Introduction
The prevalence of diabetes is rising; furthermore,
the prevalence of total diabetes (physician-diagnosed and
undiagnosed) has been calculated to be 13.4% (11.7–15.0%),
indicating that ~40% of diabetes cases remain undiagnosed (1). A previous study revealed that
long-term hyperglycemia was associated with adverse effects on
brain activity, neuronal structural changes and impaired long-term
spatial memory (2). Diabetes can
act on synaptic plasticity through mechanisms involved in
metaplasticity. Such persistent inhibition of long-term
potentiation and facilitation of long-term depression may lead to
activity-dependent synapse weakening and contribute to cognitive
impairments (3). Our previous
study showed that hyperglycemia was associated with learning and
memory decline in diabetic rats, possibly through a mechanism
involving mitogen-activated protein kinase (MAPK) signaling
pathways (4). The MAPK pathway is
necessary for the maintenance of synaptic plasticity in the dentate
gyrus, and MAPK/extracellular signal-regulated kinase (ERK)
activation is required for long-term potentiation (LTP)-dependent
transcriptional regulation (5).
Salvia miltiorrhiza is a Traditional Chinese
Medicine that is widely used throughout clinics in Eastern Asia to
treat liver fibrosis (6), diabetes
(7), diabetic nephropathy
(8), stroke and Alzheimer’s
disease (AD) (9). Biochemical and
pharmacological investigations have identified the phenolic acids
of S. miltiorrhiza as the effective constituents responsible
for its beneficial pharmacological activities (10). It has been proposed that S.
miltiorrhiza may be able to improve the condition of patients
with neurological disease, although the definite mechanisms remain
unknown (11–13). The aim of the present study was to
investigate the protective effects of S. miltiorrhiza
injection in experimental diabetic rats, and to explore the
association between MAPK phosphatase-1 (MKP-1) levels in the
hippocampus and the protective effects of S. miltiorrhiza
injection in the learning and memory ability of diabetic rats.
Materials and methods
Animal groups and induction of
diabetes
A total of 30 male Sprague Dawley rats (Experimental
Animal Center of Zhejiang University, Hangzhou, China) were used in
the experiments and were randomly divided into three groups (n=10):
Diabetes, S. miltiorrhiza-treated and normal control. The
rats of the diabetes and S. miltiorrhiza-treated groups
received a single dose of 65 mg/kg streptozotocin (STZ) (Alexis
Corporation, Lausen, Switzerland) intraperitoneally (i.p.) to
induce diabetes while the remaining 10 rats in the control group
received an injection of an equivalent volume of 0.9% saline.
Forty-eight hours after STZ injection, blood glucose levels
>16.7 mmol/l and a positive urine glucose test were regarded to
indicate the successful induction of diabetes. The S.
miltiorrhiza-treated rats then received 5 ml/kg/day S.
miltiorrhiza i.p. and rats in the other two groups received the
same volume of 0.9% saline i.p. for four weeks. The study was
approved by the Animal Care Committee of Zhejiang University
(Hangzhou, China) and conformed to the Guide for the Care and Use
of Laboratory Animals.
Behavioral testing
Four weeks after the induction of diabetes, the
learning and memory ability of the rats was assessed using the
Morris water maze. The procedure included place navigation, which
was used to test the rats’ access to learning and memory abilities,
and the length of time taken by each rat to reach the destination.
The rats were trained in the morning for four days. Subsequent to
place navigation, the platform was removed and all the rats were
placed into the water at the same point. The number of times the
rats passed through the platform in 120 sec was measured.
MKP-1 immunohistochemistry assay
Subsequent to the Morris water maze test, the rats
from each group were anesthetized and perfused intracardially with
normal saline (pH 7.0). Following saline perfusion, the animals
were perfused with 300 ml fixative containing 4% paraformaldehyde
in 0.1 M phosphate-buffered saline (PBS; pH 7.4). The brain of each
rat was then removed, fixed in the same fixative for 4 h and placed
in 30% phosphate-buffered sucrose (pH 7.4) until the tissue sank.
Tissue sections (10-μm) were cut on a freezing microtome through
coronary planes of the brain and mounted onto 0.02%
poly-L-lysine-coated slides. The tissue sections were washed in 0.1
M PBS and incubated in 1% bovine serum albumin (BSA; pH 7.0) for 30
min. The tissues were then incubated overnight at 4°C with mouse
anti-rat MKP-1 monoclonal antibodies (1:100 dilution in 0.1 M PBS
plus 1% BSA; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA).
Control sections were incubated in 0.1 M PBS plus 1% BSA. The
sections were washed three times for 5 min in 0.1 M PBS and then
incubated with a biotinylated goat anti-mouse secondary antibody
(1:200 dilution; Boster Biological Technology Ltd., Wuhan, China)
for 5 h at room temperature. The sections were washed three times
for 5 min in 0.1 M PBS and then incubated in an avidin-horseradish
peroxide solution. The sections were subsequently dehydrated
through an ethanol and xylene series, prior to the application of
the cover slips.
Statistical analysis
Hippocampal slides from each rat were examined at a
magnification of ×400 and analyzed with the University of Texas
Health Science Center at San Antonio ImageTool 3.0 (University of
Texas Medical School, San Antonio, TX, USA). The number of
MKP-1-positive cells per vision field of each rat was measured. All
data are presented as the mean ± standard deviation. Statistical
analysis was performed using one-way analysis of variance by
SPSS® statistical software, version 17.0 (SPSS Inc.,
Chicago, IL, USA) for Windows®. P<0.05 was considered
to indicate a statistically significant difference.
Results
Body weight and blood glucose
concentration
Prior to the STZ injection, no significant
difference was identified among the three groups with regard to
body weight or blood glucose concentration. After four weeks of
diabetes induction, the diabetic rats had a significantly lower
body weight and significantly higher blood glucose concentration
than the normal rats. The S. miltiorrhiza treatment was
observed to increase the body weight and decrease the blood glucose
concentration in the diabetic rats (P<0.01) (Fig. 1).
Behavioral changes of the three groups
four weeks after S. miltiorrhiza treatment
During the four-day training phase of the Morris
water maze test, the escape latency of all rats in the platform
quadrant improved gradually. The escape latency of the diabetic
rats on the fourth day was markedly longer (26.3±13.2 sec) than
that of the control rats (12.6±3.8 sec) (P<0.05). S.
miltiorrhiza injection improved the escape latency of the
diabetic rats to 18.6±0.1 sec (P<0.05). On the fourth day, the
average platform-finding frequency in the 120-sec period was
5.6±2.3 times for the normal rats, which was higher than that for
the diabetic rats (2.6±1.1 times; P<0.05). The platform-finding
frequency for the S. miltiorrhiza-treated rats was 3.8±1.7
times (P<0.05). The longer escape latency and lower
platform-finding frequency in the diabetic rats observed in the
Morris water maze tests indicated that diabetes affected the
learning and memory capacity and that S. miltiorrhiza
treatment could improve the learning and memory capacity in
diabetic rats.
Assessment of MKP-1 protein in the rat
hippocampus
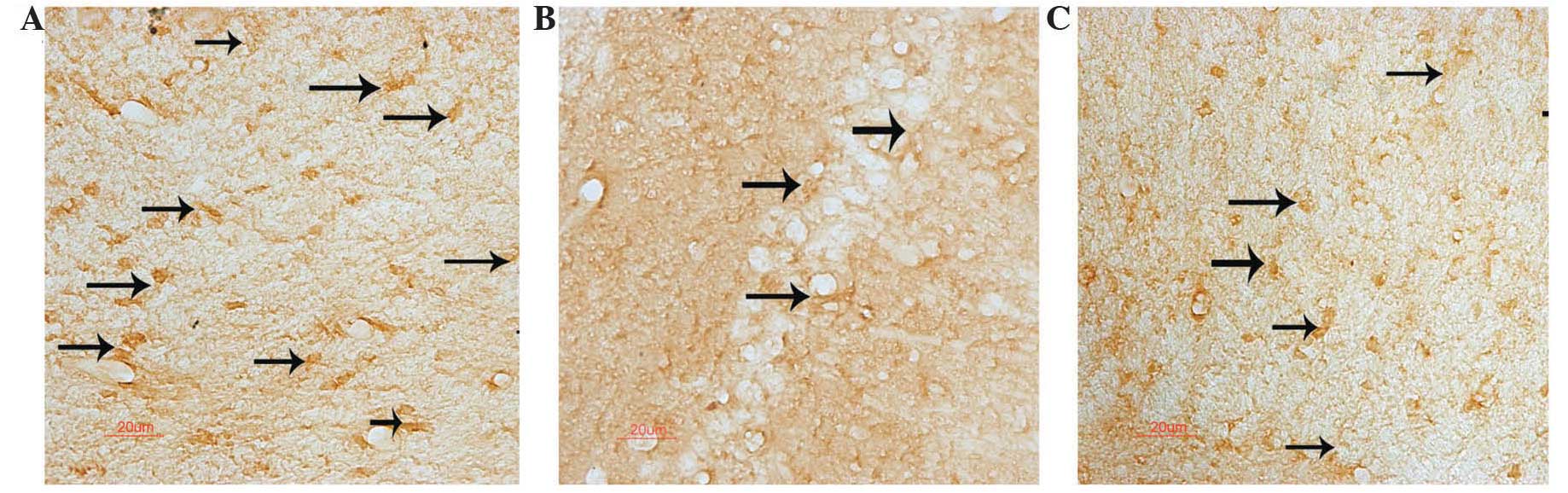
MKP-1-positive neurons with brown granules were
observed under the microscope. The brown granules were present in
the hippocampus of the normal rats, but were relatively rare in the
diabetic rats, indicating that MKP-1 had a lower expression in the
diabetic condition. However, the expression was enhanced in the
S. miltiorrhiza-treated group. The number of MKP-1-positive
neurons in the hippocampus of the normal rats was 22.8±4.3
cells/mm2, which was significantly higher than that of
the diabetic rats (13.5±3.2 cells/mm2). S.
miltiorrhiza treatment increased the number of MKP-1-positive
neurons to 18.7±4.0 cells/mm2 in the diabetic rats
(P<0.05) (Fig. 2).
Discussion
Diabetic encephalopathy is one of the complications
of diabetes and has been shown to differ in types 1 and 2 diabetes
with regard to the nature of the resulting cognitive deficits
(14). A previous study showed
that a pre-existing diabetic condition doubled the incidence of
dementia and AD and increased mortality. Elderly patients with
diabetes developed more extensive vascular pathology, which
increased dementia risk. Dementia risk was also increased in
elderly patients with diabetes and AD-type pathology, particularly
in apolipoprotein E-ɛ4 carriers (15). The present study explored the
cognitive function of STZ-induced diabetic rats and found that the
diabetic rats demonstrated a markedly longer escape latency and a
lower platform-finding frequency in 120 sec than the normal rats.
S. miltiorrhiza treatment could prevent the decline in the
learning and memory capacity of the diabetic rats.
The MAPK family consists of the ERK, c-Jun
N-terminal kinase and p38MAPK subfamilies. Previous studies have
shown that MAPK signaling is involved in long-term synaptic
plasticity and memory (16,17).
MKP-1, an immediate-early gene product, is one of the
dual-specificity phosphatases that may play an important role in
the regulation of MAPK activity, and is highly inducible in
response to extracellular stimuli, including growth factors,
hydrogen peroxide, angiotensin II and hyperglycemia (18–20).
A previous study suggested that inactivating MKP-1 in turn
increased the MAPK pathway activity and resulted in rapid LTP decay
and consequent deficits in hippocampal memory retention (5). We previously demonstrated that the
MAPK signaling pathway is involved in the decline in learning and
memory under hyperglycemia, which led to the conclusion that MKP-1
may be associated with the diabetes-related learning and memory
deficits (4). The present study
showed that MKP-1 protein expression was significantly lower in the
hippocampus of diabetic rats than that in the normal rats, while
the expression in the S. miltiorrhiza-treated rats was
higher than that in the diabetes group.
S. miltiorrhiza can activate blood flow and
eliminate stasis, improve the microcirculation and reverse the
upregulation of vascular endothelial growth factor induced by high
glucose concentrations, in addition to ameliorating mitochondrial
oxidative stress (21–24). With regard to neuronal protection,
S. miltiorrhiza can significantly improve spatial cognition
in a rat model of AD, which may be associated with a reduction in
β-amyloid precursor protein expression in the rat brain (25). It has also been found that
pretreatment with S. miltiorrhiza protects primary rat
cortical neurons against H2O2-induced
cytotoxicity. Furthermore, Tanshinone IIA, a phenolic acid of S.
miltiorrhiza, has been shown to markedly reduce the increase in
Ca2+ levels evoked by H2O2 and
reverse H2O2-induced hippocampal LTP
impairment (26). Previous studies
have demonstrated that the long-term use of S. miltiorrhiza
extract can not only decrease the volume of cerebral infarction but
also improve learning and memory capacity; the underlying mechanism
for these effects may be associated with the antioxidant property
of S. miltiorrhiza (27,28).
In the present study, S. miltiorrhiza-treated (four weeks,
i.p) diabetic rats showed improvements in body weight and blood
sugar level and an increased learning and memory capacity, as
demonstrated by reductions in escape latency and increased
platform-crossing frequency in 120 sec, compared with untreated
diabetic rats.
In conclusion, the present study demonstrated that
hyperglycemia can cause a decline in learning and memory via the
negative feedback regulation of MKP-1 towards the MAPK signaling
pathway. However, treatment with S. miltiorrhiza injection
can improve the learning and memory abilities in diabetic rats and
increase MKP-1 protein expression levels under the hyperglycemic
condition. It is possible that S. miltiorrhiza injection may
prevent progressive neuronal injury in diabetic rats by increasing
MKP-1 protein levels.
Acknowledgements
The study was supported by the Education Department
Bureau of Zhejiang Province (nos. 20070084 and Y201225157).
References
|
1
|
Leong A, Dasgupta K, Chiasson JL and Rahme
E: Estimating the population prevalence of diagnosed and
undiagnosed diabetes. Diabetes Care. 36:3002–3008. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Malone JI, Hanna S, Saporta S, et al:
Hyperglycemia not hypoglycemia alters neuronal dendrites and
impairs spatial memory. Pediatr Diabetes. 9:531–539. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Artola A: Diabetes-, stress- and
ageing-related changes in synaptic plasticity in hippocampus and
neocortex - the same metaplastic process? Eur J Pharmacol.
585:153–162. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou J, Wang L, Ling S and Zhang X:
Expression changes of growth-associated protein-43 (GAP-43) and
mitogen-activated protein kinase phosphatase-1 (MKP-1) and in
hippocampus of streptozotocin-induced diabetic cognitive impairment
rats. Exp Neurol. 206:201–208. 2007. View Article : Google Scholar
|
|
5
|
Davis S, Vanhoutte P, Pages C, Caboche J
and Laroche S: The MAPK/ERK cascade targets both Elk-1 and cAMP
response element-binding protein to control long-term
potentiation-dependent gene expression in the dentate gyrus in
vivo. J Neurosci. 20:4563–4572. 2000.PubMed/NCBI
|
|
6
|
Liu L, Wei J, Huo X, et al: The Salvia
miltiorrhiza monomer IH764-3 induces apoptosis of hepatic
stellate cells in vivo in a bile duct ligation-induced model
of liver fibrosis. Mol Med Rep. 6:1231–1238. 2012.
|
|
7
|
Huang M, Xie Y, Chen L, et al:
Antidiabetic effect of the total polyphenolic acids fraction from
Salvia miltiorrhiza Bunge in diabetic rats. Phytother Res.
26:944–948. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee SH, Kim YS, Lee SJ and Lee BC: The
protective effect of Salvia miltiorrhiza in an animal model
of early experimentally induced diabetic nephropathy. J
Ethnopharmacol. 137:1409–1414. 2011.
|
|
9
|
Yu XY, Lin SG, Chen X, et al: Transport of
cryptotanshinone, a major active triterpenoid in Salvia
miltiorrhiza Bunge widely used in the treatment of stroke and
Alzheimer’s disease, across the blood-brain barrier. Curr Drug
Metab. 8:365–378. 2007.PubMed/NCBI
|
|
10
|
Yuan Y, Liu Y, Lu D, et al: Genetic
stability, active constituent, and pharmacoactivity of Salvia
miltiorrhiza hairy roots and wild plant. Z Naturforsch C.
64:557–563. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim DH, Park SJ, Kim JM, et al: Cognitive
dysfunctions induced by a cholinergic blockade and Aβ 25–35 peptide
are attenuated by salvianolic acid B. Neuropharmacology.
61:1432–1440. 2011.
|
|
12
|
Zheng CS, Xu XJ, Ye HZ, et al:
Computational pharmacological comparison of Salvia
miltiorrhiza and Panax notoginseng used in the therapy
of cardiovascular diseases. Exp Ther Med. 6:1163–1168.
2013.PubMed/NCBI
|
|
13
|
Yu XY, Lin SG, Zhou ZW, et al: Tanshinone
IIB, a primary active constituent from Salvia miltiorrhiza,
exhibits neuro-protective activity in experimentally stroked rats.
Neurosci Lett. 417:261–265. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sima AA: Encephalopathies: the emerging
diabetic complications. Acta Diabetol. 47:279–293. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ahtiluoto S, Polvikoski T, Peltonen M, et
al: Diabetes, Alzheimer disease, and vascular dementia: a
population-based neuropathologic study. Neurology. 75:1195–1202.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thomas GM and Huganir RL: MAPK cascade
signalling and synaptic plasticity. Nat Rev Neurosci. 5:173–183.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kelleher RJ III, Govindarajan A, Jung HY,
Kang H and Tonegawa S: Translational control by MAPK signaling in
long-term synaptic plasticity and memory. Cell. 116:467–479. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Racz B, Hanto K, Tapodi A, et al:
Regulation of MKP-1 expression and MAPK activation by PARP-1 in
oxidative stress: a new mechanism for the cytoplasmic effect of
PARP-1 activation. Free Radic Biol Med. 49:1978–1988. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Calò LA, Schiavo S, Davis PA, et al:
Angiotensin II signaling via type 2 receptors in a human model of
vascular hyporeactivity: implications for hypertension. J
Hypertens. 28:111–118. 2010.PubMed/NCBI
|
|
20
|
Takehara N, Kawabe J, Aizawa Y, Hasebe N
and Kikuchi K: High glucose attenuates insulin-induced
mitogen-activated protein kinase phosphatase-1 (MKP-1) expression
in vascular smooth muscle cells. Biochim Biophys Acta.
1497:244–252. 2000. View Article : Google Scholar
|
|
21
|
Han B, Zhang X, Zhang Q, et al: Protective
effects of salvianolate on microvascular flow in a porcine model of
myocardial ischaemia and reperfusion. Arch Cardiovasc Dis.
104:313–324. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang XY, Qiang GF, Zhang L, et al:
Salvianolic acid A protects against vascular endothelial
dysfunction in high-fat diet fed and streptozotocin-induced
diabetic rats. J Asian Nat Prod Res. 13:884–894. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qian S, Huo D, Wang S and Qian Q:
Inhibition of glucose-induced vascular endothelial growth factor
expression by Salvia miltiorrhiza hydrophilic extract in
human microvascular endothelial cells: evidence for mitochondrial
oxidative stress. J Ethnopharmacol. 137:985–991. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang X, He D, Xu L and Ling S: Protective
effect of tanshinone IIA on rat kidneys during hypothermic
preservation. Mol Med Rep. 5:405–409. 2012.PubMed/NCBI
|
|
25
|
Qin RA, Yao XX and Huang ZY: Effects of
compound danshen tablets on spatial cognition and expression of
brain beta-amyloid precursor protein in a rat model of Alzheimer’s
disease. J Tradit Chin Med. 32:63–66. 2012.PubMed/NCBI
|
|
26
|
Wang W, Zheng LL, Wang F, et al:
Tanshinone IIA attenuates neuronal damage and the impairment of
long-term potentiation induced by hydrogen peroxide. J
Ethnopharmacol. 134:147–155. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin LL, Wang W, Cheng MH and Liu AJ:
Protection of different components of Danshen in cerebral
infarction in mice. CNS Neurosci Ther. 18:511–512. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chong Y, Wang T, Wang W, et al:
Down-regulation of P-glycoprotein expression contributes to an
increase in Danshensu accumulation in the cerebral
ischemia/reperfusion brain. Mol Med Rep. 5:812–816. 2012.
|