Introduction
Budd-Chiari syndrome (BCS) is a diverse group of
conditions associated with obstruction of the hepatic vein or
inferior vena cava (IVC) within or above the liver. In western
countries, BCS is often caused by prothrombotic disorders; whilst
membranous or segmental obstruction of the IVC is the most common
cause of BCS in Asia (1–4). Following obstruction of the IVC,
collateral circulation may be developed via azygous, lumbothoracic,
intercostal, inferior phrenic and abdominal veins or the portal
venous system (5,6). However, blood within the occluded IVC
may also be drained to the right atrium by another route, the
cavo-hepato-atrial pathway. In the present study, the ultrasonic
features of the unusual blood-draining pathway were
investigated.
Materials and methods
Patients
This study was approved by the Ethics Committee of
Shandong Provincial Hospital of Shandong University (Jinan, China).
Informed consent was obtained from each patient prior to digital
subtraction angiography (DSA), and the protocol was in accordance
with the Declaration of Helsinki.
This retrospective study is based on the integrated
data of each patient. A total of 11 patients with BCS with IVC
obstruction and cavo-hepato-atrial pathways underwent ultrasonic
examinations between August 2004 and June 2013. This group of
patients comprised 7 males and 4 females aged between 35–73 years
(mean age, 49.82±12.15 years). All patients had chronic BCS and the
period from first clinical symptoms to diagnosis ranged between 5
months and 10 years. However, a number patients had already
suffered from the disease for several years without clear clinical
symptoms prior to seeing a doctor; therefore, the duration of the
disease could not be determined prior to ultrasonic examination.
All patients had primary BCS.
Three patients demonstrated symptoms of right upper
abdominal distention, and one patient had decreased appetite. The
remaining patients showed no overt symptoms and underwent checkups.
Physical examinations revealed hepatomegaly in five patients and no
evident signs of ascites, leg edema or superficial venous
dilatation in all patients. The laboratory tests revealed that the
liver functions of the patients were within the normal range.
Ultrasonic examination
Ultrasonography was performed using Logiq E9 (GE
Healthcare, Vienna, Austria) and Envisor HD (Philips Healthcare,
Andover, MA, USA) with multi-frequency convex transducers (3–5
MHz). All patients fasted for >8 h prior to examination. First,
the liver, the spleen and the IVC were observed in order to
investigate whether hepatomegaly, splenomegaly, expansion of the
portal vein and ascites were present. In the presence of hepatic
vein or IVC obstruction, the afflictions and the blood-draining
pathways were observed and recorded. Doppler ultrasound was used to
observe the direction of the flow and measure the velocity of the
blood-draining vessels. Blood flow in the draining vessels and the
collaterals was shown as blue, red or bicolored depending on flow
direction away from the transducer, towards the transducer or both.
For measurement, the Doppler angle between the axis of the Doppler
beam and that of the vein examined was always <60°.
Ultrasonography was performed 1–2 weeks prior to DSA. All patients
were confirmed by DSA.
Results
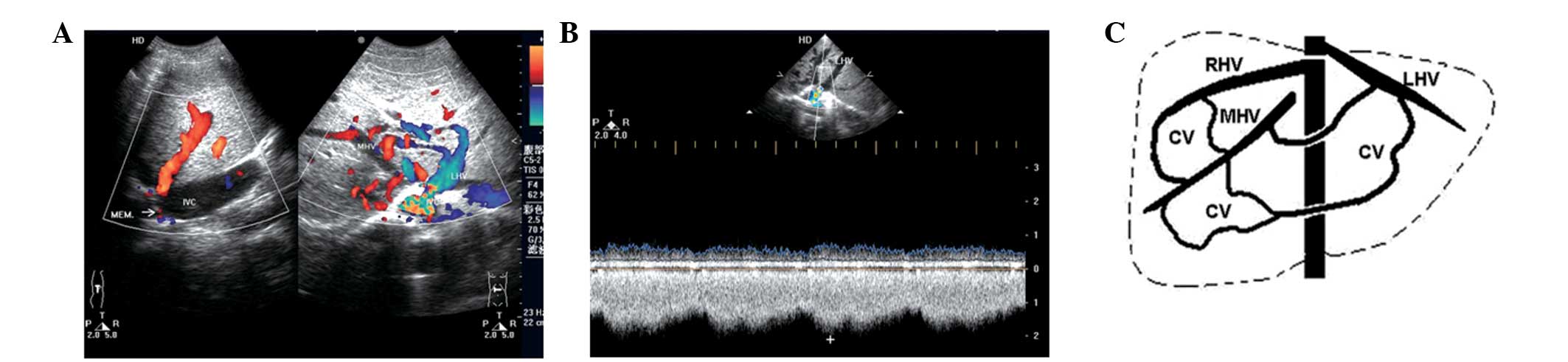
The ability to diagnose the cavo-hepato-atrial
pathway, an unusual collateral circulation with specific
hemodynamics in BCS, by ultrasonic examination was evaluated in the
present study. Ultrasonography was performed in 11 patients and the
results were retrospectively analyzed. Membranous and segmental
occlusions of IVCs were detected in seven and four cases,
respectively, and occluded hepatic veins were identified in all
patients with the exception of the draining hepatic or accessory
hepatic veins (including inferior hepatic and caudate veins) that
communicate with the IVC and the right atrium. The inlets of eight
hepatic veins, which drain to the right atrium, were found to be
narrow compared with the dilated distant parts of the lumens. The
narrowness was primarily caused by the membrane surrounding the
inlets. Blood flow from the IVC reversed to the hepatic/accessory
hepatic vein (orifice below occlusion) and then continued through
the dilated intrahepatic collaterals, onward to the other hepatic
vein (orifice above occlusion), and finally to the right atrium.
Accelerated blood flow in the inlets of draining hepatic veins to
the right atrium was detected in all patients regardless of the
luminal diameter of the inlets. All patients were associated with
at least one obstructed hepatic vein, and blood flowing to the
draining veins (Figs. 1 and
2; Table I). Minimal dilated azygous and
hemiazygous veins were only identified in 1 patient by DSA. These
results indicate that the cavo-hepato-atrial pathway is an unusual
collateral circulation with specific hemodynamics in BCS, which may
be diagnosed by ultrasonic examination.
 | Table IUltrasonographic descriptions of
Budd-Chiari syndrome with cavo-hepato-atrial pathways. |
Table I
Ultrasonographic descriptions of
Budd-Chiari syndrome with cavo-hepato-atrial pathways.
| | | | Draining vein to the
right atrium |
|---|
| | | |
|
|---|
| Patient no. | Age (years)/gender
(M/F) | Occlusion of IVC | Draining vein(s) from
IVC | Description | Status of inlet | Velocity of inlet
(cm/sec) |
|---|
| 1 | 73/M | Segmental | IRHV and CLV | RHV | Narrowing | 180 |
| 2 | 59/F | Membranous | IRHV and CLV | LHV | Narrowing | 115 |
| 3 | 35/M | Membranous | RHV | MHV | Narrowing | 131 |
| 4 | 58/M | Segmental | CLV | RHV | Patent | 63 |
| 5 | 44/M | Membranous | MHV | RHV | Narrowing | 154 |
| 6 | 51/M | Segmental | IRHV | MHV | Narrowing | 105 |
| 7 | 47/F | Membranous | RHV | MHV | Narrowing | 135 |
| 8 | 38/M | Membranous | RHV | LHV | Narrowing | 213 |
| 9 | 43/F | Membranous | IRHV | MHV | Patent | 87 |
| 10 | 63/M | Membranous | IRHV and CLV | LHV | Patent | 74 |
| 11 | 37/F | Segmental | IRHV | MHV | Narrowing | 144 |
Discussion
The cavo-hepato-atrial pathway is an unusual
blood-draining pathway of BCS, which results from IVC obstruction.
Compared with other collateral circulations (5,6),
this pathway is a direct route of drainage from the IVC to the
right atrium (7). The pathological
change is that blood pressure below the obstructed portion of the
IVC exceeds that of the hepatic veins. The continuously increasing
pressure in the IVC produces small anastomoses between adjacent
intrahepatic veins, which eventually develop into enlarged
collaterals (5). Therefore, the
hemodynamics of the IVC and hepatic/accessory hepatic veins change
accordingly. Blood from the IVC retrogradely flows to the hepatic
or accessory hepatic veins (orifice below occlusion), through the
enlarged intrahepatic collaterals and the draining hepatic veins
(orifice above occlusion), and finally to the right atrium. This
blood-draining mechanism relieves the symptoms caused by portal
hypertension and IVC hypertension (8,9), and
is also why no other collateral circulations were identified in the
majority of the patients investigated in the present study. In
addition, as shown in the literature, this blood-draining mode
efficiently compensates the IVC outflow to the heart, and treatment
of the obstruction of the IVC may be deemed unnecessary for
patients (9). Although the authors
of the present study are amenable to this view, they consider that
it may be necessary for certain patients to undergo angioplasty.
When short segmental (15 mm< occluded length ≤20 mm) or
membranous occlusions of the IVC exist and are easily managed by
interventional therapy, re-canalization of the IVC may bring the
hemodynamics back to normal and efficiently relieve the
hypertension of the IVC, the hepatic veins or accessory hepatic
veins. Although the inlets above the obstruction in certain
patients were relatively narrow, blood may be efficiently drained
into the IVC by other hepatic veins or accessory hepatic veins with
inlets below the obstruction following the surgery. Eight patients
with membranous or short segmental occlusion of IVC in the present
study underwent angioplasty for re-canalization of the IVC, and the
blood direction returned to normal. In addition, the velocity of
the inlets of the draining veins to the right atrium markedly
decreased. For patients with a long segmental occlusion (occluded
length >20 mm) of the IVC that is difficult to manage, follow-up
is required. The remaining three patients in the present study were
followed up for 1–5 years without surgery, and their ultrasonograms
and clinical manifestations did not change significantly. The
membrane that results in relative narrowness of the inlet may be
part of the IVC wall, which is derived from the IVC-wall-limited
dilatation of the inlet rather than the clearly expanded distant
lumen of the draining vein.
With the help of hemodynamics, ultrasonic
examination provides a convenient and accurate method for the
diagnosis of the rare pathway. The diagnosis is based on the
following conditions: i) obstruction of the IVC; ii) one hepatic
vein above the obstruction and another hepatic vein or accessory
hepatic vein below the obstruction are open to the IVC (in certain
cases the former hepatic vein is open directly to the right
atrium); iii) blood flow from the IVC reverses to the hepatic or
accessory hepatic vein, the intrahepatic collaterals, and the other
draining hepatic vein above the obstruction, and then flows into
the right atrium, with the intrahepatic communicating branches
receiving blood from the obstructed hepatic veins; and iv) angles
between the long axis of blood flow and the IVC may be observed due
to the existence of angulation between the long axis of the hepatic
vein and the IVC (Fig. 3).
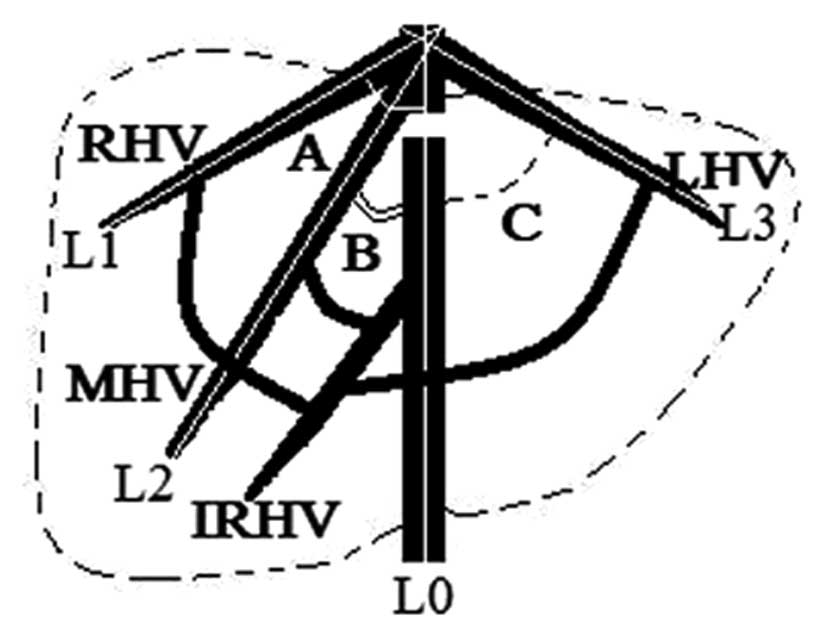 | Figure 3Diagram showing the angles between
long axes of the blood flow of the RHV (A, white arc), MHV (B,
double black arcs), LHV (C, dashed black arc) and IVC,
respectively. The white lines L0 to L3 indicate the long axes of
the blood flow of the IVC, RHV, MHV and LHV, respectively. RHV,
right hepatic vein; IVC, inferior vena cava; LHV, left hepatic
vein; MHV, middle hepatic vein; IRHV, inferior right hepatic
vein. |
In a previous study, we determined a number of
ultrasonic features of draining pathways in BCS (10). In the present study, further
investigation of the features of the cavo-hepato-atrial pathway was
conducted, which provided additional information for the diagnosis
of this rare pathway. In conclusion, the cavo-hepato-atrial pathway
is an unusual collateral circulation with specific hemodynamics in
BCS. Ultrasonic examination provides an accurate method for the
diagnosis of this rare pathway.
Acknowledgements
This study was supported by the Shandong Provincial
Science and Technology Development Project Foundation of China
(nos. 2012GSF11820 and 2013GSF11827).
References
|
1
|
Lim JH, Park JH and Auh YH: Membranous
obstruction of the inferior vena cava: comparison of findings at
sonography, CT, and venography. AJR Am J Roentgenol. 159:515–520.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sonin AH, Mazer MJ and Powers TA:
Obstruction of the inferior vena cava: a multiple-modality
demonstration of causes, manifestations, and collateral pathways.
Radiographics. 12:309–322. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Valla DC: The diagnosis and management of
the Budd-Chiari syndrome: consensus and controversies. Hepatology.
38:793–803. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Janssen HL, Garcia-Pagan JC, Elias E,
Mentha G, Hadengue A and Valla DC; European Group for the Study of
Vascular Disorders of the Liver. Budd-Chiari syndrome: a review by
an expert panel. J Hepatol. 38:364–371. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takayasu K, Moriyama N, Muramatsu Y, et
al: Intrahepatic venous collaterals forming via the inferior right
hepatic vein in 3 patients with obstruction of inferior vena cava.
Radiology. 154:323–328. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cho OK, Koo JH, Kim YS, Rhim HC, Koh BH
and Seo HS: Collateral pathways in Budd-Chiari syndrome: CT and
venographic correlation. AJR Am J Roentgenol. 167:1163–1167. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Redmond PL, Kadir S and Cameron JL:
Transhepatic venous collaterals in a patient with the Budd-Chiari
syndrome. Cardiovasc Intervent Radiol. 11:285–287. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kamba M, Ochi S, Ochi H, Maruyama S, Sato
H and Suto Y: Asymptomatic membranous obstruction of the inferior
vena cava forming intrahepatic collateral pathways. J
Gastroenterol. 30:783–785. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Akaki S, Kanazawa S, Gochi A, et al:
Asymptomatic membranous obstruction of the inferior vena cava due
to large intrahepatic collaterals. Cardiovasc Intervent Radiol.
18:403–405. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gai YH, Cai SF, Guo WB, Zhang CQ, Liang B,
Jia T and Zhang GQ: Sonographic classification of draining pathways
of obstructed hepatic veins in Budd-Chiari syndrome. J Clin
Ultrasound. 42:134–142. 2014. View Article : Google Scholar : PubMed/NCBI
|