesthetized via intramuscular injection of xylazine/ketamine HCl
(10/90 mg/kg). The head was then fixed in a stereotactic frame
along the midline incision scalp, and periosteal stripping was
performed to expose the left parietal region. A bone window
measuring 5 mm in diameter was established at 1.5 cm posterior to
the bregma and 2.5 mm beside the midline. A 40-g metal sterile rod
fell freely from a height of 30 cm to hit the duramater and create
a contusion in the left parietal lobe. The bone window was then
closed with bone wax and the scalp incision sutured, following
which the animals were removed from the stereotactic frame. The
body temperature was maintained at 37±0.5°C using a heating pad
during the surgical procedure. The animals were sacrificed at 72 h
following TBI and the ipsilateral cortices were removed intact.
Tissues were dissected immediately and stored in liquid nitrogen
until required for further analysis.
Measurement of Evans blue (EB) dye
extravasation
BBB permeability was quantitatively evaluated using
the extravasation of EB dye as a marker of albumin extravasation
(12). Briefly, 2% EB dye was
slowly injected intravenously 2 h prior to sacrifice. At 24, 48, 72
and 96 h following TBI, the rats were deeply anesthetized with 10%
chloral hydrate and perfused with heparinized saline via the
cardiac ventricle until colorless perfusion fluid was obtained from
the atrium. The ipsilateral cortex was quickly removed and placed
on ice, and a coronal section of the injured hemisphere through the
impact site was dissected using a double-blade scalpel. Brain
samples were weighed and then immersed in 5 l/kg formamide at 50°C
for 72 h. The supernatant was collected and the fluorescence was
measured using a multiplate reader (Synergy; BioTek, Inc.,
Winooski, VT, USA). The fluorescent intensity was normalized
against wet tissue weight, and the EB dye tissue content was
quantified based on a linear standard line.
Immunofluorescence
Ipsilateral cortices were removed and post-fixed
overnight in 4% paraformaldehyde at 4°C. Immunofluorescence signals
of occludin, claudin-5 and ZO-1 were then determined in
perfused-fixed paraffin-embedded sections. The paraffin-embedded
sections were deparaffinized and placed through a series of
alcohols with decreasing concentrations. Slides were blocked for 1
h at room temperature and incubated in anti-claudin-5,
anti-occludin and anti-ZO-1 antibodies (1:200; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight. The slides
were then rinsed three times for 5 min in phosphate-buffered saline
containing 0.1% Tween-20, and incubated with secondary anti-rabbit
immunoglobulin G, conjugated with Alexa Fluor 488 (Invitrogen Life
Technologies, Carlsbad, CA, USA), for 30 min. For image analysis,
the slides were mounted following subsequent washing procedures and
examined under an Olympus BX-51 epifluorescence microscope
(magnification, ×100; Olympus, Tokyo, Japan).
Western blot analysis
Cell suspensions were prepared from dissected
ipsilateral cortices and transferred to a fresh tube. The
suspensions were homogenized using a handheld mortar and pestle and
then agitated for 10 min. Extracts were clarified by centrifugation
and then diluted in a reducing agent. Proteins were resolved on a
12% Bis-Tris polyacrylamide gel and electrotransferred onto a
nitrocellulose membrane. The membrane was incubated with
anti-claudin-5, anti-occludin or anti-ZO-1 antibodies (1:200; Santa
Cruz Biotechnology, Inc.), followed by incubation with
goat-anti-rabbit horseradish peroxidase-conjugated secondary
antibodies (1:1,000; Santa Cruz Biotechnology, Inc.) in blocking
buffer. The membrane was then developed using enhanced
chemiluminescence detection and film exposure (Amersham, Little
Chalfont, UK). GAPDH was used as the internal control.
Quantitative polymerase chain reaction
(qPCR)
Total RNA was isolated from the tissues of
ipsilateral cortices using TRIzol reagent (Gibco-BRL, Gaithersburg,
MD, USA), and treated with 200 units Moloney Murine Leukemia Virus
reverse transcriptase (Promega Corporation, Madison, WI, USA) for
first-strand cDNA synthesis. A SYBR Green Detection kit (Ameritech
Biomedicines, Houston, TX, USA) and Applied Biosystems Prism 7500
detection system (Applied Biosystems, Foster City, CA, USA) were
used to amplify the transcribed cDNA for 40 cycles. The real time
thermal cycler program consisted of three stages: Stage one, 95°C
for 5 min; stage two, 94°C for 20 sec, followed by 57°C for 20 sec
and 72°C for 20 sec (repeated 40 times); and stage three, 72°C for
5 min, followed by 55°C for 10 sec and 95°C for 15 sec. The copy
numbers of netrin-1, claudin-5, occludin and ZO-1 mRNA were
normalized against the internal control, β-actin. The sequences of
the PCR primers used were as follows: Netrin-1 forward,
5′-CTACTGCAAGGAGGGCTTCTA-3′ and reverse,
5′-GCGCTACAGGAATCTTAATG-3′; occludin forward,
5′-ACAAAGAGCTCTCTCGTCTCG-3′ and reverse,
5′-CATAGTCTCCCACCATCCTC-3′; claudin-5 forward,
5′-CACAGAGAGGGGTCGTTGAT-3′ and reverse, 5′-CTGCCCTTTCAGGTTAGCAG-3′;
ZO-1 forward, 5′-AGTTCTGCCCTCAGCTACCA-3′ and reverse,
5′-GCTTAAAGCTGGCAGTGTC-3′; and β-actin forward,
5-CCTAGACTTCGAGCAAGAGA-3′ and reverse
5′-AGAGGTCTTTACGGATGTCA-3′.
Microvessel isolation
Ipsilateral cortices from rats in the TBI group were
removed and homogenized, prior to being transferred to a 40-ml
syringe with a 300-μm nylon mesh. The homogenates were filtered
through the mesh, which was repeated with a 115-μm nylon mesh. The
filtrate was transferred to a graduated cylinder with an equal
volume of 40% dextran, and centrifuged for 15 min at 5,000 × g. The
supernatant was carefully aspirated, following which the pellet was
resuspended and filtered through a 20-μm nylon mesh. The filter was
inverted and rinsed in a Petri dish to remove the microvessels.
Finally, the brain microvascular endothelial cells were cultured in
rat brain endothelial cell growth medium (Cell Applications, Inc.,
San Diego, CA, USA).
Construction of the netrin-1 gene
delivery system
A pZsGreen1-N1-netrin-1 vector (with netrin-1 gene
insert) was prepared as previously described (13). A pZsGreen1-N1 vector without the
netrin-1 insertion was used as a control vector. Microvascular
endothelial cells from the ipsilateral cortex were transfected with
the recombinant pZsGreen1-N1-netrin-1 plasmid or the empty vector
using Lipofectamine 2000 (Invitrogen Life Technologies, Grand
Island, NY, USA).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. The results were analyzed with GraphPad Prism version
4.00 for Windows software (GraphPad Software, Inc., San Diego, CA,
USA), using the Student’s t-test and one-way analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference. Post-hoc pairwise comparisons were performed using
Tukey’s method.
Results
TBI markedly increases extravasation of
EB dye
EB dye does not permeate an intact BBB; however,
following TBI, EB dye easily permeates a compromised BBB. The
severity of BBB disruption at the site of TBI, expressed as EB dye
extravasation per gram of hemispheric tissue, is shown in Fig. 1. EB dye extravasation was
significantly increased following TBI when compared with the
sham-operated group. A peak increase in permeability was observed
at 72 h following TBI.
TBI reduces the expression of TJ
proteins
To investigate the effect of TBI on TJ proteins,
immunofluorescence and western blot analysis were performed to
detect the protein expression levels. Immunofluorescence analysis
of TJ-associated proteins demonstrated changes in the localization
and expression levels of claudin-5, occludin and ZO-1 following TBI
in the rats. As shown in Fig. 2A,
marked staining for claudin-5, occludin and ZO-1 (as shown by the
black arrow) was observed at the cell-cell junctions in the
sham-operated group, while fluorescent staining of the
interendothelial TJ-associated proteins was reduced in the TBI
group at 72 h. As shown in Fig. 2B and
C, the results from the western blot analysis were consistent
with the observations obtained from the immunofluorescence
analysis. The expression levels of claudin-5, occludin and ZO-1 in
the TBI group were significantly lower compared with those in
sham-operated group. These results demonstrated that TBI reduced
the expression of TJ-associated proteins.
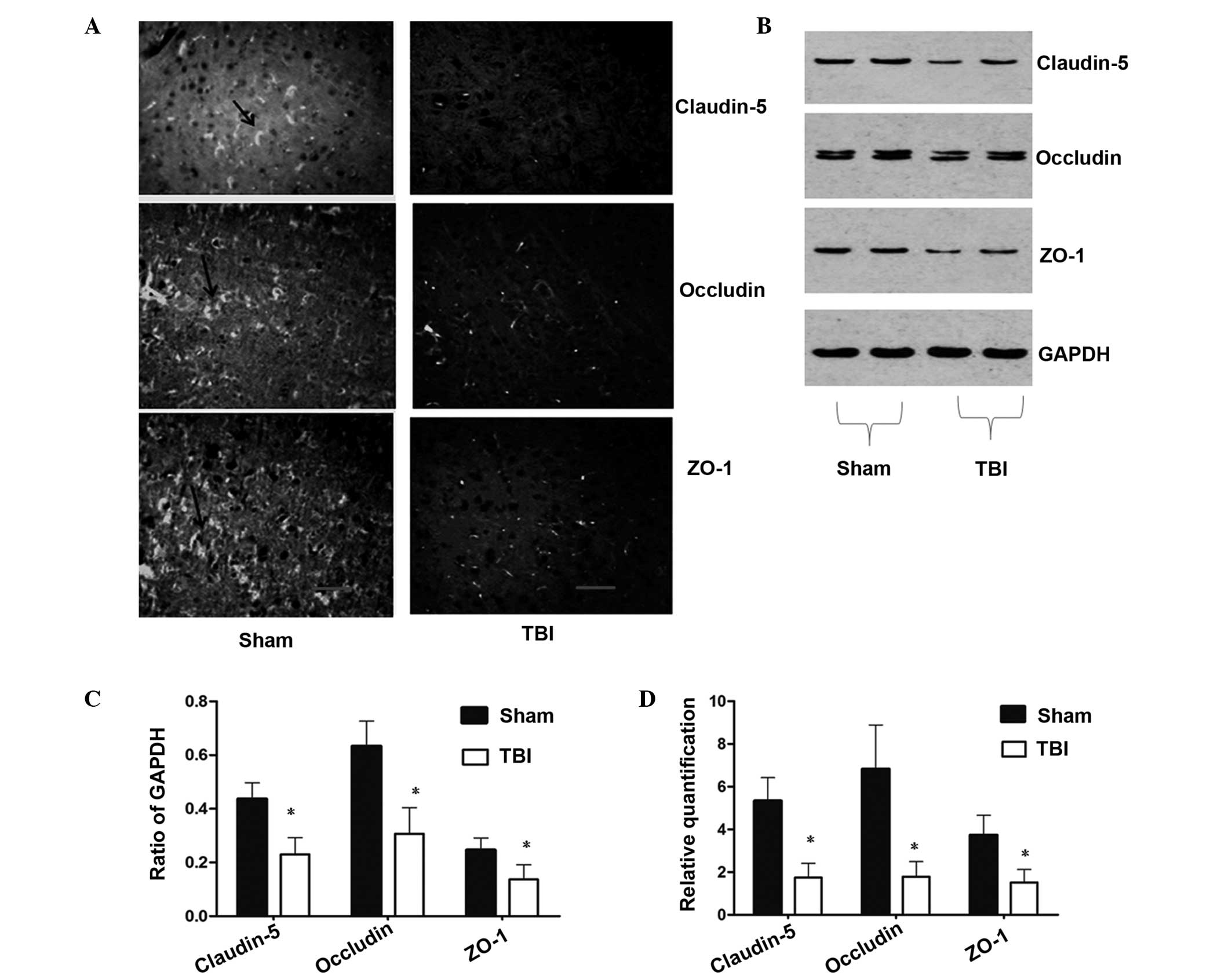 | Figure 2Changes in the expression levels of
claudin-5, occludin and ZO-1 at 72 h following TBI. (A)
Distribution and expression of claudin-5, occludin and ZO-1 in the
ipsilateral cortex, as shown by immunofluorescence (magnification,
×100; scale bar, 50 μm). (B) Western blot analysis revealed reduced
expression levels of claudin-5, occludin and ZO-1 in the
ipsilateral cortex following TBI. (C) Densitometric analysis of the
results from the western blot analysis, where the data were
normalized against GAPDH expression. (D) qPCR analysis demonstrated
that the expression levels of claudin-5, occludin and ZO-1 in the
ipsilateral cortex were significantly reduced at 72 h following TBI
when compared with the sham-operated rats. Data are expressed as
the mean ± the standard error of the mean. *P<0.05,
vs. sham-operated groups. ZO, zonula occluden; TBI, traumatic brain
injury; qPCR, quantitative polymerase chain reaction. |
The qPCR results demonstrated that the mRNA
expression levels of claudin-5, occludin and ZO-1 were
significantly reduced in the TBI group when compared with the
sham-operated group (P<0.05). Compared with sham-operated group,
the relative gene expression levels of claudin-5, occludin and ZO-1
were decreased by 67.17, 73.76 and 59.25%, respectively (Fig. 2D), in the TBI group.
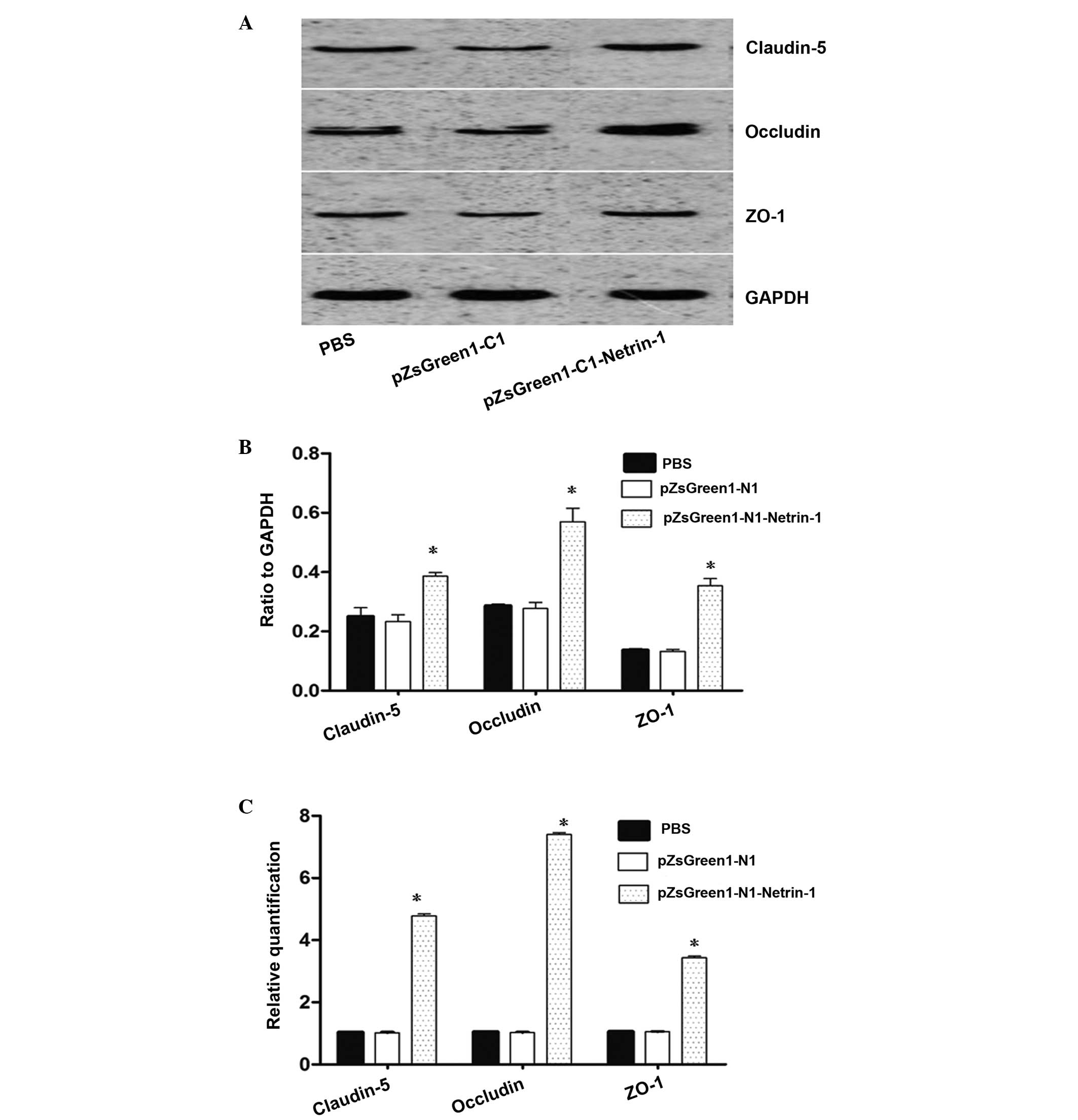
pZsGreen1-N1-netrin-1 gene transfer
increases the expression of claudin-5, occludin and ZO-1 following
TBI
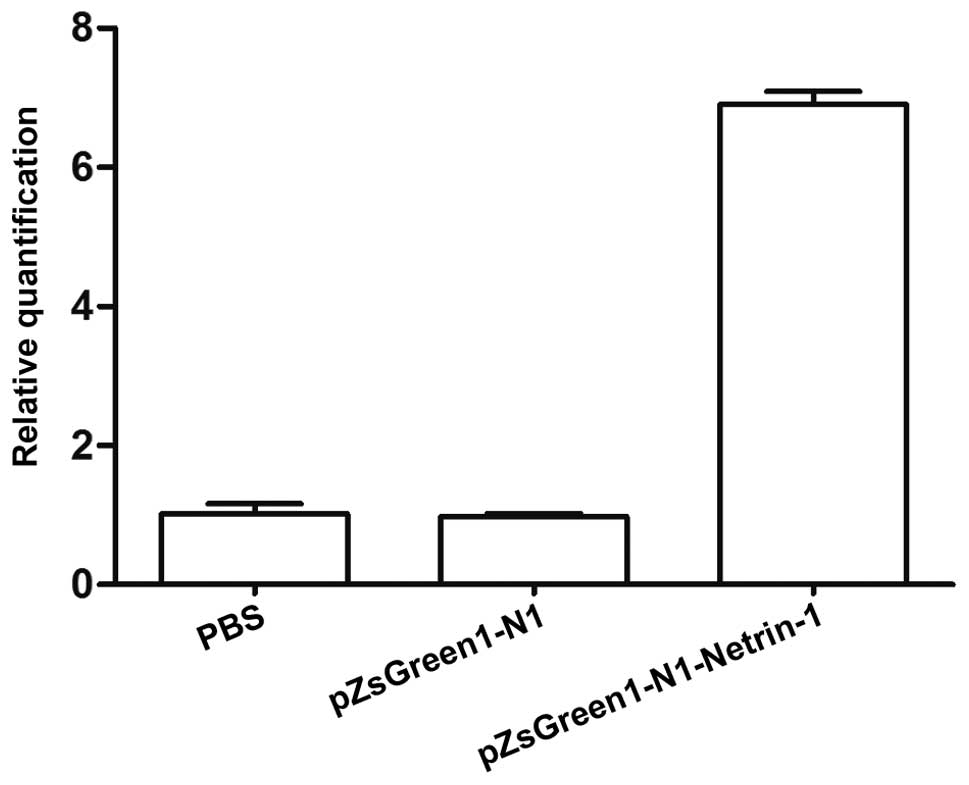
As shown in Fig. 3,
the mRNA expression levels of netrin-1 in the endothelial cells
transfected with pZsGreen1-N1-netrin-1 were significantly higher
compared with the control group. To assess the association between
netrin-1 and claudin-5, occludin and ZO-1 expression, the
expression levels of TJ proteins in endothelial cells from TBI rats
transfected or non-transfected with pZsGreen1-N1-netrin-1 were
analyzed. The results from Fig. 4A and
B indicate that the protein expression levels of claudin-5,
occludin and ZO-1 in the brain microvascular endothelial cells
increased following pZsGreen1-N1-netrin-1 transfection. Compared
with the non-transfected and control-vector-treated endothelial
cells, there was a >3–6 fold increase in the mRNA expression
levels of claudin-5, occludin and ZO-1 in the pZsGreen1-N1-netrin-1
treated cells (Fig. 4C).
Discussion
The ability to maintain the BBB integrity depends on
adequate structural support from the TJ-associated proteins, which
include claudin-5, occludin and ZO-1. Occludin was the first
integral membrane protein identified within the TJs of endothelial
cells. A deletion construct lacking the N terminus and
extracellular domains of occludin has been shown to exhibit a
marked effect on TJ integrity (14). Occludin has an important role in
maintaining TJ assembly and barrier function. Transmembrane
protein, claudin-5, has also been shown to directly regulate the
integrity and proper functioning of the BBB (15,16).
For example, mice with a claudin-5 deletion succumb as neonates due
to the size-selective loosening of the BBB for molecules <800 Da
(17). Drugs that increase
claudin-5 expression increase transendothelial electrical
resistance and decrease the BBB permeability (18). ZO-1 is a peripheral protein
localized at junction sites that interacts directly with the
majority of TJ transmembrane proteins, including occludins and
claudins. Epithelial cells deficient in ZO-1 do not form TJs due to
the lack of claudin polymerization (19), and delayed barrier establishment
(20). Therefore, ZO-1 appears to
be crucial for the formation and function of TJs. TJ-associated
proteins, including claudin-5, occludin and ZO-1, have critical
roles in the maintenance of BBB functions; thus, the expression
levels of these TJ proteins following TBI were investigated in the
present study. The results demonstrated that the levels of mRNA
transcription and protein expression of these three TJ-associated
proteins were significantly reduced following TBI (Fig. 2). Furthermore, BBB permeability was
markedly increased in the injured brain regions following TBI when
compared with the sham-operated group, as shown by the results from
the EB dye extravasation (Fig. 1).
These observations indicate that the changes in the distribution
and the decreased expression levels of claudin-5, occludin and ZO-1
were consistent with the results of the BBB permeability changes
following TBI.
Netrin-1 hyperstimulation is able to promote focal
neovascularization in the adult brain in vivo (21). In addition, Liu et al
(22) previously demonstrated that
netrin-1 may regulate the BBB (22). Thus, the various roles of netrin-1
may engage in the recovery processes of TBI. Understanding the
mechanisms underlying TJ-associated proteins development and
functioning, and more specifically the effects that netrin-1
exhibits on the BBB, is of utmost importance. In the present study,
the association between netrin-1 and the expression levels of
TJ-associated proteins was investigated. The results revealed that
pZsGreen1-N1-mediated netrin-1 transcription was detected in brain
microvascular endothelial cells at an mRNA level following gene
transfer. Notably, a significant enhancement in the expression
levels of claudin-5, occludin and ZO-1 were observed in the
endothelial cells isolated from TBI following pZsGreen1-N1-netrin-1
gene transfer (Fig. 4). Since
brain endothelial cells play a critical role in the structural and
transport maintenance of the BBB, and the BBB permeability depends
on the integrity of the TJs and the expression of claudin-5,
occludin and ZO-1, the results from the present study indicate that
overexpression of netrin-1 may improve the TJs of brain endothelial
cells and contribute to the recovery of the BBB following TBI. The
development of novel therapies for the treatment of TBI may involve
netrin-1 in the repair of the BBB; thus, future study should focus
on the association between netrin-1 and the integrity of TJs and
the recovery of the BBB.
In conclusion, overexpression of netrin-1 increases
the expression levels of TJ-associated proteins following TBI,
which provides a solid foundation for further study investigating
the role of netrin-1 in the integrity of TJs and the function of
the BBB.
Acknowledgements
The study was supported by a grant from the Medical
Research Subject of the 11th Five-Year Plan of Nanjing
Military Region (no. 06MA76).
References
|
1
|
Fanning AS, Mitic LL and Anderson JM:
Transmembrane proteins in the tight junction barrier. J Am Soc
Nephrol. 10:1337–1345. 1999.PubMed/NCBI
|
|
2
|
Furuse M, Sasaki H and Tsukita S: Manner
of interaction of heterogeneous claudin species within and between
tight junction strands. J Cell Biol. 147:891–903. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hirase T, Staddon JM, Saitou M, et al:
Occludin as a possible determinant of tight junction permeability
in endothelial cells. J Cell Sci. 110:1603–1613. 1997.PubMed/NCBI
|
|
4
|
Anderson J, Fanning A, Lapierre L and Van
Itallie CM: Zonula occludens (ZO)-1 and ZO-2: membrane-associated
guanylate kinase homologues (MAGuKs) of the tight junction. Biochem
Soc Trans. 23:470–475. 1995.PubMed/NCBI
|
|
5
|
Haskins J, Gu L, Wittchen ES, Hibbard J
and Stevenson BR: ZO-3, a novel member of the MAGUK protein family
found at the tight junction, interacts with ZO-1 and occludin. J
Cell Biol. 141:199–208. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huber JD, Egleton RD and Davis TP:
Molecular physiology and pathophysiology of tight junctions in the
blood-brain barrier. Trends Neurosci. 24:719–725. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park KW, Crouse D, Lee M, et al: The
axonal attractant netrin-1 is an angiogenic factor. Proc Natl Acad
Sci USA. 101:16210–16215. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wilson BD, Ii M, Park KW, et al: Netrins
promote developmental and therapeutic angiogenesis. Science.
313:640–644. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shapira Y, Setton D, Artru AA and Shohami
E: Blood-brain barrier permeability, cerebral edema, and neurologic
function after closed head injury in rats. Anesth Analg.
77:141–148. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Soares HD, Thomas M, Cloherty K and
McIntosh TK: Development of prolonged focal cerebral edema and
regional cation changes following experimental brain injury in the
rat. J Neurochem. 58:1845–1852. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feeney DM, Boyeson MG, Linn RT, Murray HM
and Dail WG: Responses to cortical injury: I. Methodology and local
effects of contusions in the rat. Brain Res. 211:67–77. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Belayev L, Busto R, Zhao W and Ginsberg
MD: Quantitative evaluation of blood-brain barrier permeability
following middle cerebral artery occlusion in rats. Brain Res.
739:88–96. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zeng Y, Liu Z, Yang J, et al: ARID1A is a
tumour suppressor and inhibits glioma cell proliferation via the
PI3K pathway. Head Neck Oncol. 5:62013.
|
|
14
|
Bamforth SD, Kniesel U, Wolburg H,
Engelhardt B and Risau W: A dominant mutant of occludin disrupts
tight junction structure and function. J Cell Sci. 112:1879–1888.
1999.PubMed/NCBI
|
|
15
|
Feldman GJ, Mullin JM and Ryan MP:
Occludin: structure, function and regulation. Adv Drug Deliv Rev.
57:883–917. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Piontek J, Winkler L, Wolburg H, et al:
Formation of tight junction: determinants of homophilic interaction
between classic claudins. FASEB J. 22:146–158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nitta T, Hata M, Gotoh S, et al:
Size-selective loosening of the blood-brain barrier in
claudin-5-deficient mice. J Cell Biol. 161:653–660. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Honda M, Nakagawa S, Hayashi K, Kitagawa
N, et al: Adrenomedullin improves the blood-brain barrier function
through the expression of claudin-5. Cell Mol Neurobiol.
26:109–118. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Umeda K, Ikenouchi J, Katahira-Tayama S,
et al: ZO-1 and ZO-2 independently determine where claudins are
polymerized in tight-junction strand formation. Cell. 126:741–754.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Umeda K, Matsui T, Nakayama M, et al:
Establishment and characterization of cultured epithelial cells
lacking expression of ZO-1. J Biol Chem. 279:44785–44794. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fan Y, Shen F, Chen Y, et al:
Overexpression of netrin-1 induces neovascularization in the adult
mouse brain. J Cereb Blood Flow Metab. 28:1543–1551. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu N, Huang H, Lin F, Chen A, Zhang Y,
Chen R and Du H: Effects of treadmill exercise on the expression of
netrin-1 and its receptors in rat brain after cerebral ischemia.
Neuroscience. 194:349–358. 2011. View Article : Google Scholar : PubMed/NCBI
|