Introduction
In recent years, significant advances in HIV
treatment have been made towards reducing mortality in HIV-infected
patients (1). However, patients
treated with antiretroviral therapy, including protease inhibitors
(PIs), develop metabolic side-effects, including hyperlipidemia,
insulin resistance, lipoatrophy and lactic acidosis (2).
The molecular mechanism of PI-induced insulin
resistance has not yet been elucidated. Previous studies have
suggested that PI-induced insulin resistance and diabetes are
associated with the inhibition of glucose transporter 4 (GLUT4)
translocation (3,4) and that lopinavir inhibits the
phosphorylation of insulin receptor substrate (IRS) (5).
A previous study demonstrated that increased
inflammation in adipose tissue is a prominent mechanism of insulin
resistance (6). In addition,
increased levels of pro-inflammatory cytokines secreted from
adipose tissue activate a variety of cellular events that impede
insulin action in adipose tissue (7). Suppressor of cytokine signaling
(SOCS) 1 is one of the main molecules involved in inflammatory
signaling; it has Src homology 2 domains, interacts with Janus
kinase and inhibits the kinase activity of inflammatory cytokines
(8). Among the SOCS family
members, SOCS1 and SOCS3 induce insulin resistance by inhibiting
the phosphorylation of IRS (9).
The insulin signal transduction system also includes
protein tyrosine phosphatases (PTPs), enzymes that dephosphorylate
tyrosine kinases. PTP1B is a negative regulator that has an
important role in the metabolic system, immune system and
oncogenesis (10).
In the present study, it was hypothesized that PIs
affect insulin signaling by regulating the expression of SOCS or
PTPs. Therefore, the aim of the study was to investigate the
mechanism of the dysregulation of insulin signaling induced by
lopinavir and darunavir, which are widely used protease inhibitors.
In particular, changes in the activities of SOCS and PTP1B caused
by PI treatment were analyzed.
Materials and methods
Materials
Lopinavir and darunavir were purchased from Toronto
Research Chemicals Inc. (Toronto, Ontario, Canada) and dissolved in
ethyl acetate and methanol, respectively. Since the levels of IRS1
expression and IRS1 phosphorylation by insulin were comparable in
preliminary experiments, methanol was used as a vehicle control in
the following experiments. Insulin from bovine pancreas was
obtained from Sigma-Aldrich (St. Louis, MO, USA). The primary
antibodies used were anti-phospho (Ser307)-IRS1 and anti-IRS1
antibodies (Upstate Biotechnology Inc., Lake Placid, NY, USA), and
anti-SOCS1, anti-SOCS3 and anti-PTP1B antibodies (Santa Cruz
Biotechnology, Santa Cruz, CA, USA). The phospho-tyrosine-specific
monoclonal antibody 4G10 (Upstate Biotechnology, Darmstadt,
Germany) was used in the immunoprecipitation assay.
Cell culture, pretreatment with PIs and
insulin stimulation
3T3-L1 preadipocytes were obtained from the American
Type Culture Collection (Manassas, VA, USA) and cultured and
maintained as previously described (11). Differentiated adipocytes were
obtained by plating preadipocytes in differentiation medium
containing insulin, dexamethasone, isobutyl methyl xanthine and a
thiazolidinedione (AM-1; DS Pharma Biomedical Co. Ltd., Osaka,
Japan) for an additional 7 days.
Adipocytes were pretreated with PIs by adding 30 μM
lopinavir, 30 μM darunavir or a vehicle control (0.1% ethyl acetate
or 0.1% methanol, respectively) for 48 h. Following PI
pretreatment, adipocytes were stimulated with 100 nM of insulin for
30 min.
Western blotting and
immunoprecipitation
Following insulin stimulation, ice-cold
phosphate-buffered saline (PBS) was added, and cells were lysed
with NP-40 lysis buffer containing 1% Nonidet P-40, 25 mM Tris-HCl
(pH 7.5), 150 mM sodium chloride, 1 mM EDTA, 5 mM sodium fluoride,
1 mM sodium orthovanadate, 1 mM leupeptin and 1 mM
phenylmethylsulfonyl fluoride. The lysates were resuspended in
loading buffer as described by Laemmli (12). Sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed
with 10–12% (w/v) acrylamide gels (12). The separated proteins were
transferred onto a nitrocellulose membrane for immunoblotting. The
membrane was blocked in blocking buffer for 1 h, then incubated
with a primary antibody, followed by a horseradish peroxidase
(HRP)-labeled secondary antibody (Sigma-Aldrich). The protein bands
were then visualized using a chemiluminescence reagent (Immobilon
Western chemiluminescent HRP Substrate; Millipore, Billerica, MA,
USA).
For the immunoprecipitation studies, cell lysates
were mixed with 4 μg anti-IRS1 antibody for 1 h. Cell lysates were
then mixed with protein G-coupled Sepharose beads (GE Healthcare UK
Ltd., Little Chalfont, UK) and rotated for 1 h at 4°C. The beads
were washed 3 times with ice-cold NP-40 lysis buffer and the
precipitated proteins were boiled for 5 min and eluted with loading
buffer. SDS-PAGE and western blot analysis were performed with 4G10
antibody as described above.
Immunodetection of GLUT4
The 3T3-L1 adipocytes grown on coverslips were
pretreated with protease inhibitors and stimulated with insulin as
described above. Following insulin stimulation, cells were placed
on ice, washed twice in ice-cold PBS and fixed with 4% (w/v)
paraformaldehyde in PBS for 15 min. The reaction was quenched with
0.1 M glycine in PBS for 10 min. Samples were then blocked with PBS
containing 5% bovine serum albumin for 10 min and incubated with 5
μg/ml anti-GLUT4 antibody (LifeSpan Biosciences, Inc, Seattle, WA,
USA) for 16 h at 4°C and for 45 min with secondary Alexa
594-conjugated anti-mouse immunoglobulin antibodies (Molecular
Probes, Inc, Eugene, OR, USA) at room temperature. Coverslips were
washed twice with PBS and mounted with Dako mounting solution (Dako
Japan, Tokyo, Japan). Images were captured using a fluorescence
microscope (ECLIPSE TE2000-U; Nikon, Kanagawa, Japan) by argon
laser (excitation, 594 nm) at room temperature with a ×40 objective
lens at the same setting.
Results
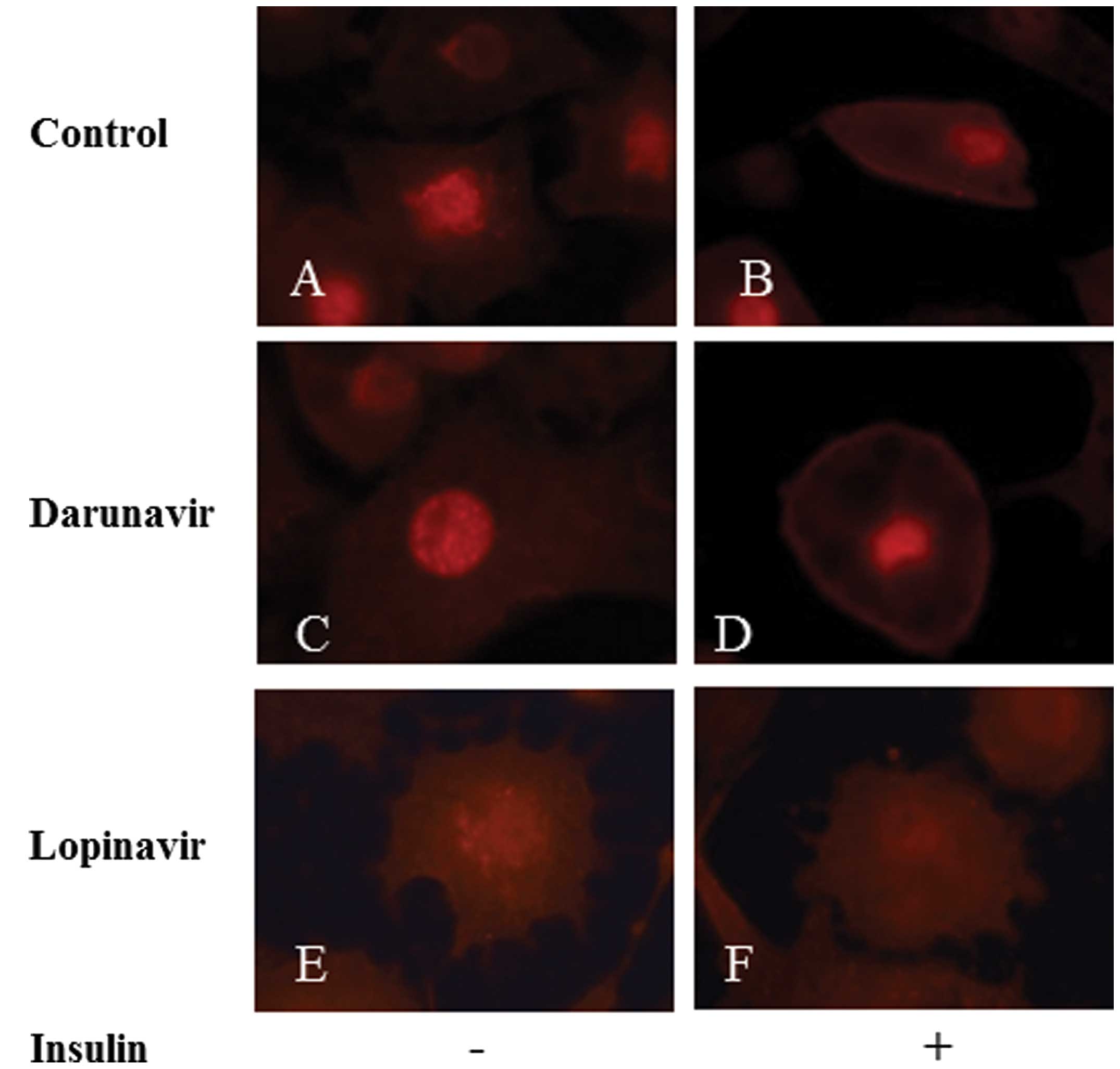
GLUT4 recruitment to the plasma membrane
is inhibited by lopinavir and darunavir
3T3-L1 adipocytes were pretreated with lopinavir,
darunavir or a vehicle control and were stimulated with insulin for
30 min. GLUT4 localization was then observed using
immunofluorescence. In the control adipocytes, GLUT4 was localized
diffusely in the cytosol without insulin stimulation (Fig. 1A) and then translocated to the
plasma membrane following insulin treatment (Fig. 1B). In adipocytes treated with
darunavir, the distribution of GLUT4 was similar to that in the
control cells in the absence of insulin (Fig. 1C). However, following insulin
stimulation, GLUT4 was recruited to the cellular membrane, but some
GLUT4 was observed to remain in the cytosol (Fig. 1D). However, in lopinavir-treated
adipocytes, only a small quantity of GLUT4 was recruited to the
plasma membrane following insulin treatment (Fig. 1E and F). These results indicate
that PIs, in particular lopinavir, inhibit insulin-induced GLUT4
recruitment to the plasma membrane.
Lopinavir inhibits IRS1
phosphorylation
3T3-L1 adipocytes were pretreated with a control
vehicle, lopinavir or darunavir, and then stimulated with insulin
for 30 min. The levels of IRS1 expression did not differ in control
adipocytes and adipocytes treated with lopinavir or darunavir prior
to and following insulin stimulation (Fig. 2). In the absence of insulin
stimulation, IRS1 at Ser307 was not phosphorylated in the control
adipocytes or the lopinavir- or darunavir-pretreated adipocytes.
However, following insulin stimulation, IRS1 at Ser307 was
phosphorylated in control adipocytes, and it was phosphorylated in
a similar manner in darunavir- and lopinavir-pretreated adipocytes.
In addition, IRS1 was also tyrosine-phosphorylated following
insulin stimulation in control adipocytes. However, in
darunavir-treated adipocytes, tyrosine phosphorylation of IRS1 was
reduced. Furthermore, it was almost completely inhibited in
lopinavir-pretreated adipocytes.
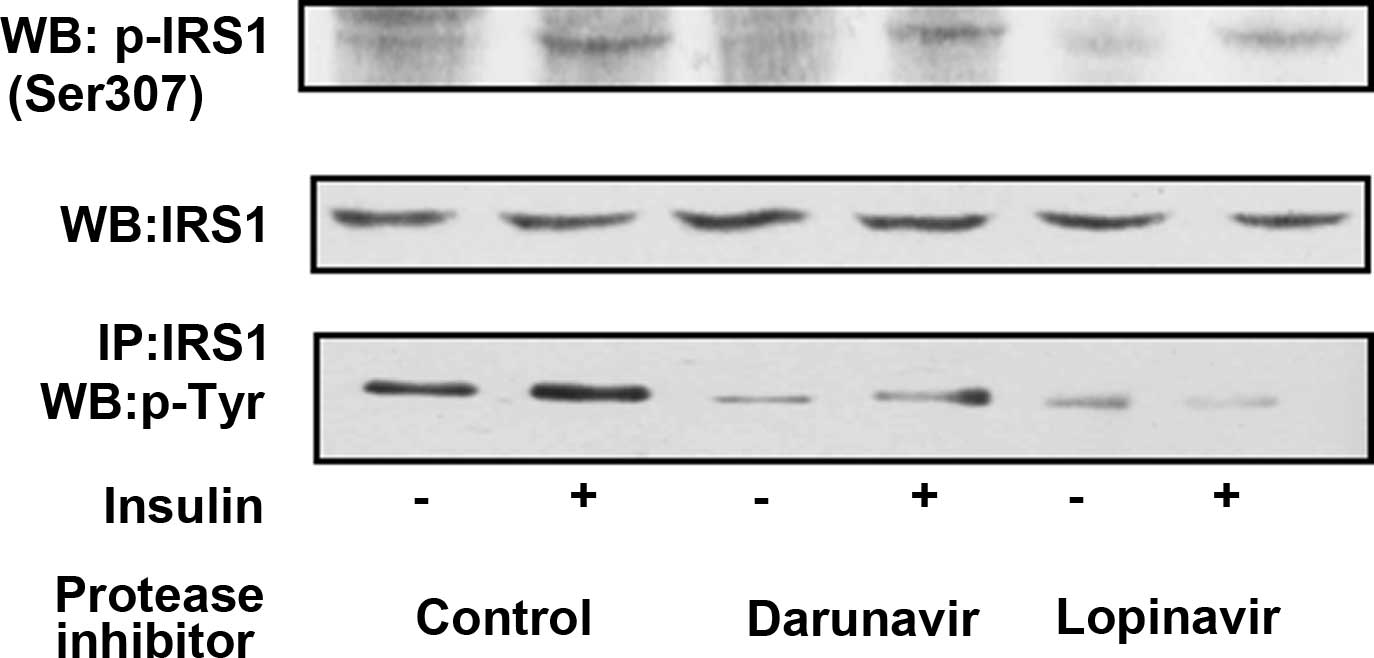 | Figure 2Insulin-induced IRS1 phosphorylation
was inhibited in adipocytes pretreated with lopinavir. Mature
3T3-L1 adipocytes were pretreated with darunavir (30 μM), lopinavir
(30 μM) or a control vehicle, and then were stimulated with or
without 100 nM of insulin for 30 min. The cell lysates were
resolved using SDS-PAGE and visualized by immunoblotting with a
1:2,000 dilution of anti-phospho (Ser307)-IRS1 antibody (top panel)
or with a 1:2,000 dilution of anti- IRS1 antibody (middle panel).
In addition, the cell lysates were immunoprecipitated with IRS1
antibody, and the immunoprecipitated proteins were resolved using
SDS-PAGE and visualized by immunoblotting with a 1:2,000 dilution
of anti-phospho-tyrosine (4G10) antibody (bottom panel). IRS1,
insulin receptor substrate 1; SDS-PAGE, sodium dodecyl
sulfate-polyacrylamide gel electrophoresis; WB, western blot; IP,
immunoprecipitation. |
Lopinavir and darunavir do not affect
SOCS expression
SOCS family members are negative regulators of
insulin signaling. The expression of SOCS1 and SOCS3 was compared
between control adipocytes and PI-pretreated adipocytes. In the
absence and presence of insulin stimulation, the expression levels
of SOCS1 or SOCS3 did not change among the cells (Fig. 3). Analysis of the results from the
immunoprecipitation assay demonstrated that neither SOCS1 nor SOCS3
was associated with IRS1 in the control adipocytes and PI-treated
adipocytes prior to and following insulin stimulation (data not
shown). These results indicate that PIs did not influence the
expression of SOCS1 and SOCS3 and were not associated with
them.
Lopinavir promotes PTP1B expression
The levels of PTP1B expression were compared among
control adipocytes and PI-pretreated adipocytes. The expression
levels of PTP1B were enhanced in adipocytes pretreated with
protease inhibitors, in particular lopinavir (Fig. 4). However, no significant
differences were identified in the levels of PTP1B expression among
the adipocytes prior to and following insulin treatment.
Discussion
To investigate the regulation of insulin signaling,
3T3-L1 adipocytes and 30 μM lopinavir and darunavir were used. The
mean Cmin values of lopinavir and darunavir are 4.6
μg/ml (7.3 μM) and 1.8 μg/ml (3.1 μM), respectively, and the
Cmax values of lopinavir and darunavir are 10.0 μg/ml
(15.9 μM) and 8.2 μg/ml (13.8 μM) (13,14).
Glucose uptake is inhibited with 10–100 μM PIs (3). The concentration of lopinavir and
darunavir that was used in the present study is consistent with the
dosage used in clinical settings.
PIs mediate their antiviral effect by cleaving HIV
protease, the pol gene product (15). Protease inhibitors have several
targets in insulin signaling (16). In the present study, it was found
that PIs upregulate PTP1B expression. This target is considered to
be critical since the levels of PTP1 expression are consistent with
the degree of inhibition of IRS1 tyrosine phosphorylation and GLUT4
translocation that are associated with insulin resistance in the
clinical setting.
Indinavir (100 μM) has been shown to significantly
inhibit GLUT4 activity in Xenopus oocytes (3). In the present study, the effects of
lopinavir and darunavir on insulin resistance were investigated by
analyzing the changes of GLUT4 recruitment to the plasma membrane
using immunofluorescence. However, translocation of GLUT4 was not
investigated for other PIs, including lopinavir and darunavir, by
immunofluorescence in previous studies. The immunofluorescence
results in the present study following treatment with lopinavir or
darunavir appear to be consistent with previous results.
IRS1 phosphorylation, which is activated by insulin
signaling, was also investigated in this study. Increased IRS-1
phosphorylation of serine and threonine residues, in particular
Ser307, contributes to the defective IRS-1 tyrosine phosphorylation
in insulin-resistance (17).
Ser307 phosphorylation was not observed to be significantly
enhanced in the PI-treated adipocytes. However, tyrosine
phosphorylation of IRS-1 was inhibited in adipocytes treated with
PIs, in particular with lopinavir. Ismail et al (18) demonstrated that pretreatment with
indinavir induced a significant reduction in insulin-induced
tyrosine phosphorylation of IRS-1, and these results were
consistent with the results from the present study. This study
focused on PTP1B, which inhibits IRS1 tyrosine phosphorylation, and
it was found that PTP1B expression was enhanced in the presence of
PIs. Following insulin binding, the insulin receptor tyrosine
kinase becomes activated and phosphorylates IRS1 protein on
tyrosine residues, which serve as binding sites for
phosphatidylinositol 3-kinase (PI3K). PI3K catalyzes the
phosphorylation of phosphatidylinositol at the 3′-position and
generates 3′-phophatidylinositol products. Subsequent signaling
pathways induce the translocation of the glucose transporter GLUT4.
Enhancement of PTP1B expression may lead to the dephosphorylation
of tyrosine residues on several substrates, including IRS-1,
resulting in the downregulation of insulin signaling (19). Ben-Romano et al (20) demonstrated that a direct inhibitory
effect on insulin-induced glucose uptake occurs following a
specific interaction of protease inhibitors with GLUT4, whereas
prolonged exposure to nelfinavir interferes with PKB
phosphorylation. In a study by Schütt et al (21), impaired insulin secretion by
nelfinavir or saquinavir was found to be associated with decreased
insulin-induced IRS-1 phosphorylation, although amprenavir and
indinavir had no effect on insulin secretion. Ismail et al
(18) reported that the levels of
PTP1B were not altered in adipocytes treated with indinavir, which
is not in accordance with the results from the present study and
the reason for this has yet to be elucidated. However, it may be
hypothesized that the PIs may affect multiple sites in insulin
signaling and that, therefore, the regulatory effects may differ
among PIs.
In the present study, lopinavir had a stronger
inhibitory effect on insulin signaling compared with darunavir.
This is the first study, to the best of out knowledge, to compare
insulin sensitivity between darunavir and lopinavir. In a previous
study comparing insulin sensitivity between atazanavir and
lopinavir in vitro and clinically, the area under the curve
of glucose increased significantly with lopinavir/ritonavir, but
not with atazanavir/ritonavir during oral glucose tolerance tests
(22). In another study
investigating HIV-negative healthy volunteers receiving
darunavir/ritonavir or atazanavir/ritonavir it was found that the
glucose parameters did not differ between the two groups (23). Björnholm et al (24) reported that reduced
insulin-stimulated IRS-1 tyrosine phosphorylation led to impaired
insulin-induced glucose transport in the skeletal muscle of obese
diabetic patients. Assuming that there was no difference in the
impact of boosted ritonavir in insulin signaling among lopinavir,
atazanavir, and darunavir, this suggests that the results from the
present study are consistent with these clinical results.
Although lopinavir and darunavir inhibited insulin
signaling in adipocytes, lopinavir had a stronger inhibitory effect
on the recruitment of GLUT4 to the cellular membrane and the
tyrosine phosphorylation of IRS-1 compared with darunavir.
References
|
1
|
May MT, Sterne JA, Costagliola D, et al;
Antiretroviral Therapy (ART) Cohort Collaboration. HIV treatment
response and prognosis in Europe and North America in the first
decade of highly active antiretroviral therapy: a collaborative
analysis. Lancet. 368:451–458. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Honda M and Oka S: Current therapy for
human immunodeficiency virus infection and acquired
immunodeficiency syndrome. Int J Hematol. 84:18–22. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murata H, Hruz PW and Mueckler M: The
mechanism of insulin resistance caused by HIV protease inhibitor
therapy. J Biol Chem. 275:20251–20254. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nolte LA, Yarasheski KE, Kawanaka K,
Fisher J, Le N and Holloszy JO: The HIV protease inhibitor
indinavir decreases insulin- and contraction-stimulated glucose
transport in skeletal muscle. Diabetes. 50:1397–1401. 2001.
View Article : Google Scholar
|
|
5
|
Djedaini M, Peraldi P, Drici MD, et al:
Lopinavir co-induces insulin resistance and ER stress in human
adipocytes. Biochem Biophys Res Commun. 386:96–100. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wellen KE and Hotamisligil GS:
Inflammation, stress, and diabetes. J Clin Invest. 115:1111–1119.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weisberg SP, McCann D, Desai M, Rosenbaum
M, Leibel RL and Ferrante AW Jr: Obesity is associated with
macrophage accumulation in adipose tissue. J Clin Invest.
112:1796–1808. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dalpke A, Heeg K, Bartz H and Baetz A:
Regulation of innate immunity by suppressor of cytokine signaling
(SOCS) proteins. Immunobiology. 213:225–235. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ueki K, Kondo T and Kahn CR: Suppressor of
cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance
through inhibition of tyrosine phosphorylation of insulin receptor
substrate proteins by discrete mechanisms. Mol Cell Biol.
24:5434–5446. 2004. View Article : Google Scholar
|
|
10
|
Asante-Appiah E and Kennedy BP: Protein
tyrosine phosphatases: the quest for negative regulators of insulin
action. Am J Physiol Endocrinol Metab. 284:E663–E670.
2003.PubMed/NCBI
|
|
11
|
Tordjman KM, Leingang KA, James DE and
Mueckler MM: Differential regulation of two distinct glucose
transporter species expressed in 3T3-L1 adipocytes: effect of
chronic insulin and tolbutamide treatment. Proc Natl Acad Sci USA.
86:7761–7765. 1989. View Article : Google Scholar
|
|
12
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eron JJ, Feinberg J, Kessler HA, et al:
Once-daily versus twice-daily lopinavir/ritonavir in
antiretroviral-naive HIV-positive patients: a 48-week randomized
clinical trial. J Infect Dis. 189:265–272. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Curran A, Gutirerrez M, Deig E, et al:
Efficacy, safety and pharmacokinetics of 900/100 mg of
darunavir/ritonavir once daily in treatment-experienced patients. J
Antimicrob Chemother. 65:2195–2203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Patick AK and Potts KE: Protease
inhibitors as antiviral agents. Clin Microbiol Rev. 11:614–627.
1998.PubMed/NCBI
|
|
16
|
Bogachus LD and Turcotte LP: HIV protease
inhibitors induce metabolic dysfunction in part via increased
JNK1/2 pro-inflammatory signaling in L6 cells. Antiviral Res.
92:415–423. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun XJ and Liu F: Phosphorylation of IRS
proteins Yin-Yang regulation of insulin signaling. Vitam Horm.
80:351–387. 2009.PubMed/NCBI
|
|
18
|
Ismail WI, King JA, Anwar K and Pillay TS:
Indinavir and nelfinavir inhibit proximal insulin receptor
signaling and salicylate abrogates inhibition: potential role of
the NFkappa B pathway. J Cell Biochem. 114:1729–1737. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goldstein BJ, Bittner-Kowalczyk A, White
MF and Harbeck M: Tyrosine dephosphorylation and deactivation of
insulin receptor substrate-1 by protein-tyrosine phosphatase 1B.
Possible facilitation by the formation of a ternary complex with
the Grb2 adaptor protein. J Biol Chem. 275:4283–4289. 2000.
View Article : Google Scholar
|
|
20
|
Ben-Romano R, Rudich A, Tirosh A, et al:
Nelfinavir-induced insulin resistance is associated with impaired
plasma membrane recruitment of the PI 3-kinase effectors Akt/PKB
and PKC-zeta. Diabetologia. 47:1107–1117. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schütt M, Zhou J, Meier M and Klein HH:
Long-term effects of HIV-1 protease inhibitors on insulin secretion
and insulin signaling in INS-1 beta cells. J Endocrinol.
183:445–454. 2004.PubMed/NCBI
|
|
22
|
Noor MA, Flint OP, Maa JF and Parker RA:
Effects of atazanavir/ritonavir and lopinavir/ritonavir on glucose
uptake and insulin sensitivity: demonstrable differences in vitro
and clinically. AIDS. 20:1813–1821. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tomaka F, Lefebvre E, Sekar V, et al:
Effects of ritonavir-boosted darunavir vs. ritonavir-boosted
atazanavir on lipid and glucose parameters in HIV-negative, healthy
volunteers. HIV Med. 10:318–327. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Björnholm M, Kawano Y, Lehtihet M and
Zierath JR: Insulin receptor substrate-1 phosphorylation and
phosphatidylinositol 3-kinase activity in skeletal muscle from
NIDDM subjects after in vivo insulin stimulation. Diabetes.
46:524–527. 1997.PubMed/NCBI
|