Introduction
Reperfusion, as the most important technique for
salvaging ischemic myocardium, may also lead to detrimental
myocardial ischemia-reperfusion injury (MIRI) (1). Ischemic or pharmacological
preconditioning, which is respectively induced by a brief ischemia
or the application of bioactive substances prior to a sustained
ischemia, has been established as an effective cardioprotective
mechanism (2,3). However, ischemic events cannot be
anticipated in the clinical setting; therefore, the utility of
ischemic and pharmacological preconditioning is notably
limited.
Ischemic postconditioning, which is induced by a
short series of repetitive cycles of reperfusion and ischemia
applied immediately at the onset of prolonged reperfusion, has been
reported to be cardioprotective against MIRI. However, in the
clinical setting, ischemic postconditioning may be difficult to
introduce, i.e., repetitive inflations and deflations of the
balloon during primary angioplasty may lead to coronary endothelial
damages, plaque rupture and embolic events. Therefore, the concept
of pharmacological postconditioning by administering bioactive
agents, which mimic the protective effects of ischemic
postconditioning, deserves more attention for its feasible,
effective and safer characteristics (4,5).
Tanshinone IIA, one of the major effective
components in conventional Chinese medicine Danshen, which is
derived from the dried root or rhizome of Salvia
miltiorrhiza Bge., has been widely used in adjunctively
treating cardiovascular diseases in China for a long time (6). Previous studies have demonstrated
that pharmacological preconditioning with tanshinone IIA may
protect the heart from MIRI by reducing myocardial infarct size
when applied prior to sustained ischemia in rats (7). Furthermore, the phosphatidylinositol
3-kinase (PI3K)/Akt signaling pathway has been shown to be involved
in the cardioprotective effect of ischemia- or pharmacological pre-
and postconditioning, including tanshinone preconditioning by
inhibiting the opening of the mitochondrial permeability transition
pore (mPTP) (4,8,9).
However, it remains unclear whether pharmacological
postconditioning with tanshinone IIA is able to attenuate MIRI.
Such protection would widen and facilitate the clinical application
of tanshinone IIA as an adjunct to the early reperfusion therapy of
acute myocardial infarction. Therefore, the present study was
designed to examine the hypothesis that tanshinone IIA, applied
prior to prolonged reperfusion following a sustained ischemia, may
exert a cardioprotective effect against MIRI by activating the
PI3K/Akt pathway.
Methods
Animals and materials
Male Sprague-Dawley (SD) rats (Shanghai SLAC
Laboratory Animal Co., Ltd., Shanghai, China), weighing 250–300 g,
were used in this study which conformed to the Guide for the Care
and Use of Laboratory Animals published by the US National
Institutes of Health (NIH Publication no. 85–23, revised 1996) and
was approved by the Experimental Animal Care Committee of Fujian
Medical University Union Hospital (Fuzhou, China). All the rats
were sedated with 75 mg/kg ketamine and 7.5 mg/kg diazepam
intraperitoneally. Sodium tanshinone IIA silate was obtained from
Shanghai No.1 Biochemical and Pharmaceutical Co., Ltd. (Shanghai,
China). Antibodies for phospho-Akt (p-Akt) and total-Akt (t-Akt)
were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA), and for phospho-endothelial nitric oxide synthase (p-eNOS)
and total-eNOS (t-eNOS) were obtained from Cell Signaling
Technology, Inc. (Boston, MA, USA). Evan’s blue,
triphenyltetrazolium chloride (TTC) and LY294002, a specific
inhibitor of PI3K, were purchased from Sigma (St. Louis, MO,
USA).
Experimental procedure
A total of 88 SD rats were included in the
experiment and their left main coronary arteries (LCA) were
occluded for 30 min to induce ischemia (I), followed by sustained
relaxation for 5 or 120 min to reperfuse (R). All the animals were
randomly divided into seven groups (Fig. 1): the sham-surgery group (sham)
without ischemia (n=8); the control group (control), receiving I/R
without any other intervention (n=16); the ischemic
postconditioning group (post), treated the same as the control,
with the addition of providing three cycles of 10 sec R and 10 sec
I prior to 120 min R (n=16); the low-dose tanshinone group (tan-L),
treated the same as the control, with the addition of an
intravenous injection of 5 mg/kg tanshinone IIA during 25–30 min I
(n=8); the medium-dose tanshinone group (tan-M), treated the same
as the control, with the addition of an intravenous injection of 10
mg/kg tanshinone IIA (n=16); the high-dose tanshinone group
(tan-H), treated the same as the control, with the addition of
receiving an injection of 20 mg/kg tanshinone IIA (n=8); the
medium-dose tanshinone plus LY294002 group (tan+LY), treated the
same as the tan-M group, with the addition of an intravenous
injection of 0.3 mg/kg LY294002, 5–10 min prior to reperfusion
(n=16).
 | Figure 1Experimental procedures and animal
groups. The rats, exposed to I (dark bar) and R (open bar), were
divided into seven groups (n=8 or 16/group): the sham group,
subject to surgery but not ligation of the LCA (arrow); the control
group, received 30 min I and 5 min or 120 min R; the post group,
received 3 cycles of 10 sec R and 10 sec I prior to 120 min R; the
tan-L, tan-M and tan-H groups, subject to intravenous injection of
5, 10 and 20 mg/kg of Tan 5 min prior to R, respectively (straight
line); the tan+LY group, treated similarly to the Tan-M group, with
the addition of an injection 0.3 mg/kg LY, a specific inhibitor of
PI3K, 5–10 min prior to R (straight line). In each group, eight rat
hearts were harvested following 120 min R. In the control, post,
tan-M and tan+LY groups, an additional eight rat hearts were
harvested following 5 min R (triangles). Post, ischemic
postconditioning; Tan, tanshinone IIA; LY, LY294002; I, ischemia;
R, reperfusion; LCA, left main coronary artery. |
Surgical preparation
The surgical preparations were performed as
previously described by Fang et al (10). The chest was opened through the
fourth intercostal space and a single 7-0 Prolene suture was placed
under the LCA, 1–2 mm from its origin. A small polyethylene tube
was placed between the ends for reversible coronary artery
occlusion. The sham group rats were treated in the same manner,
with the exception that the suture was not ligated. At the end of
120 min reperfusion, the left ventricles at risk in eight rats of
each group were determined by injection 1 ml 0.1% Evan’s blue after
the LCA was ligated again. Cardiac arrest was induced by an
intravenous bolus injection of 10% KCl and the left ventricle was
transversely sectioned into four slices to assess the infarct size.
For the control, post, tan-M and tan+LY groups, an additional eight
rats of each group were sacrificed 5 min following reperfusion and
the myocardium samples were collected and stored at −80°C for the
assessment of mitochondrial permeability transition (MPT), which
occurs due to the opening of mPTPs, and western blot examination
was performed (Fig. 1).
Infarct size assessment
The slices of the left ventricle were incubated in a
1% solution of TTC at 36.5°C for 10–15 min. Following this, the
slices were fixed in a 10% formaldehyde solution for 24 h. Images
of the surfaces that faced the apex were captured by a digital
camera. The extents of the blue normal area, plum survival area and
the grey area of necrosis were quantified by planimetry using
ImageJ 1.36 software from the National Institutes of Health. The
area of necrosis (AN), area at risk (AAR; including the grey and
red area) and the left ventricular area (LV) were measured. The
infarct size was expressed as a percentage of the AAR (AN/AAR) and
the risk area was expressed as the AAR/LV.
Western blot analysis
The samples (60 μg protein/lane) were
electrophoresed by 12% sodium dodecyl sulfate polyacrylamide gel
electrophoresis, and then electrophoretically transferred onto
Hybond-C Extra membranes (Amersham, Pittsburgh, PA, USA). Following
treatment with the blocking buffer, the membranes were incubated
with primary antibodies (anti-t-Akt and anti-p-Akt; anti-t-eNOS and
anti-p-eNOS) and the horseradish peroxidase-conjugated secondary
antibody (Sigma). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
was used as an internal control. The immunoreactive bands were
detected by Enhanced Chemiluminescence Plus reagent (Amersham)
using an X-ray film (Kodak; Rochester, NY, USA). Target signals
were normalized relative to the GAPDH expression and assessed using
ImageJ 1.36 software.
Assessment of mPTP opening
The preparation of mitochondria was adapted from a
previously described procedure (11). Isolated mitochondria from
cardiomyocytes (1 mg protein) were resuspended in swelling buffer
(71 mmol/l sucrose, 215 mmol/l mannitol and 10 mmol/l succinate in
3 mmol/l HEPES, pH 7.4) to a final volume of 2 ml, and incubated at
25°C for 2 min. MPT due to opening of mPTPs, which was induced by
2, 20 and 20 μmol/l CaCl2, induced mitochondrial
swelling, and was measured spectrophotometrically (DU 800; Beckman
Coulter, Inc., Brea, CA, USA) as a reduction in the optical density
at 540 nm (OD540) in isolated mitochondria (11–13).
Statistical analysis
Statistical analysis was performed using the SPSS
software, version 16.0 (SPSS, Inc., Chicago, IL, USA). All values
are expressed as the mean ± standard deviation. The differences
among the groups were tested by one-way analysis of variance. When
a statistical difference was identified, the least significant
difference procedure was applied. P<0.05 was considered to
indicate a statistically significant result.
Results
Infarct size
No ischemic and necrotic areas were found in the
sham-surgery group. No significant difference was identified among
the other groups in AAR/LV (F=0.737, P=0.600). Infarct size
(AN/AAR) in the post, tan-M and tan-H groups was significantly
smaller than that in the control group (25.3±4.3, 29.2±4.5 and
28.5±3.0 vs. 46.9±3.6%, respectively; P<0.01). No significant
differences were observed between the control group and the tan-L
group (46.9±3.6 vs. 43.0±4.0%), and among the post, medium- and
high-dose tanshinone IIA groups. The reduction in infarct size
induced in the tan-M group was abrogated completely by LY294002
treatment (29.2±4.5 vs. 45.3±4.3%; P<0.01) and the infarct size
following LY294002 treatment was comparable with that of the
control group (Fig. 2).
 | Figure 2Effects of various treatments on
infarct size. AAR is expressed as a percentage of the LV (AAR/LV)
and AN as a percentage of the AAR (AN/AAR). Compared with the
control group, the post, tan-M, tan-H groups had a significantly
reduced infarct size (AN/AAR). The reduction in infarct size
induced by 10 mg/kg tanshinone IIA postconditioning was abrogated
completely by LY, a specific inhibitor of PI3K. All values are
expressed as the mean ± standard deviation (%; n=8/group).
*P<0.01 vs. the control, tan-L and tan+LY groups.
Post, ischemic postconditioning; tan-L, low-dose tanshinone IIA (5
mg/kg); tan-M, medium-dose tanshinone IIA (10 mg/kg); tan-H,
high-dose tanshinone IIA (20 mg/kg); LY, LY294002; AAR, area at
risk; LV, left ventricular area; AN, area of necrosis. |
Expression levels of p-Akt and p-eNOS in
the myocardium
Compared with the control group, the post and tan-M
groups had higher p-Akt/t-Akt and p-eNOS/t-eNOS expression ratios
(p-Akt/t-Akt, 1.0±0.0 vs. 2.3±0.3 and 2.2±0.3; p-eNOS/t-eNOS,
1.0±0.0 vs. 2.1±0.2 and 1.8±0.2, respectively; all P<0.01). No
statistically significant difference was observed in the
p-Akt/t-Akt ratio between the post and tan-M groups; however, the
p-eNOS/t-eNOS ratio in the tan-M group was lower than that in the
post group (P<0.01). Compared with the phosphorylation level in
the tan-M group, that in the LY294002 group was significantly lower
(p-Akt/t-Akt, 2.2±0.3 vs. 1.2±0.3; P<0.01; p-eNOS/t-eNOS,
1.8±0.2 vs. 1.2±0.2, P<0.01; Fig.
3).
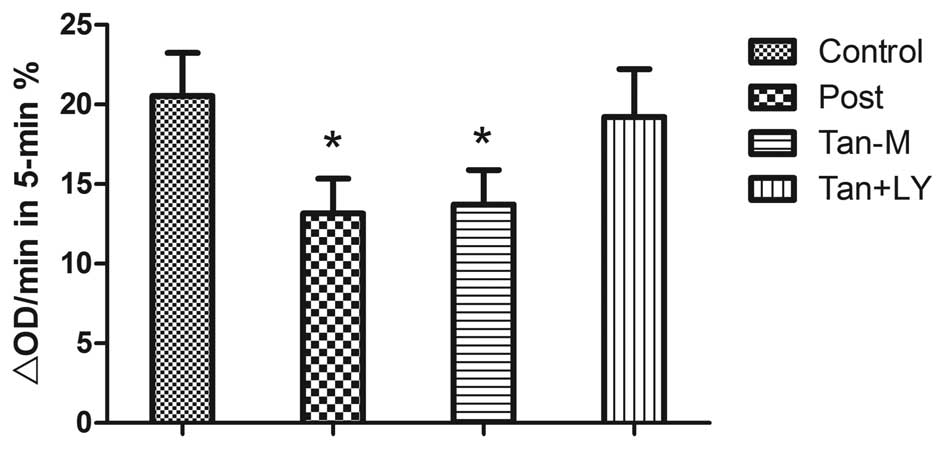
Ca2+ induced mPTP opening
MPT was expressed as a reduction in OD540
during 5 min (ΔOD/min). In the post and tan-M groups, ΔOD/min was
lower than that in the control group (13.2±2.2 and 13.7±2.2 vs.
20.5±2.7%, respectively; P<0.01). No statistically significant
difference was observed between the post and tan-M groups. The
reduction in MPT afforded by the Tan-M group was eliminated by
LY294002 treatment (13.7±2.2 vs. 19.2±3.0%; P<0.01; Fig. 4).
Discussion
In the present study, it was demonstrated that
pharmacological postconditioning with tanshinone IIA, one of the
active ingredients in the Chinese medicine Danshen, protects the
myocardium from ischemia-reperfusion injury by activating the
PI3K/Akt-eNOS pathway, and the blockage of mPTP opening may be
involved in this cardioprotective effect.
In animal models, a variety of pharmacological
treatment strategies have been demonstrated to reduce infarct size
by activating the reperfusion injury salvage kinase (RISK) pathway,
which includes PI3K/Akt and extra-cellular signal-regulated protein
kinase 1/2 pathways, when applied at the onset of reperfusion
(14). However, a number of these
strategies have produced disappointing results when translated into
the clinical setting (15). This
may be as a result of the marked differences that exist between the
clinical setting and studies in animal models. However, the
pharmacological agents applied in these studies are also important
factors, which may lead to negative clinical results. Therefore,
studies investigating more clinically feasible and effective
pharmacological strategies are required to confer cardioprotection
by activating pro-survival kinases, serving as an adjunct to early
reperfusion therapy for acute myocardial infarction.
Tanshinone IIA, a key component of the Chinese
medicine Danshen obtained from Salvia miltiorrhiza, has been
used in clinical practice for cardiovascular and cerebrovascular
diseases in Asia. A number of studies have demonstrated that
tanshinone IIA protects cardiac myocytes against oxidative
stress-triggered damage and apoptosis (16), prevents myocardial hypertrophy
(17), protects against sudden
cardiac death (18) and attenuates
myocardial ischemic (19) and
reperfusion injury (20). However,
the majority of studies (7,20)
have focused on applying tanshinone IIA prior to ischemia, which
limits its use in clinical practice. In the present study, the
clinical application of tanshinone IIA was widened, as the data
obtained demonstrate that when applied prior to prolonged
reperfusion following sustained ischemia, tanshinone IIA at both
medium and high doses, which have been used in other studies for
cardioprotection, resulted in a reduction in infarct size
comparable to that of ischemic postconditioning. In the present
study, three dosages (5, 10 and 20 mg/kg) were utilized. Since
low-dose tanshinone IIA failed to reduce the infarct size, and
medium-dose tanshinone IIA elicited a similar cardioprotective
effect in infarct size reduction as high-dose tanshinone IIA, the
dosage of 10 mg/kg tanshinone IIA may serve as a more
cost-effective optimal dose and was used to further examine the
molecular mechanisms involved in this process.
Several studies have demonstrated that the
activation of the RISK pathway is involved in cardioprotection in
animal models (14). The RISK
pathway, first reported by Hausenloy and Yellon, is one of crucial
mechanisms in the regulation of cell survival (14). eNOS is an important downstream
target of PI3K/Akt (21). Previous
studies have demonstrated that the mPTP, a non-specific pore
associated with contact sites between the outer and inner
mitochondrial membranes, is a key end-effector of reperfusion
injury; its opening may lead to MPT, ultimately causing severe
apoptosis and cell necrosis (22).
The present study also investigated whether
tanshinone IIA attenuates ischemia-reperfusion injury through the
PI3K/Akt/eNOS-mPTP pathway. The results demonstrated that at 5 min
reperfusion, the myocardial protein levels of p-Akt/t-Akt and
p-eNOS/t-eNOS were comparably elevated in ischemic and tanshinone
IIA postconditioning groups, as compared with those in the control
group, suggesting that Akt phosphorylation may be activated by the
two postconditioning methods at the early reperfusion. Furthermore,
a specific PI3K inhibitor, LY294002, was used to reduce Akt and its
downstream eNOS phosphorylation levels, which eliminated the
cardioprotection induced by tanshinone IIA. This demonstrated that
tanshinone IIA confers cardioprotection through the PI3K/Akt/eNOS
pathway when applied prior to reperfusion following sustained
ischemia. It was also identified that p-eNOS/t-eNOS in the tan-L
group was lower than that in the post group, which indicates that
tanshinone IIA may also activate other downstream signaling
proteins (not only eNOS) of PI3K to confer cardioprotection. In
addition, MPT was detected at 5 min reperfusion, the same time
point when pro-survival signal kinases were upregulated by
tanshinone IIA postconditioning. The results demonstrated that
ischemic and tanshinone IIA postconditioning both lowered the
ΔOD/min, indicating attenuated mitochondrial swelling. However,
these effects were blocked by LY294002, which suggested that
inhibition of mPTP opening by tanshinone IIA postconditioning may
be regulated by the PI3K/Akt pathway.
Notably, a number of studies (8,14,23,24)
have indicated that the PI3K/Akt pathway is involved in the
cardioprotective effect afforded by ischemia- or pharmacological
pre- and postconditioning by inhibiting mPTP opening. One previous
study has also revealed that pretreatment with tanshinone IIA may
inhibit mPTP opening dose-dependently, and its cardioprotective
effect may be via the inhibition of pore opening during reperfusion
(25). More recently, Zhang et
al (20) reported that
tanshinone IIA pretreatment elicits cardioprotection in diabetic
rats via the PI3K/Akt-dependent pathway. The results from the
present study are consistent with these previous findings.
There are several limitations to consider in the
present study. Firstly, specific mPTP openers or inhibitors were
not used to confirm the causal correlation between tanshinone
postconditioning and mPTP opening. Secondly, further studies are
required to determine the efficacy of tanshinone postconditioning
in clinical practice.
The present study demonstrated that pharmacological
postconditioning with tanshinone IIA protects the rat myocardium
from ischemia-reperfusion injury through the PI3K/Akt-eNOS pathway,
and the blockage of MPT may have an important role in this process.
Since tanshinone IIA has been safely used in clinical practice,
particularly in patients with ischemic heart diseases in Asia, the
concept of tanshinone postconditioning by the PI3K/Akt-mPTP pathway
may represent a practical solution to reduce ischemia-reperfusion
injury as an adjuvant to current reperfusion strategies.
Acknowledgements
This study is supported mainly by the National
Natural Science Foundation of China (grant no. 81100151), the
Science Foundation for Distinguished Young Scholars of Fujian
Province, China (grant no. 2013J06015) and partially by the
National Natural Science Foundation of China (grant no. 81170196)
and the Science and Technology Project of Education Department of
Fujian Province, China (grant no. JA12132).
References
|
1
|
Braunwald E and Kloner RA: Myocardial
reperfusion: a double-edged sword? J Clin Invest. 76:1713–1719.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Murry CE, Jennings RB and Reimer KA:
Preconditioning with ischemia: a delay of lethal cell injury in
ischemic myocardium. Circulation. 74:1124–1136. 1986. View Article : Google Scholar
|
|
3
|
Maulik N, Engelman RM, Wei Z, et al:
Interleukin-1 alpha preconditioning reduces myocardial ischemia
reperfusion injury. Circulation. 88:II387–II394. 1993.PubMed/NCBI
|
|
4
|
Zhao ZQ, Corvera JS, Halkos ME, et al:
Inhibition of myocardial injury by ischemic postconditioning during
reperfusion: comparison with ischemic preconditioning. Am J Physiol
Heart Circ Physiol. 285:H579–H588. 2003.PubMed/NCBI
|
|
5
|
Tissier R, Waintraub X, Couvreur N, et al:
Pharmacological postconditioning with the phytoestrogen genistein.
J Mol Cell Cardiol. 42:79–87. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao S, Liu Z, Li H, et al: Cardiovascular
actions and therapeutic potential of tanshinone IIA.
Atherosclerosis. 220:3–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu W, Yang J and Wu LM: Cardioprotective
effects of tanshinone IIA on myocardial ischemia injury in rats.
Pharmazie. 64:332–336. 2009.PubMed/NCBI
|
|
8
|
Tsang A, Hausenloy DJ, Mocanu MM and
Yellon DM: Postconditioning: a form of ‘modified reperfusion’
protects the myocardium by activating the phosphatidylinositol
3-kinase-Akt pathway. Circ Res. 95:230–232. 2004.
|
|
9
|
Lim SY, Davidson SM, Hausenloy DJ and
Yellon DM: Preconditioning and postconditioning: the essential role
of the mitochondrial permeability transition pore. Cardiovasc Res.
75:530–535. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fang J, Chen L, Wu L and Li W:
Intra-cardiac remote ischemic post-conditioning attenuates
ischemia-reperfusion injury in rats. Scand Cardiovasc J.
43:386–394. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fang J, Wu L and Chen L: Postconditioning
attenuates cardiocyte ultrastructure injury and apoptosis by
blocking mitochondrial permeability transition in rats. Acta
Cardiol. 63:377–387. 2008. View Article : Google Scholar
|
|
12
|
Wang G, Liem DA, Vondriska TM, et al:
Nitric oxide donors protect murine myocardium against infarction
via modulation of mitochondrial permeability transition. Am J
Physiol Heart Circ Physiol. 288:H1290–H1295. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baines CP, Song CX, Zheng YT, et al:
Protein kinase Cepsilon interacts with and inhibits the
permeability transition pore in cardiac mitochondria. Circ Res.
92:873–880. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hausenloy DJ and Yellon DM: New directions
for protecting the heart against ischaemia-reperfusion injury:
targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway.
Cardiovasc Res. 61:448–460. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yellon DM and Hausenloy DJ: Myocardial
reperfusion injury. N Engl J Med. 357:1121–1135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fu J, Huang H, Liu J, et al: Tanshinone
IIA protects cardiac myocytes against oxidative stress-triggered
damage and apoptosis. Eur J Pharmacol. 568:213–221. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tu EY, Zhou YG, Wang ZH, Liang QS and Yang
GT: Effects of tanshinone II A on the myocardial hypertrophy signal
transduction system protein kinase B in rats. Chin J Integr Med.
15:365–370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shan H, Li X, Pan Z, et al: Tanshinone IIA
protects against sudden cardiac death induced by lethal arrhythmias
via repression of microRNA-1. Br J Pharmacol. 158:1227–1235. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pan C, Lou L, Huo Y, et al: Salvianolic
acid B and tanshinone IIA attenuate myocardial ischemia injury in
mice by NO production through multiple pathways. Ther Adv
Cardiovasc Dis. 5:99–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y, Wei L, Sun D, et al: Tanshinone
IIA pretreatment protects myocardium against ischaemia/reperfusion
injury through the phosphatidylinositol 3-kinase/Akt-dependent
pathway in diabetic rats. Diabetes Obes Metab. 12:316–322. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gao F, Gao E, Yue TL, et al: Nitric oxide
mediates the antiapoptotic effect of insulin in myocardial
ischemia-reperfusion: the roles of PI3-kinase, Akt, and endothelial
nitric oxide synthase phosphorylation. Circulation. 105:1497–1502.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Halestrap AP, Kerr PM, Javadov S and
Woodfield KY: Elucidating the molecular mechanism of the
permeability transition pore and its role in reperfusion injury of
the heart. Biochim Biophys Acta. 1366:79–94. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bopassa JC, Ferrera R, Gateau-Roesch O,
Couture-Lepetit E and Ovize M: PI 3-kinase regulates the
mitochondrial transition pore in controlled reperfusion and
postconditioning. Cardiovasc Res. 69:178–185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Argaud L, Gateau-Roesch O, Raisky O, et
al: Postconditioning inhibits mitochondrial permeability
transition. Circulation. 111:194–197. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang SZ, Ye ZG, Xia Q, Zhang W and Bruce
I: Inhibition of mitochondrial permeability transition pore: A
possible mechanism for cardioprotection conferred by pretreatment
with tanshinone IIA. Conf Proc IEEE Eng Med Biol Soc. 3:2276–2279.
2005.PubMed/NCBI
|