Introduction
The herbicidal properties of paraquat (PQ) were
first identified in the 1950s, with PQ first marketed as a
therapeutic agent in 1962. Contact of paraquat with soil causes
immediate absorption and complete loss of its activity (1); thus, PQ is widely used around the
world in agriculture. Presently, PQ is the second highest-selling
weed killer globally and is available in a 20% solution form that
requires dilution prior to agricultural use (2). PQ is a highly toxic compound and PQ
poisoning is difficult to treat clinically due to the lack of
effective treatments. Patients with severe PQ-induced poisoning may
succumb to multiple organ failure involving the circulatory and
respiratory systems. Ingestion of ~30 ml of PQ usually leads to
circulatory failure within two days, whereas patients who ingest
less than one mouthful often survive the early phase of PQ
poisoning (3). In China, the use
of PQ has been virtually banned in the future (4). However, a number of PQ poisoning
cases remain, the majority of which are suicide attempts. Numerous
patients have been treated at the Qilu Hospital of Shandong
University (Jinan, China); however, cases of extreme bradycardia
caused by acute PQ poisoning have rarely been observed. In the
current study, a case of extreme bradycardia as a result of acute
PQ poisoning is presented.
Case report
A 59-year-old male was admitted to the Department of
Poisoning and Occupational Disease at the Qilu Hospital of Shandong
University three days following an attempted suicide by PQ
poisoning via oral admission. The patient became intoxicated, and
ingested ~50 ml PQ at 13:20 on August 17, 2013. Minutes later, the
patient was found by his family and was found to have vomited. On
the third day the patient was transferred to our hospital for
further treatment. The patient was lethargic on the way to the
hospital, following administration of an emetic.
The patient was treated at the local hospital for
alcohol consumption, without gastolavage and hemoperfusion. On the
second day, the patient admitted to ingesting PQ; thus, was
subsequently treated with steroid pulse therapy. The patient had no
previous history of disease. During physical examination on
admission to our local hospital, the patient was conscious with a
blood pressure of 138/82 mmHg, a pulse rate of 61 bpm, a
respiratory rate of 18 breaths/min, a blood oxygen saturation level
of 98% and pharyngeal swelling was observed. The concentration of
PQ in the urea was ~30 μg/ml. Analysis of the blood revealed a
white blood cell count of 16.98×109/l, a serum creatine
level of 141 μmol/l and a blood sugar level of 19.8 mmol/l. In
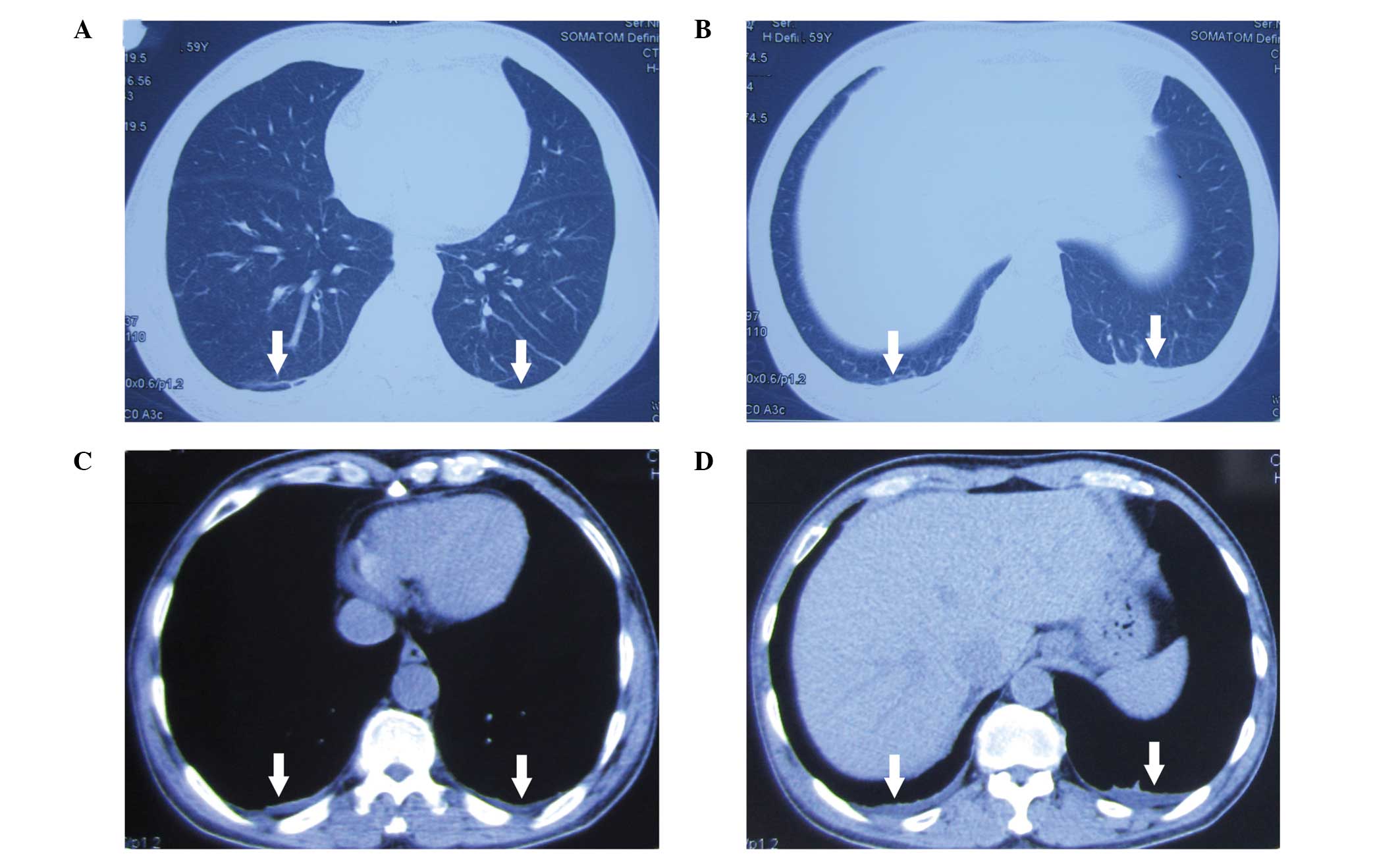
addition, effusion shadows and an unclear boundary in the double
lower lobe were observed in the lungs following a computed
tomography scan (Fig. 1).
The patient was administered 1,000 mg
methylprednisolone, which was gradually reduced according to the
health condition. Furthermore, whole gastrointestinal lavage,
protection of the gastrointestinal mucosa, free radical scavenging,
protection of the liver and kidneys, myocardial nutrition and
maintenance of the water and electrolyte balance were applied via
the administration of the ‘Qilu scheme’ (Department of Poisoning
and Occupational Diseases) (5).
However, on August 23, bradycardia occurred, and the patient had a
pulse of 35 bpm. The heart rate of the patient gradually increased
to 53 bpm following an intramuscular injection of atropine, but
decreased to 40 bpm after 3 h. The following day, isoprenaline was
administered continuously via an intravenous drip, while 0.6 mg
atropine was administered orally three times a day. However, the
heart rate remained low at 35–45 bpm. An ultrasonic cardiogram
revealed enlargement of the right atrium, widening of the ascending
aorta, moderate mitral valve regurgitation, light-medium tricuspid
regurgitation and mild pulmonary valve regurgitation (Fig. 2A). Furthermore, the ultrasound
revealed that the thyroid gland was normal in size; however, a cyst
(0.15 × 0.13 cm) was observed in the left lower pole (Fig. 2B). Additional biochemical analyses
revealed that the creatine kinase (CK) level was 50 IU/l, the CK-MB
level was 0.9 ng/ml, the cardiac troponin I level was 0 ng/ml, the
triglyceride (TG) level was 2.59 mmol/l, the free triiodothyronine
(T3) level was 1.18 pg/ml, the free thyroxine
(T4) level was 10.90 pmol/l and the thyroid stimulating
hormone (TSH) level was 2.550 μIU/ml. Atropine and isopropyl
adrenaline treatments were stopped and thyroid tablets were
administered (80 mg per day). The heart rate increased to 50–55 bpm
and was steady. Changes in the electrocardiograms are shown in
Fig. 3. Following the treatment,
the main examination index returned to normal with mild obstructive
ventilation dysfunction observed in lung function. The patient was
discharged from hospital, but received a follow-up examination one
month later. The heart rate of the patient was 63 bpm and the level
of N-terminal pro-brain natriuretic peptide was 22.17 pg/ml, the
cortisol level was 15.75 μg/dl, the thyroglobulin antibody level
was 18.26 IU/ml and the antithyroid peroxidase antibodies level was
14.08 IU/ml. With the exception of a degree of pulmonary fibrosis,
the other examinations, including blood routine examinations and
blood biochemical tests, were found to be normal. A written
informed patient consent was obtained for this study.
Discussion
PQ is a herbicide that is highly toxic to humans.
Following the rapid oral absorption of PQ, a peak level is reached
at 60–90 min following ingestion regardless of the plasma PQ levels
(6). Misdiagnosis with alcohol
poisoning during the early stages may result in patients not
receiving gastric lavage and hemoperfusion, and as a consequence,
the majority of the PQ is absorbed into the body. In the present
study, the pulse rate of the patient decreased to 29 bpm from 61
bpm on admission to hospital. Isoprenaline was administered
continuously via an intravenous drip, while atropine was
administered orally; however, the condition of the patient did not
improve. Treatment with atropine and isopropyl adrenaline was
terminated and thyroid tablets were administered. The heart rate
increased to 50–55 bpm and was steady. To the best of our
knowledge, cases of bradycardia following PQ poisoning are rare. It
was hypothesized that the following factors contributed to the
decrease in the heart rate. Firstly, PQ poisoning may lead to toxic
myocarditis (7) or dysfunction of
the sinus node. Secondly, eating disorders and serious nutritional
shortfalls may decrease the transport of T4 and
T3 into tissues (8).
Furthermore, in patients with existing heart conditions, low
T3 levels may lower the neuroendocrine profile and
ventricular performance (9).
Finally, acute treatment with large doses of glucocorticoids
results in a reduction of basal TSH levels. Pfister et al
(10) demonstrated that free
T3 and low-T3 syndrome are predictors of
mortality, which are independent from other known cardiovascular
risk parameters.
In conclusion, low levels of T3 are
usually produced under the influence of a number of factors. When
thyroid hormone levels are too low, a decrease in target organ
function commonly occurs, and in particular, the heart rate may
decrease. In addition, individuals with heart disease are more
susceptible to being affected by levels of thyroid hormones.
However, a number of other possible causes may lead to heart rate
reduction; thus, further investigation is required.
Acknowledgements
The study was supported by grants from the Taishan
Scholar Program of China Shandong Province and the Natural Science
Foundation of China Shandong Province (no. Y2008C123) and Shandong
University Qilu Hospital Clinical New Technology Innovation
Project, China (nos. 013527Z1 and 2013527Z2).
References
|
1
|
Bullivant CM: Accidental poisoning by
paraquat: Report of two cases in man. Br Med J. 21:1272–1273. 1966.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arts J, Schuit G, Schipper A and Kleij van
der B: A case report of PQ poisoning. Eur J Hosp Pharm. 12:22–24.
2006.
|
|
3
|
Bertram A, Haenel SS, Hadem J, Hoeper MM,
et al: Tissue concentration of PQ on day 32 after intoxication and
failed bridge to transplantation by extracorporeal membrane
oxygenation therapy. BMC Pharmacol Toxicol. 14:452013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yin Y, Guo X, Zhang SL and Sun CY:
Analysis of paraquat intoxication epidemic (2002–2011) within
China. Biomed Environ Sci. 26:509–512. 2013.PubMed/NCBI
|
|
5
|
Jian X, Zhang H, Sui H, Guo G, et al: Qilu
Scheme of PQ poisoning treatment. Chinese Journal of Industrial
Medicine. 27:119–121. 2014.(In Chinese).
|
|
6
|
Kang MS, Gil HW, Yang JO, Lee EY and Hong
SY: Comparison between kidney and hemoperfusion for paraquat
elimination. J Korean Med Sci. 24:S156–S160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Magnani JW and Dec GW: Myocarditis current
trends in diagnosis and treatment. Circulation. 113:876–890. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Van der Heyden JT, Docter R, Van Toor H
and Wilson JH: Effects of caloric deprivation on thyroid hormone
tissue uptake and generation of low-T3 syndrome. Am J Physiol.
251:E156–E163. 1986.PubMed/NCBI
|
|
9
|
Pingitore A, Galli E, Barison A, Lervasi
A, et al: Acute effects of triiodothyronine (T3) replacement
therapy in patients with chronic heart failure and low-T3 syndrome:
a randomized, placebo-controlled study. J Clin Endocrinol Metab.
93:1351–1358. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pfister R, Strack N, Wielckens K, Malchau
G, et al: The relationship and prognostic impact of low-T3 syndrome
and NT-pro-BNP in cardiovascular patients. Int J Cardiol.
144:187–190. 2010. View Article : Google Scholar : PubMed/NCBI
|