Introduction
Traumatic brain injury (TBI) is caused by both
primary and secondary injury. Primary injury occurs from the forces
at the time of injury and is known to be irreversible. However, it
is the complex secondary mechanisms initiated at the time of trauma
that have an important role in the delayed progression of the brain
damage, which may present novel opportunities for therapeutic
strategies. One of the secondary injury processes that may promote
delayed neuronal death is post-traumatic inflammation, which has
been shown to increase blood-brain barrier (BBB) permeability,
cerebral edema and intracranial pressure, resulting in neuronal
dysfunction following TBI (1).
During post-traumatic inflammation, metabolic
products of arachidonic acid, known as prostanoids, including
prostaglandins, prostacyclin and thromboxanes, are released. These
aggravate the injury process and have a central role in the central
nervous system (CNS) in brain injury (2). Prostaglandin E2 (PGE2) synthesis is
regulated by cyclooxygenase (COX) that is present in at least two
isoforms, COX-1, the constitutive form, and COX-2, the inducible
form (3). COX-2 regulates the
critical metabolic step in the biosynthesis of PGE2, which is
considered to have a significant role in the inflammatory response
following TBI. In addition, nuclear factor κB (NF-κB) is an
important transcription factor complex that modulates the
expression of numerous genes involved in immune and inflammatory
responses, including COX-2 and tumor necrosis factor-α (TNF-α),
which are considered to be significant in secondary injury
(4). Following TBI, the
overproduction of inflammatory mediators within the injured brain,
including PGE2, COX-2, NF-κB and TNF-α, are believed to contribute
to the cerebral damage, cell death and BBB dysfunction (5). Therefore, inflammation is a
significant contributor to secondary injury, and control of the
inflammatory response benefits BBB and neurological outcome.
Progesterone is considered to have a neuroprotective
effect, and this has been demonstrated in a variety of animal
models, including ischemic and traumatic brain insult models
(6). Previous studies have shown
that post-injury treatment with progesterone decreases brain edema
(7), attenuates free radical
damage (8), reduces neuronal loss
(9), inhibits the inflammatory
response (10), restores BBB
function (11), increases the
levels of endothelial progenitor cells (12) and promotes behavioral recovery
(13). In addition, the
neuroprotective effect has been confirmed in clinical trials in
which progesterone treatment was administered following TBI
(14,15). Due to these promising results, a
large multicenter phase III clinical trial (ProTECT III) is being
conducted in order to further assess the safety and efficacy of
this treatment for adults with moderate to severe acute TBI
(16). Previous studies have
demonstrated that progesterone reduces BBB degeneration (8,11,17–19);
however, the mechanism by which progesterone mediates its
neuroprotective effects on the BBB has yet to be elucidated. A
number of studies have demonstrated that the protective effect of
progesterone BBB is associated with reductions of lipid
peroxidation and matrix metalloproteinase (MMP) expression
(8,11,17–19).
Since inflammatory mediators, including PGE2, COX-2, NF-κB and
TNF-α, following TBI are associated with the disruption of the BBB,
it was hypothesized in the present study that progesterone may
promote BBB recovery from TBI by reducing the expression levels of
inflammatory mediators. Therefore, the present study investigated
whether progesterone inhibits the expression of the important
inflammatory molecules PGE2, COX-2, NF-κB and TNF-α following TBI
in rats. The ability of progesterone to protect BBB function,
reduce brain edema and improve neurological function in the
brain-injured rats was also evaluated.
Materials and methods
Animals and groups
A total of 90 adult male Sprague-Dawley rats
(weighing between 250 and 300 g) were purchased from the Beijing
Experimental Animal Center (Beijing, China). The animals were
housed in a light controlled room under a 12 h light-dark cycle and
maintained at a temperature of 25°C. Animals were given
unrestricted access to food and water. All procedures were approved
by the Animal Care Committee of Hebei United University (Tangshan,
China) and were in accordance with the guidelines of the National
Institutes of Health (Bethesda, MD, USA) on the care and use of
animals.
The rats were randomly divided into three
experimental groups: a sham-operated group (SHAM), a TBI group
(TBI) and a progesterone treatment group (TBI-PROG), with 30 rats
in each group. Animals in each group were assigned to subgroups for
immunohistochemical analysis, enzyme-linked immunosorbent assay
(ELISA), detection of Evan’s blue (EB) dye extravasation,
determination of brain water content and modified neurological
severity score (n=18 per assay; 6 rats from each group).
TBI model
The TBI model was established on experimental rats
by modified Feeney’s weight-dropping method, as previously
described (20). In this method,
the rats were anesthetized with an intraperitoneal injection of 40
mg/kg sodium pentobarbital. A right parietal craniotomy (5 mm
diameter) was drilled under aseptic conditions, 1 mm posteriorly
and 2 mm laterally to the bregma. A steel rod (weighing 40 g) with
a flat end diameter of 4 mm was allowed to fall from a height of 25
cm onto a piston resting on the exposed intact cranial dura. Rats
in the SHAM group were subjected to skull fenestration without
brain injury. Rats in the TBI-PROG group received injections of
progesterone (Sigma, St Louis, MO, USA), in accordance a previous
study (21). Progesterone was
dissolved in 22.5% 2-hydroxypropyl-β-cyclodextrin and administered
at a dose of 16 mg/kg by intraperitoneal injection 1 h post injury
to ensure more rapid absorption following TBI. Subsequent
injections of 16 mg/kg were administered subcutaneously 6 h and 12
h following TBI. Rats in the SHAM and TBI groups received
equivalent volumes of 2-hydroxypropyl-β-cyclodextrin at the same
time following the surgery.
Immunohistochemistry
The animals were decapitated 24 h following injury
for tissue assays. The rats were anesthetized and perfused through
the heart with 4% paraformaldehyde. Brains were carefully removed
and immersed in fixative. A 3 mm-thick coronal slice of each brain,
containing the area surrounding the injury site, was dehydrated and
embedded in paraffin. Coronal sections of the brain (10 μm
thickness) were cut and rinsed in phosphate-buffered saline (PBS).
The sections were incubated in PBS-Triton (PBS-T) containing 0.1%
H2O2 for 30 min to block the endogenous
peroxidase. The sections were rinsed three times for 5 min in PBS-T
and incubated with 1.5% normal goat serum for 30 min. The sections
were then incubated overnight (4°C) using anti-COX-2 (Santa Cruz
Biotechnology, Santa Cruz, CA, USA) or anti-NF-κB (Santa Cruz
Biotechnology) primary antibodies, diluted 1:100 with PBS.
Following extensive rinsing steps in PBS, sections were reincubated
in biotinylated goat anti-rabbit antibody (Zhongshan Biotechnology
Co., Ltd., Beijing, China) for 1 h at room temperature. The
immunocomplex was visualized using diaminobenzidine as a chromogen
in a reaction with peroxidase. For negative controls, the primary
antibody was omitted.
Enzyme-linked immunosorbent assay
(ELISA)
The animals were decapitated 24 h after brain
injury. The brain tissues were isolated from the skull and washed
with ice-cold physiological saline to remove surface blood. The
cortex was then separated, weighed and placed in a homogenizer. The
tissue was homogenized with 1 ml ice-cold physiological saline per
100 mg brain tissue. Hypothermal centrifugation was performed at
302 × g for 10 min, and the supernatant was obtained. An ELISA kit
(Shanghai Saimo Biotechnology, Shanghai, China) on a microplate
reader (Hyperion MR III; Hyperion Inc., Miami, FL, USA) was used to
determine the levels of PGE2 and TNF-α.
Detection of EB dye extravasation
The BBB permeability was determined by EB
extravasation 24 h following TBI. A dose of 2 ml/kg 2% EB was
injected intravenously through the tail vein. Animals were
anesthetized after 1 h and perfused using saline to remove
intravascular EB dye. Animals were then decapitated, the brains
removed and homogenized in PBS. The protein was precipitated by
adding trichloroacetic acid, and the samples were cooled and
centrifuged. The resulting supernatant was measured for absorbance
of EB at 610 nm using a spectrophotometer (model VIS722; Aoxi,
Shanghai, China).
Determination of brain water content
Coronal sections of the frontal cortex of the brain
(4 mm thick) were weighed to obtain the wet weight, placed in an
oven at 90°C for 2 days and then weighed again to obtain the dry
weight. The formula used to calculate the water content was as
follows: Brain water (%) = [(wet weight-dry weight)/wet weight] ×
100.
Modified neurological severity score
(mNSS)
The neurological function of the rats was assessed
using the mNSS test (22), which
includes motor, sensory, reflex and balance tests. In the mNSS
test, the neurological function was graded on a scale of 0–18. The
test was performed 24 h following TBI in a blinded manner. The
higher the score, the more severe the injury. A score of 0
indicates no neurological deficit, whilst a score of 18 indicates
the most severe impairment.
Statistical analysis
All results are expressed as mean ± standard error
of the mean. SPSS software, version 16.0 was used for statistical
analysis of the data (SPSS, Inc., Chicago, IL, USA). A one-way
analysis of variance was performed to determine the differences
among the groups followed by a least significance difference in
order to compare the differences between groups. P<0.05 was
considered to indicate a statistically significant result.
Results
Progesterone treatment inhibits COX-2 and
NF-κB expression
Immunohistochemical analysis revealed that the
expression levels of COX-2 and NF-κB in the cortex were low in the
SHAM group. Following TBI, significant increases in COX-2 and NF-κB
expression levels were observed in the cortex of rats in the TBI
group compared with those in the SHAM group (P<0.05). The
expression levels of COX-2 and NF-κB were decreased by progesterone
administration in the TBI-PROG group compared with those in the TBI
group (P<0.05; Fig. 1).
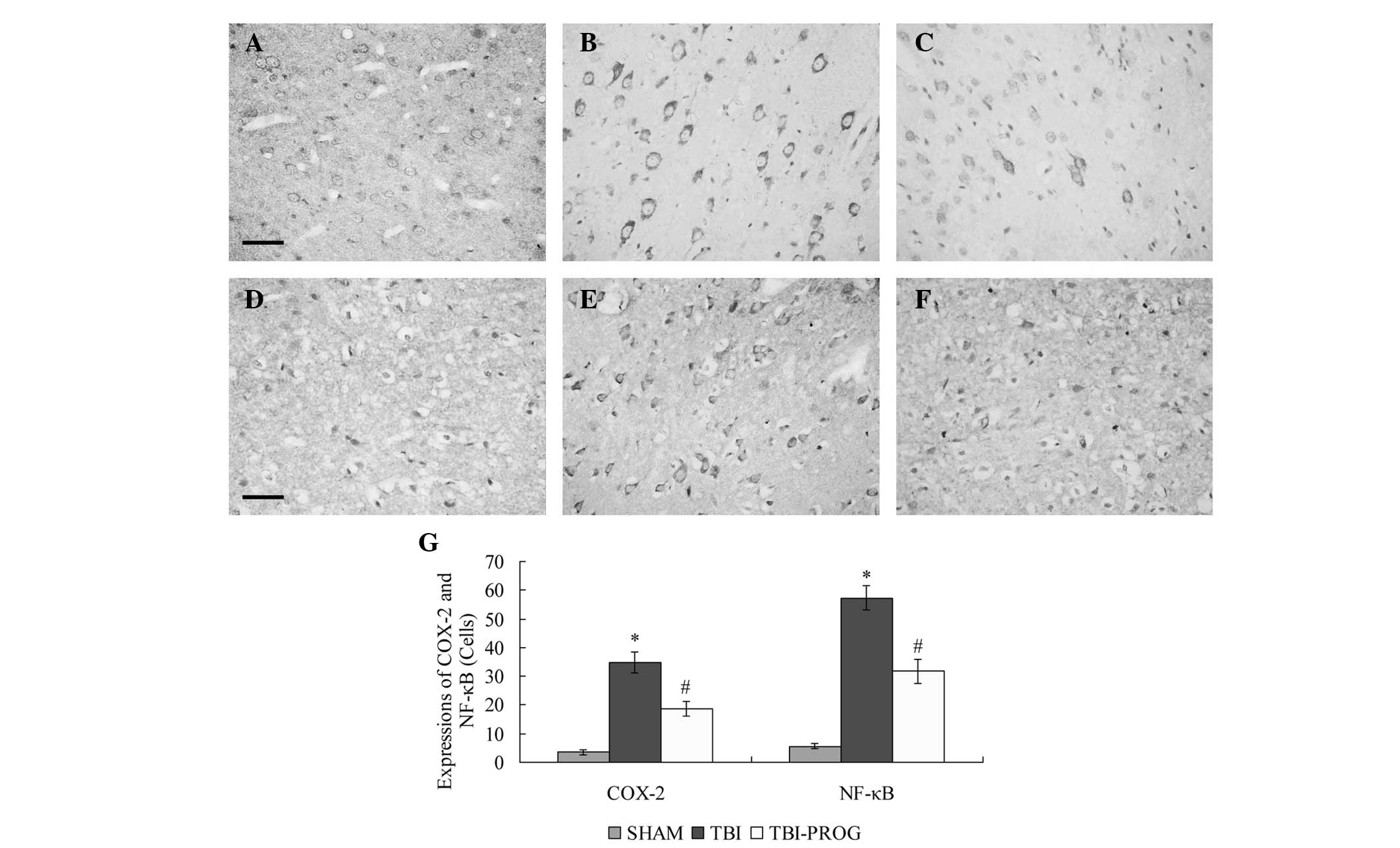 | Figure 1Immunohistochemical analysis of COX-2
and NF-κB in the cortex of rats in the SHAM, TBI and TBI-PROG
groups. Immunohistochemistry of (A–C) COX-2 and (D–F) NF-κB
expression. (A, D) The SHAM group showed few positive cells, (B,E)
The TBI group showed strongly stained positive cells, and (C, F)
the TBI-PROG group showed fewer positive cells compared with the
TBI group (scale bar, 50 μm; magnification, ×400). (G)
Administration of progesterone significantly inhibited the
TBI-induced upregulation of COX-2 and NF-κB expression in the
cortex (n=6/group); *P<0.05, compared with the SHAM
group; #P<0.05, compared with the TBI group. COX-2,
cyclooxygenase 2; NF-κB, nuclear factor κB; TBI, traumatic brain
injury, PROG, progesterone. |
Progesterone treatment decreases PGE2 and
TNF-α levels
The expression levels of PGE2 and TNF-α were low in
the brains of rats in the SHAM group. Compared with those in the
SHAM group, the PGE2 and TNF-α levels were markedly increased
following TBI (P<0.05), whilst the administration of
progesterone significantly decreased the PGE2 and TNF-α expression
levels compared with those in the TBI group (P<0.05; Table I).
 | Table IEffects of PROG on PGE2, TNF-α,
blood-brain barrier permeability and WC in the cerebral cortex
following TBI in rats. |
Table I
Effects of PROG on PGE2, TNF-α,
blood-brain barrier permeability and WC in the cerebral cortex
following TBI in rats.
| Group | PGE2 (ng/g) | TNF-α (ng/g) | EB (μg/g) | WC (%) |
|---|
| SHAM | 2.3±0.51 | 0.6±0.18 | 3.2±0.54 | 74.3±0.92 |
| TBI | 7.1±0.94a | 1.7±0.32a | 14.6±1.79a | 82.6±2.13a |
| PROG | 4.5±0.68b | 1.2±0.29b | 8.3±1.87b | 78.4±1.47b |
Progesterone treatment decreases BBB
permeability
Low EB extravasation was observed in rats in the
SHAM group. However, rats in the TBI group demonstrated a
significant increase in BBB permeability compared with that of the
rats in the SHAM group (P<0.05). Progesterone significantly
inhibited EB extravasation in the animals with brain injury
(P<0.05; Table I).
Progesterone treatment reduces brain
water content
The brain water content in the TBI group was
significantly increased compared with that in the SHAM group
(P<0.05). Following progesterone administration, the brain water
content in the TBI-PROG group was significantly reduced compared
with that in the TBI group (P<0.05; Table I).
Progesterone treatment improves
neurological outcome
The mNSSs for rats in the SHAM group were low
(0.5±0.54). The mNSSs for rats in the TBI-PROG group (10.5±1.37)
were significantly lower following TBI compared with those of rats
in the TBI group (14.8±2.75; P<0.05). These results indicate
that progesterone treatment improves the neurological functional
outcome following TBI in rats.
Discussion
In the present study, it was demonstrated that TBI
results in an increased expression of PGE2, COX-2, NF-κB and TNF-α
in the cortex in rats, and the increase in these inflammatory
mediators in the brain following TBI was associated with BBB
dysfunction, brain edema and the development of functional
deficits. Progesterone was observed to significantly reduce
post-injury inflammatory response, BBB permeability and cerebral
edema, as well as improve functional outcome.
Inflammatory processes are considered major
components of the secondary injury cascade following TBI (23). PGE2, the metabolic product of
arachidonic acid, is a key regulator. Overexpression of PGE2
produced by COX-2 is an important determinant of the cytotoxicity
associated with inflammation following injury to the brain
(24). PGE2 may affect
pathological processes through the modulation of glutamate release,
cerebral vasoconstriction and neuroendocrine function (24). Furthermore, PGE2 has been shown to
be associated with the generation of highly reactive oxygen species
(ROS), which have potential deleterious effects on lipids, proteins
and DNA, and lead to a breakdown of the BBB following TBI (25). In addition, NF-κB is an upstream
regulator of inflammation that activates TBI-induced inflammatory
molecules, including COX-2 and TNF-α (26). TNF-α is involved in BBB injury and
edema formation through a mechanism involving MMP upregulation
(11). In combination, this
indicates that secondary inflammation exacerbates BBB dysfunction
following TBI.
Progesterone has been shown to be a potent
antagonist of CNS inflammation and cerebral edema following TBI
(6). Previous studies in aged rats
showed that progesterone decreases acute inflammation by preventing
the expression of inflammatory mediators, including COX-2, IL-6 and
NF-κB following TBI (27). Pettus
et al (28) suggested that
progesterone administered following TBI may reduce the initial
cytotoxic surge of inflammatory factors, including complement
factor C3, glial fibrillary acidic protein and NF-κB. Progesterone
may also reduce the injury-induced expression of inflammatory
mediators interleukin-1 β (IL-1β) and TNF-α (29). The fact that progesterone reduces
inflammation following TBI may also contribute to the widely
observed beneficial effects of the hormone on edema (28). Administration of progesterone
following brain injury has been shown to attenuate edema, even when
the progesterone treatment was delayed for up to 24 h following
injury (30). In the present
study, it was found that progesterone treatment inhibited the
expression of the inflammatory mediators PGE2, COX-2, NF-κB and
TNF-α that accompanied TBI, and it was observed that brain edema in
the injured rat brain following TBI was decreased by progesterone
administration, which is in agreement with previous studies
(27–29).
The protective effect of progesterone on the BBB has
been previously investigated and a number of studies have suggested
that treatment with progesterone following TBI attenuates BBB
permeability following brain injury (8,11,17–19).
Ishrat et al (19) studied
the changes of MMPs and inflammation following permanent middle
cerebral artery occlusion (pMCAO). The authors found that ischemic
injury significantly increased the expression levels of MMP-9,
MMP-2, TNF-α and IL-6, and EB extravasation, but progesterone
attenuated BBB disruption by reducing MMP levels and the
inflammatory response. A previous study has also shown BBB leakage
to be significantly decreased in progesterone-treated animals
following TBI, and this decrease was accompanied by a reduction in
lipid peroxidation (8). Although
previous studies have demonstrated that the protective effect of
progesterone on BBB may be associated with a reduction of lipid
peroxidation, reduction of MMP expression and attenuation of TNF-α
and IL-6 levels, little is known about the effect of progesterone
on BBB permeability and the expression levels inflammatory
mediators COX-2, PGE2 and NF-κB in the brain. In the present study,
it was demonstrated that TBI in rats results in reductions in the
expression levels of PGE2, COX-2, NF-κB and TNF-α in the cortex, as
well as BBB dysfunction, and that the administration of
progesterone significantly reduces the expression levels of these
inflammatory mediators. Inflammation is known to contribute to
secondary BBB damage, which may explain the mechanism of the effect
of progesterone on the BBB. In addition, in the present study it
was also demonstrated using the mNSS test, which is a
well-established scoring system to determine the neurologic
performance of rats following brain injury (22), that the administration of
progesterone is able to enhance functional recovery. Since BBB
disruption is considered to be associated with the development of
neurological deficits following TBI (31), the improvement of neurological
function by progesterone may be linked with the protection of BBB
function.
In conclusion, the present study demonstrated that
progesterone treatment is able to significantly inhibit the
inflammatory response, attenuate BBB disruption and brain edema and
improve functional recovery following traumatic brain injury. Since
the inflammatory response is a cause of considerable secondary BBB
damage following brain trauma, the inhibition of inflammation may
be another crucial mechanism by which progesterone protects the BBB
and improves neurological outcome.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (81201048) and the Natural Science
Foundation of Hebei Province (H2012401009), China.
References
|
1
|
Kumar A and Loane DJ: Neuroinflammation
after traumatic brain injury: opportunities for therapeutic
intervention. Brain Behav Immun. 26:1191–1201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Knöferl MW, Diodato MD, Schwacha MG,
Cioffi WG, Bland KI and Chaudry IH: Cyclooxygenase-2-mediated
regulation of Kupffer cell interleukin-6 production following
trauma-hemorrhage and subsequent sepsis. Shock. 16:479–483.
2001.PubMed/NCBI
|
|
3
|
Vane JR, Bakhle YS and Botting RM:
Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 38:97–120.
1998. View Article : Google Scholar
|
|
4
|
Morganti-Kossmann MC, Satgunaseelan L, Bye
N and Kossmann T: Modulation of immune response by head injury.
Injury. 38:1392–1400. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Allan SM and Rothwell NJ: Inflammation in
central nervous system injury. Philos Trans R Soc Lond B Biol Sci.
358:1669–1677. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deutsch ER, Espinoza TR, Atif F, Woodall
E, Kaylor J and Wright DW: Progesterone’s role in neuroprotection,
a review of the evidence. Brain Res. 1530:82–105. 2013.
|
|
7
|
Shahrokhi N, Khaksari M, Soltani Z,
Mahmoodi M and Nakhaee N: Effect of sex steroid hormones on brain
edema, intracranial pressure, and neurologic outcomes after
traumatic brain injury. Can J Physiol Pharmacol. 88:414–421. 2010.
View Article : Google Scholar
|
|
8
|
Roof RL, Hoffman SW and Stein DG:
Progesterone protects against lipid peroxidation following
traumatic brain injury in rats. Mol Chem Neuropathol. 31:1–11.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Djebaili M, Hoffman SW and Stein DG:
Allopregnanolone and progesterone decrease cell death and cognitive
deficits after a contusion of the rat pre-frontal cortex.
Neuroscience. 123:349–359. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grossman KJ, Goss CW and Stein DG: Effects
of progesterone on the inflammatory response to brain injury in the
rat. Brain Res. 1008:29–39. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang J, Jiang C, Li X, Liu C, Cheng N and
Hao Y: The protective mechanism of progesterone on blood-brain
barrier in cerebral ischemia in rats. Brain Res Bull. 79:426–430.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Z, Wang B, Kan Z, Zhang B, Yang Z, Chen
J, et al: Progesterone increases circulating endothelial progenitor
cells and induces neural regeneration after traumatic brain injury
in aged rats. J Neurotrauma. 29:343–353. 2012. View Article : Google Scholar
|
|
13
|
Wali B, Sayeed I and Stein DG: Improved
behavioral outcomes after progesterone administration in aged male
rats with traumatic brain injury. Restor Neurol Neurosci. 29:61–71.
2011.PubMed/NCBI
|
|
14
|
Wright DW, Kellermann AL, Hertzberg VS,
Clark PL, Frankel M, Goldstein FC, et al: ProTECT: a randomized
clinical trial of progesterone for acute traumatic brain injury.
Ann Emerg Med. 49:391–402. 402:2007.PubMed/NCBI
|
|
15
|
Xiao G, Wei J, Yan W, Wang W and Lu Z:
Improved outcomes from the administration of progesterone for
patients with acute severe traumatic brain injury: a randomized
controlled trial. Crit Care. 12:R612008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
ProTECT III. Progesterone for the
Treatment of Traumatic Brain Injury (ProTECT III). http://clinicaltrials.gov/ct2/show/record/NCT00822900uri.
Accessed November 14, 2013
|
|
17
|
Wang X, Zhang J, Yang Y, Dong W, Wang F,
Wang L and Li X: Progesterone attenuates cerebral edema in neonatal
rats with hypoxic-ischemic brain damage by inhibiting the
expression of matrix metalloproteinase-9 and aquaporin-4. Exp Ther
Med. 6:263–267. 2013.PubMed/NCBI
|
|
18
|
Shahrokhi N, Haddad MK, Joukar S, Shabani
M, Keshavarzi Z and Shahozehi B: Neuroprotective antioxidant effect
of sex steroid hormones in traumatic brain injury. Pak J Pharm Sci.
25:219–225. 2012.PubMed/NCBI
|
|
19
|
Ishrat T, Sayeed I, Atif F, Hua F and
Stein DG: Progesterone and allopregnanolone attenuate blood-brain
barrier dysfunction following permanent focal ischemia by
regulating the expression of matrix metalloproteinases. Exp Neurol.
226:183–190. 2010. View Article : Google Scholar
|
|
20
|
Feeney DM, Boyeson MG, Linn RT, Murray HM
and Dail WG: Responses to cortical injury: I. Methodology and local
effects of contusions in the rat. Brain Res. 211:67–77. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goss CW, Hoffman SW and Stein DG:
Behavioral effects and anatomic correlates after brain injury: a
progesterone dose-response study. Pharmacol Biochem Behav.
76:231–242. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M
and Chopp M: Therapeutic benefit of intravenous administration of
bone marrow stromal cells after cerebral ischemia in rats. Stroke.
32:1005–1011. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Israelsson C, Bengtsson H, Kylberg A,
Kullander K, Lewén A, Hillered L and Ebendal T: Distinct cellular
patterns of upregulated chemokine expression supporting a prominent
inflammatory role in traumatic brain injury. J Neurotrauma.
25:959–974. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Buccellati C, Folco GC, Sala A, Scelsi R,
Masoero E, Poggi P, et al: Inhibition of prostanoid synthesis
protects against neuronal damage induced by focal ischemia in rat
brain. Neurosci Lett. 257:123–126. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Beaumont A, Marmarou A, Fatouros P and
Corwin F: Secondary insults worsen blood brain barrier dysfunction
assessed by MRI in cerebral contusion. Acta Neurochir Suppl.
81:217–219. 2002.PubMed/NCBI
|
|
26
|
Ghosh S and Karin M: Missing pieces in the
NF-kappaB puzzle. Cell. 109(Suppl): S81–S96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cutler SM, Cekic M, Miller DM, Wali B,
VanLandingham JW and Stein DG: Progesterone improves acute recovery
after traumatic brain injury in the aged rat. J Neurotrauma.
24:1475–1486. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pettus EH, Wright DW, Stein DG and Hoffman
SW: Progesterone treatment inhibits the inflammatory agents that
accompany traumatic brain injury. Brain Res. 1049:112–119. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He J, Evans CO, Hoffman SW, Oyesiku NM and
Stein DG: Progesterone and allopregnanolone reduce inflammatory
cytokines after traumatic brain injury. Exp Neurol. 189:404–412.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roof RL, Duvdevani R, Heyburn JW and Stein
DG: Progesterone rapidly decreases brain edema: treatment delayed
up to 24 hours is still effective. Exp Neurol. 138:246–251. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McIntosh TK, Smith DH, Meaney DF, Kotapka
MJ, Gennarelli TA and Graham DI: Neuropathological sequelae of
traumatic brain injury: relationship to neurochemical and
biomechanical mechanisms. Lab Invest. 74:315–342. 1996.PubMed/NCBI
|















