Introduction
Continuous subcutaneous insulin infusion (CSII)
therapy with an external pump and multiple daily injection (MDI)
therapy are two of the currently selected methods of insulin
treatment for diabetes. MDI therapy for diabetes requires bolus
injections of rapid or short-acting insulin prior to each meal and
a long-acting insulin injection once or twice per day for basal
insulin coverage. Control of postprandial glycemia with
rapid-acting insulin has been shown to be more effective than that
with normal human insulin (1,2).
Long-acting insulin, such as insulin glargine, is suitable as a
basal insulin therapy in diabetes (3,4).
CSII therapy produces a higher efficacy than MDI and improvements
in insulin pump technology have resulted in an increase in patient
preference (5–10). However, occasionally patients may
have to temporarily discontinue CSII therapy due to skin problems,
pump malfunction or physical activity. It has been shown that MDI
therapy is at least equivalent in its action to CSII (10).
Type 2 diabetes mellitus (T2DM) is characterized by
a progressive reduction in β-cell secretion of insulin and mass
together with insulin resistance (11). The incidence rate of T2DM has been
reported to be 9.7 % in China (12) and Chinese patients with T2DM have
shown more significant β-cell deterioration than patients of other
genealogies (13). Insulin
preparation and medication have undergone rapid progress; however,
the amount of insulin injected falls short of what is
physiologically secreted in individuals without T2DM. Furthermore,
insulin therapy may cause weight increases, hypoglycemia and
iatrogenic hyperinsulinemia, which can increase insulin resistance
and the potential risks of vascular disease (14). High doses of insulin can induce
insulin resistance in diabetic rats, whilst intermediate doses can
maximally improve insulin sensitivity (10); therefore, studies investigating the
optimal insulin dosage are required. Numerous investigations in
patients with type 1 diabetes mellitus (T1DM) have shown that CSII
therapy was more efficacious than MDI therapy (15,16),
since CSII decreased the dose of insulin required to a greater
degree (17–19). Phillip et al (20) demonstrated that the higher dose of
insulin required with MDI in patients with T1DM, the more marked
the insulin decrease subsequent to switching to CSII. Conversely,
Monami et al (21) showed
by meta-analysis of insulin replacement in patients with T2DM that
the daily dose of insulin with CSII was not significantly different
from that with MDI therapy. Similarly, a study of Indian patients
with T2DM showed no significant differences in the total daily dose
of insulin when therapy was transitioned from MDI to CSII for six
months (22).
The dysfunction of β cells has been found to be a
major contributing factor in Chinese patients with T2DM (13); therefore, insulin remains the first
choice of therapy. However, as previously discussed, this treatment
may result in weight increase, hypoglycemia and iatrogenic
hyperinsulinemia, thus increasing the potential risks of insulin
resistance, vascular disease and sleep apnea syndrome (14,23–26).
Bruttomesso et al (15,17)
and Hoogma et al (27)
found that patients with T1DM using CSII required a lower insulin
dosage, as compared with those using MDIs. In the present study, it
was hypothesized that insulin dosage adjustment may also improve
therapy for patients with T2DM. In China, MDI therapy of bolus
insulin aspart and basal insulin glargine and CSII are mainly used
for glycemic control and treatment of T2DM in hospitalized patients
with T2DM. There currently have not been enough relevant
investigations demonstrating the optimal insulin dosage in Chinese
patients with T2DM. In this study, the insulin dosage
characteristics of 200 hospitalized patients with T2DM who were
treated with CSII and MDI therapy were investigated.
Patients and methods
Subjects
Two hundred patients with T2DM (80 females and 120
males) were recruited for this study. All subjects were
hospitalized between January 2011 and July 2013 in the Department
of Endocrinology at Linyi People’s Hospital (Linyi, China). The
diagnosis of T2DM was established on the basis of the 1999 Diabetes
Diagnostic Criteria of the World Health Organization. Subjects
hospitalized for insulin treatment due to poor glycemic control and
newly diagnosed patients with high blood glucose were enrolled.
Subjects prescribed other oral hypoglycemia agents, with the
exception of metformin, were excluded and none of the subjects
received basal insulin regimen intensification prior the
antidiabetic treatment.
Subjects with impaired renal or hepatic function and
those who were pregnant or breast-feeding, had malignancies,
impaired cardiac function, hypoglycemia unawareness, acute
infection or acute complications (such as diabetic nonketotic
hyperosmolar coma, diabetic ketoacidosis and diabetic lactic
acidosis) were excluded from the study. Investigators and patients
were blinded to the therapy sequence up to the point of subject
randomization. Subjects were randomly divided into a CSII or MDI
group, according to their hospitalization number. One hundred
subjects (60 males and 40 females) were randomly assigned to
continued therapy with CSII and 100 subjects (60 males and 40
females) to therapy with MDI for 12 weeks. Subjects in the MDI
group were administered insulin aspart immediately prior to each
meal and then insulin glargine before bed. In the CSII group, all
patients received insulin; 32 had peripheral neuropathy, 18 had
retinopathy and six had large vessel diseases. In the MDI group,
all patients received insulin; 31 subjects had peripheral
neuropathy, 18 had retinopathy and seven had large vessel
diseases.
Ethics statement
The investigation was approved by the Medical Ethics
Committee of Linyi People’s Hospital. All subjects were patients
admitted to the Department of Endocrinology and all patients signed
a consent form allowing their information to be stored in the
hospital database and used for this study. The consent form was
approved by the Medical Ethics Committee of Linyi People’s
Hospital.
Investigation design and method
No oral hypoglycemic drugs, with the exception of
metformin, were used during the insulin treatment in the present
study. The initial dosage for all subjects was 0.3–0.4 IU/kg/day.
The MDI group was treated with insulin aspart (Novo Nordisk,
Bagsværd, Denmark) injected subcutaneously prior to each of the
three meals in the day, as well as a single basal bedtime injection
of insulin glargine (Lantus®, Sanofi-Aventis
Pharmaceuticals, Mumbai, India) daily, for 12 weeks. The initial
glargine-based insulin injections accounted for 60% of the total
daily dose and the insulin aspart accounted for 40%. The CSII group
was treated with insulin aspart using insulin pumps (Medtronic,
Northridge, CA, USA). In the CSII group, the initial basal dose,
which accounted for 60% of the total daily amount, was divided into
the following four periods of the day: midnight-4:00 a.m.,
4:00–9:00 a.m., 9:00 a.m.-9:00 p.m. and 9:00 p.m.-midnight. The
three pre-meal doses together accounted for 40% of the total daily
dose and were administered for 12 weeks. Blood glucose levels were
monitored using a stable blood glucose-monitoring device from
LifeScan (Johnson & Johnson Company, Milpitas, CA, USA). Blood
glucose levels were measured from finger-stick blood samples eight
times per day (before each of the three meals, 2 h later and at
10:00 pm and 3:00 am). The diet of all subjects was regulated
according to the China Guideline for T2DM. The basal insulin dose
was preferentially adjusted to control the blood glucose when the
pre-meal blood glucose level was >9 mmol/l or the postprandial
glucose (PBG) was <6 mmol/l according to the features of the PBG
state in patients with T2DM (15,26).
Pre-meal doses were distributed evenly among the three meals. Basal
insulin doses were titrated to target fasting glucose between 4.0
and 7.0 mmol/l. Pre-meal insulin doses were adjusted according to
2-h PBG levels to achieve the target of ≤11.0 mmol/l. If the
subject achieved two consecutive days at this level, the length of
time required to achieve the target, total daily insulin doses,
daily basal insulin doses, blood glucose fluctuations and
hypoglycemic episodes were calculated. Hypoglycemic episodes were
classified as ‘severe hypoglycemia’ when patients were not able to
treat the episode themselves and blood glucose was ≤3.9 mmol/l,
‘symptomatic hypoglycemia’ when patients were able to treat the
episode and blood glucose was ≤3.9 mmol/l, and ‘relative
hypoglycemia’ when patients exhibited symptoms of hypoglycemia but
blood glucose was either >3.9 mmol/l or not measured (28). Hypoglycemic episodes were evaluated
as all events (all episodes occurring over a 24-h period) and
nocturnal events (episodes occurring between 11:00 p.m. and 6:00
a.m.). Once glycemic control was stabilized for 3 days, the daily
insulin dose was calculated when the pre-meal glucose and PBG were
<7.01 and 11.09 mmol/l, respectively. The state of hypoglycemia
was defined as blood glucose levels ≤3.9 mmol/l or where symptoms
of hypoglycemia resolved with administration of oral carbohydrates,
and a decrease in basal insulin or regular insulin dose according
to pre-meal glucose and PBG.
Statistical analysis
All statistical data analyses were performed using
SPSS version 13. (SPSS, Inc., Chicago, IL, USA). Descriptive data
analyses of the qualitative variables were performed with
proportions and percentages. Quantitative variables are presented
as the mean ± standard deviation. The variables were analyzed with
the independent samples t-test between the CSII and MDI groups. A
value of P<0.05 was considered to be statistically
significant.
Results
Clinical data
No statistically significant differences were
observed between the CSII and MDI groups in gender, age, hemoglobin
A1c (HbA1c), fasting serum C-peptide, body mass index, fasting
blood glucose (FBG) or other clinical data (P>0.05) (Table I).
 | Table IDemographic characteristics of the
subjects. |
Table I
Demographic characteristics of the
subjects.
| Group |
|---|
|
|
|---|
| Parameter | MDI | CSII |
|---|
| Male/female
(n/n) | 60/40 | 60/40a |
| Newly diagnosed
patients (n) | 36 | 36a |
| Duration of diabetes
(years) | 6.78±5.71 | 6.89±5.79a |
| Age (years) | 51.38±11.73 | 50.58±12.67a |
| BMI
(kg/m2) | 24.41±3.62 | 24.89±3.47a |
| HbAlc (%) | 10.86±1.36 | 10.79±1.42a |
| FBG (mmol/l) | 7.61±3.12 | 8.68±3.32a |
| 2-h PBG (mmol/l) | 15.42±4.78 | 15.60±5.71a |
| FCP (ng/ml) | 0.71±0.44 | 0.72±0.45a |
Insulin doses and incidence of
hypoglycemia
Neither the MDI nor the CSII groups had severe
hypoglycemic episodes during the treatment duration, and no
statistically significant differences were observed in nocturnal
hypoglycemic episodes. Good glycemic level control was achieved in
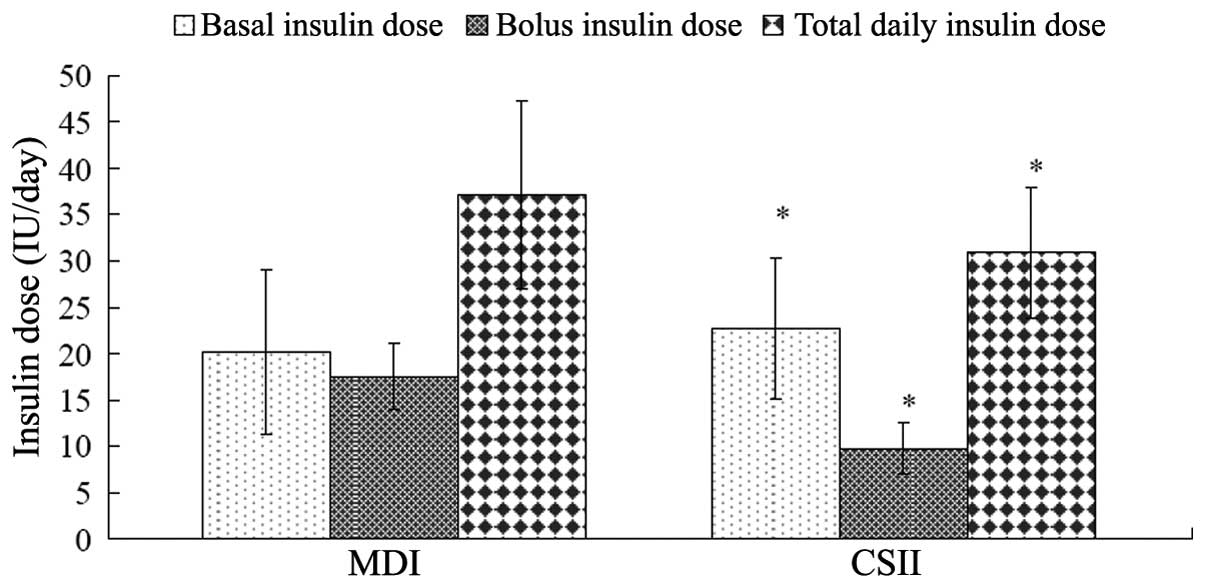
the 100 subjects in the MDI group after 6.88±2.31 days. The mean
total daily dosage of insulin was 37.12±10.18 IU (0.58±0.17
IU/kg/day), and the total daily basal and bolus doses were
19.35±7.84 and 17.55±3.52 IU (50.80±8.32 and 49.11±8.32% of the
total daily dose), respectively. Good glycemic level control was
achieved in the 100 subjects in the CSII group after 5.43±2.30
days. The decrease in HbA1c in the two groups was reached earlier
in patients in the CSII group compared with those in the MDI group.
The mean total daily insulin dose in the CSII group was 31.68±8.88
IU (0.48±0.16 IU/kg/day), and the total daily basal and bolus doses
were 22.77±7.65 and 9.78±2.74 IU (69.13±6.99 and 30.87±6.99% of the
total daily dose), respectively (Table II). A significant difference in
the dose of insulin was observed between the CSII and MDI groups
(P<0.001). Insulin requirements decreased 19.12±2.3% after 12
weeks for good glycemic level control in the two groups.
Significant differences were found between the two groups in the
total dose of insulin and the basal and bolus doses of insulin per
day (P<0.001) (Fig. 1 and
Table II). The incidence of
hypoglycemia was 5.93% in the MDI group and 1.62% in the CSII group
(P<0.01) (Table II).
 | Table IIComparison of insulin doses upon
achievement of good blood glucose control at the start of the 12
weeks of therapy. |
Table II
Comparison of insulin doses upon
achievement of good blood glucose control at the start of the 12
weeks of therapy.
| Group | n | Rate (IU/kg/day) | Basal insulin dose
(%) | Bolus insulin dose
(%) | Incidence of
hypoglycemia (%) |
|---|
| CSII | 100 | 0.48±0.16a | 69.13±6.99a | 30.87±6.99a | 1.62b |
| MDI | 100 | 0.58±0.17 | 50.89±8.32 | 49.11±8.32 | 5.93 |
Treatment efficacy
The mean HbA1c showed a statistically significant
decrease during the course of the experiment for the MDI and CSII
groups (Table III). In the MDI
group, mean HbA1c decreased from 10.79±1.42 to 7.51±1.28%. In the
CSII group, mean HbA1c decreased from 10.86±1.36 to 7.11±1.32%. No
significant differences were identified between the groups.
 | Table IIIComparison of efficacy between the
CSII and MDI groups for 12 weeks. |
Table III
Comparison of efficacy between the
CSII and MDI groups for 12 weeks.
| Group |
|---|
|
|
|---|
| Parameter | MDI | CSII |
|---|
| HbAlc |
| Baseline (%) | 10.79±1.42 | 10.86±1.36 |
| End-point (%) | 7.51±1.28 | 7.11±1.32a |
| Change (%) | −3.47±1.53 | −3.75±1.48 |
| P-value | <0.005 | <0.005 |
| FBG |
| Baseline
(mmol/l) | 8.68±3.32 | 8.61±3.12a |
| End-point
(mmol/l) | 6.85±1.26 | 6.76±1.13 |
| Change
(mmol/l) | −1.83±2.61 | −1.85±2.36 |
| P-value | <0.005 | <0.005 |
| 2-h PBG |
| Baseline
(mmol/l) | 15.60±5.71 | 15.42±4.78a |
| End-point
(mmol/l) | 9.95±2.16 | 9.87±2.63 |
| Change
(mmol/l) | −5.65±4.48 | −5.56±4.15 |
| P-value | <0.005 | <0.005 |
The mean FBG levels in the two groups showed
statistically significant baseline to end-point decreases, from
8.61±3.12 to 6.76±1.13 mmol/l in the CSII group and from 8.68±3.32
to 6.85±1.26 mmol/l in the MDI group (P<0.005 for both). No
statistically significant differences in baseline to end-point FBG
level decreases were observed between the two groups (P>0.05)
(Table III).
The mean 2-h PBG level decreased significantly from
15.42±4.78 mmol/l at baseline to 9.87±2.63 mmol/l at the end-point
in the CSII group and from 15.60±5.71 to 9.95±2.16 mmol/l in the
MDI group (P<0.005 for both). No statistically significant
differences in baseline to end-point 2-h PBG level decreases were
observed between the two groups (P>0.05) (Table III).
Changes in insulin doses
The total daily insulin dose in the CSII group
decreased from 0.48±0.16 to 0.39±0.23 IU/kg/day after 12 weeks
(−0.09±0.22 IU/kg/day), and from 0.58±0.17 to 0.47±0.19 IU/kg/day
after 12 weeks in the MDI group (−0.11±0.21 IU/kg/day); however,
the basal and bolus insulin doses as percentages of the total daily
dose remained unchanged at the end of the 12-week period (Table IV).
 | Table IVComparison of insulin doses upon
achievement of good blood glucose control after 12 weeks. |
Table IV
Comparison of insulin doses upon
achievement of good blood glucose control after 12 weeks.
| Group |
|---|
|
|
|---|
| Parameter | MDI | CSII |
|---|
| Rate
(IU/kg/day) | 0.47±0.19 | 0.39±0.23a |
| Basal insulin dose
(%) | 50.88±8.42 | 69.23±6.89a |
| Bolus insulin dose
(%) | 49.12±8.11 | 30.77±6.99a |
Blood glucose levels
Blood glucose levels in the CSII group were lower
than those in the MDI group (P>0.05) at each time-point;
however, good glycemic control was reached in both groups and no
statistical differences were observed in FBG levels, 2 h
post-breakfast, 2 h post-lunch or 2 h post-supper blood glucose
levels or blood glucose levels at 3:00 a.m. between the two groups
(Table V). Blood glucose
fluctuations for the CSII group were lower than those for the MDI
group (P<0.001) (Table V).
Insulin requirements decreased 19.12±2.3% after 12 weeks for good
glycemic level control in the two groups.
 | Table VComparison of blood glucose levels at
different times and blood glucose fluctuations after 12 weeks. |
Table V
Comparison of blood glucose levels at
different times and blood glucose fluctuations after 12 weeks.
| Group |
|---|
|
|
|---|
| Parameter | MDI | CSII |
|---|
| Rate
(IU/kg/day) | 0.47±0.19 | 0.39±0.23a |
| Basal insulin dose
(%) | 50.88±8.42 | 69.23±6.89a |
| Bolus insulin dose
(%) | 49.12±8.11 | 30.77±6.99a |
| Blood glucose
levels (mmol/l) |
| Fasting | 6.17±0.71 | 6.06±0.51 |
| 2 h after
breakfast | 8.96±1.51 | 8.76±1.11 |
| 2 h after
lunch | 9.23±1.21 | 9.05±0.91 |
| 2 h after
supper | 8.59±1.19 | 8.19±0.89 |
| 3:00 a.m. | 6.26±0.89 | 6.21±0.61 |
| Blood glucose
fluctuation (mmol/l) | 0.23±0.06 | 0.19±0.04b |
Discussion
Blood glucose daily fluctuations contribute to
oxidative stress, which can cause long-term complications in
patients with diabetes (29).
Avoiding glucose fluctuations in patients with diabetes is an
emerging challenge (30). The data
presented indicate that fluctuations in daily blood glucose were
narrower for patients undergoing CSII therapy than for those
undergoing MDI therapy. The results were consistent with the theory
that basal insulin adjustment using CSII therapy in patients with
diabetes provides less variable blood glucose levels than
long-acting insulin (31). The
variability in blood glucose control appears to be particularly
significant with regard to long-acting insulin. However, no
difference was detected in daily blood fluctuations between CSII
and MDI therapies in a previous study of patients with T2DM
(32).
Hypoglycemia is one of the main factors for patients
with diabetes requiring insulin to achieve tight glycemic control
and a reduced likelihood of complications. No statistically
significant differences in nocturnal hypoglycemic episodes and
hypoglycemia were detected between the two groups in the present
study (P>0.05), suggesting that the safety of the MDI therapy
may be comparable to that of CSII therapy in subjects with
T2DM.
The data presented in this study showed that, in
patients with T2DM treated with insulin therapy for 12 weeks, the
total daily dose of insulin in the MDI group was significantly
greater than that in the CSII group. The basal dose regulation in
the MDI group was not convenient and the mutation variation rates
of subcutaneous absorption of bolus insulin aspart and basal
insulin glargine were considerably greater in the MDI group than
those in the CSII group. Three factors contribute to PBG in
diabetes: Increases in glycogen output, FBG and absorption of
intestinal glucose. In general, increasing the range of 2 h-PBG
depends upon increasing the gastrointestinal glucose absorption and
glycogen output. However, gastrointestinal glucose absorption is
the same in patients with T2DM and healthy subjects (31) Basal insulin may restrict the
glycogen output (33) by
decreasing both pre-meal blood glucose and FBG levels and partially
restraining postprandial hyperglycemia. Although decreasing PBG may
be treated using basal insulin, a lower pre-meal insulin dose is
still required as a supplement to control PBG. The pre-meal insulin
dose may only restrain the absorption-related increase in glucose
and some of the output of glycogen. A lower pre-meal dose may
decrease the additive effect of the basal dose, therefore avoiding
the requirement for adjustments to be made the basal dose. The
study by Suzuki et al (33)
indicated that an increase in basal insulin dose may be an
effective method to control HbA1c and FBG in patients with T2DM and
showed the dominance of basal insulin treatment.
Due to the adjustment therapy method used in the
present study, the total daily bolus insulin dose of the CSII group
was 30.87±6.99%; this contrasted with the total daily bolus dose
used in the treatment of Korean patients (64.11±12.10%) (34). In the present study, the mean total
daily dose of insulin was 31.68±8.88 IU (0.48±0.11 IU/kg/day) for
the 100 patients in the CSII group, while in a study of 46 Indian
patients with T2DM the daily insulin dose was 44.0±23.7 IU/day
(18). In the present study, the
total daily bolus dose (17.55±3.52 IU) of the MDI group subjects
comprised 49.11±8.32% of the total daily dose, and the mean total
daily dose of insulin was 37.12±10.18 IU/day (0.58±0.17 IU/kg/day);
these values were both higher than those of the CSII group
subjects. In all subjects, the mean total daily dose of insulin was
34.86±9.76 IU and the dose of insulin per unit body weight was
0.53±0.17 IU/kg/day. The total daily bolus and basal insulin doses
were 13.75±5.01 and 21.12±7.91 IU (40.07±11.88 and 59.93±11.88% of
total daily dose), respectively. These values differed from those
in a previous report where the basal/total daily ratio of insulin
was 0.23±0.08 and the mean daily dose of insulin was 38.22±14.92
IU/day (35). From studying 200
patients with T2DM and other related research reports (21,22,34),
pre-meal and basal dose proportions have been shown to be
associated with the total insulin dose. Pre-meal blood glucose
levels can be controlled by preferentially regulating basal
insulin, making the required total daily dose of insulin lower.
After 12 weeks of application, MDI-treated patients
with T2DM had a higher total insulin dose requirement and
hypoglycemia incidence and took longer to achieve the targeted
glycemic control compared with the CSII-treated patients. Following
CSII treatment in patients with T2DM, decreases in bolus dose and
increases in basal insulin dose can form an effective method to
achieve good glycemic control with a lower total daily dose.
However, where factors exist to prevent the use of therapy with
insulin pumps, once daily glargine at bedtime combined with aspart
administration at each of the three meals should be an effective
alternative.
Acknowledgements
This study was supported by the Shandong Province
Science and Technology Development Plan (2012YD18019). The authors
would like to thank all the staff working at the Departments of
Endocrinology and Clinical Medicine in the Linyi People’s
Hospital.
References
|
1
|
Lindholm A, McEwen J and Riis AP: Improved
postprandial glycemic control with insulin aspart. A randomized
double-blind cross-over trial in type 1 diabetes. Diabetes Care.
22:801–805. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Anderson JH Jr, Brunelle RL, Koivisto VA,
et al: Reduction of postprandial hyperglycemia and frequency in
hypoglycemia in IDDM patients on insulin-analog treatment.
Multicenter Insulin Lispro Study Group. Diabetes. 46:265–270. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raskin P, Klaff L, Bergenstal R, et al: A
16-week comparison of the novel insulin analog insulin glargine
(HOE901) and NPH human insulin used with insulin lispro in patients
with type 1 diabetes. Diabetes Care. 23:1666–1671. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lepore M, Pampanelli S, Fanelli C, et al:
Pharmacokinetics and pharmacodynamics of subcutaneous injection of
long-acting human insulin analog glargine, NPH insulin, and
ultralente human insulin and continuous subcutaneous infusion of
insulin lispro. Diabetes. 49:2142–2148. 2000. View Article : Google Scholar
|
|
5
|
Pickup J and Keen H: Continuous
subcutaneous insulin infusion at 25 years: evidence base for the
expanding use of insulin pump therapy in type 1 diabetes. Diabetes
Care. 25:593–598. 2002.PubMed/NCBI
|
|
6
|
Reynolds LR: Reemergence of insulin pump
therapy in the 1990s. South Med J. 93:1157–1161. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bode BW, Steed RD and Davidson PC:
Reduction in severe hypoglycemia with long-term continuous
subcutaneous insulin infusion in type 1 diabetes. Diabetes Care.
19:324–327. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bode BW, Weinstein R, Bell D, et al:
Comparison of insulin aspart with buffered regular insulin and
insulin lispro in continuous subcutaneous insulin infusion: a
randomized study in type I diabetes. Diabetes Care. 25:439–444.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bode B and Strange P: Efficacy, safety,
and pump compatibility of insulin aspart used in continuous
subcutaneous insulin infusion therapy in patients with type 1
diabetes. Diabetes Care. 24:69–72. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsui E, Barnie A, Ross S, et al: Intensive
insulin therapy with insulin lispro: a randomized trial of
continuous subcutaneous insulin infusion versus multiple daily
insulin injection. Diabetes Care. 24:1722–1727. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Elbein SC, Wegner K and Kahn SE: Reduced
beta-cell compensation to the insulin resistance associated with
obesity in members of caucasian familial type 2 diabetic kindreds.
Diabetes Care. 23:221–227. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang W, Lu J, Weng J, et al; China
national Diabetes and Metabolic Disorders Study Group. Prevalence
of diabetes among men and women in China. N Engl J Med.
362:1090–1101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
An YL, Gao Y, Zhu Q, et al: Features of
insulin secretion and insulin resistance in newly diagnosed type 2
diabetes of different body mass indices. Zhonghua Yi Xue Za Zhi.
24:256–260. 2008.(In Chinese).
|
|
14
|
Juan CC, Fang VS, Kwok CF, et al:
Exogenous hyperinsulinemia causes insulin resistance,
hyperendothelinemia, and subsequent hypertension in rats.
Metabolism. 48:465–471. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bruttomesso D, Pianta A, Crazzolara D, et
al: Continuous subcutaneous insulin infusion (CSII) in the Veneto
region: efficacy, acceptability and quality of life. Diabet Med.
19:628–634. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weissberg-Benchell J, Antisdel-Lomaglio J
and Seshadri R: Insulin pump therapy: a meta-analysis. Diabetes
Care. 26:1079–1087. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bruttomesso D, Crazzolara D, Maran A, et
al: In Type 1 diabetic patients with good glycaemic control, blood
glucose variability is lower during continuous subcutaneous insulin
infusion than during multiple daily injections with insulin
glargine. Diabet Med. 25:326–332. 2008. View Article : Google Scholar
|
|
18
|
Jothydev Kesavadev J, Balakrishnan S,
Ahammed S and Jothydev S: Reduction of glycosylated hemoglobin
following 6 months of continuous subcutaneous insulin infusion in
an Indian population with type 2 diabetes. Diabetes Technol Ther.
11:517–21. 2009.PubMed/NCBI
|
|
19
|
Torres I, Ortego J, Valencia I, et al:
Benefits of continuous subcutaneous insulin infusion in type 1
diabetes previously treated with multiple daily injections with
once-daily glargine and pre-meal analogues. Exp Clin Endocrinol
Diabetes. 117:378–385. 2009. View Article : Google Scholar
|
|
20
|
Phillip M, Battelino T, Rodriguez H, et
al; European Society for Paediatric Endocrinology; Lawson Wilkins
Pediatric Endocrine Society; International Society for Pediatric
and Adolescent Diabetes; American Diabetes Association; European
Association for the Study of Diabetes. Use of insulin pump therapy
in the pediatric age-group: consensus statement from the European
Society for Paediatric Endocrinology, the Lawson Wilkins Pediatric
Endocrine Society, and the International Society for Pediatric and
Adolescent Diabetes, endorsed by the American Diabetes Association
and the European Association for the Study of Diabetes. Diabetes
Care. 30:1653–1662. 2007.
|
|
21
|
Monami M, Lamanna C, Marchionni N and
Mannucci E: Continuous subcutaneous insulin infusion versus
multiple daily insulin injections in type 2 diabetes: a
meta-analysis. Exp Clin Endocrinol Diabetes. 117:220–222. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kesavadev J, Balakrishnan S, Ahammed S and
Jothydev S: Reduction of glycosylated hemoglobin following 6 months
of continuous subcutaneous insulin infusion in an Indian population
with type 2 diabetes. Diabetes Technol Ther. 11:517–521. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carotenuto M, Esposito M, Parisi L, et al:
Depressive symptoms and childhood sleep apnea syndrome.
Neuropsychiatr Dis Treat. 8:369–373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Verrotti A, Agostinelli S, D’Egidio C, et
al: Impact of a weight loss program on migraine in obese
adolescents. Eur J Neurol. 20:394–397. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carotenuto M, Santoro N, Grandone A, et
al: The insulin gene variable number of tandem repeats (INS VNTR)
genotype and sleep disordered breathing in childhood obesity. J
Endocrinol Invest. 32:752–755. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carotenuto M, Bruni O, Santoro N, et al:
Waist circumference predicts the occurrence of sleep-disordered
breathing in obese children and adolescents: a questionnaire-based
study. Sleep Med. 7:357–361. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hoogma RP, Hammond PJ, Gomis R, et al;
5-Nations Study Group. Comparison of the effects of continuous
subcutaneous insulin infusion (CSII) and NPH-based multiple daily
insulin injections (MDI) on glycaemic control and quality of life:
results of the 5-nations trial. Diabet Med. 23:141–147. 2006.
View Article : Google Scholar
|
|
28
|
Workgroup on Hypoglycemia, American
Diabetes Association. Defining and reporting hypoglycemia in
diabetes: a report from the American Diabetes Association Workgroup
on Hypoglycemia. Diabetes Care. 28:1245–1249. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Marcovecchio ML, Lucantoni M and Chiarelli
F: Role of chronic and acute hyperglycemia in the development of
diabetes complications. Diabetes Technol Ther. 13:389–394. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ceriello A and Ihnat MA: ‘Glycaemic
variability’: a new therapeutic challenge in diabetes and the
critical care setting. Diabet Med. 27:862–867. 2010.
|
|
31
|
Bragd J, von Döbeln A, Lins PE, et al:
Basal insulin substitution with glargine or continuous subcutaneous
insulin infusion in adult type 1 diabetes patients-a randomized
controlled trial. Diabetes Technol Ther. 12:689–693. 2010.
View Article : Google Scholar
|
|
32
|
Tone A, Iseda I, Higuchi C, et al:
Comparison of insulin detemir and insulin glargine on glycemic
variability in patients with type 1 and type 2 diabetes. Exp Clin
Endocrinol Diabetes. 118:320–324. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Suzuki D, Toyoda M, Kondo M, et al:
Efficacy of long-acting insulin analog insulin glargine at high
dosage for basal-bolus insulin therapy in patients with type 2
diabetes. Tokai J Exp Clin Med. 37:35–40. 2012.PubMed/NCBI
|
|
34
|
Cai X, Han X, Luo Y and Ji L: Analysis of
insulin doses of Chinese type 2 diabetic patients with intensive
insulin treatment. PLoS One. 7:e389622012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kelley D, Mokan M and Veneman T: Impaired
postprandial glucose utilization in non-insulin-dependent diabetes
mellitus. Metabolism. 43:1549–1557. 1994. View Article : Google Scholar : PubMed/NCBI
|