Introduction
Class II malocclusion is one of the most common
orthodontic problems. Mandibular retrognathia is the major etiology
of class II malocclusion (1). A
variety of functional appliances have been used to stimulate
mandibular growth in adolescence with mandibular retrognathia.
Mandibular advancement achieved by the fitting of a functional
appliance may promote condylar remodeling and subsequently
accelerate mandibular growth (2,3).
Type 1 diabetes mellitus, which is characterized by
a lack of insulin production, accounts for 5–10% of diabetes and a
peak incidence occurs at 10–14 years of age (4,5).
Previous studies have shown that a deficiency of insulin may alter
the mechanical response of alveolar bone during orthodontic tooth
movement in animal models, while insulin treatment normalized this
abnormal response (6,7). However, whether the deficiency of
insulin in patients with type 1 diabetes mellitus affects condylar
remodeling during treatment with functional appliances remains
unclear.
A previous study identified that insulin can exert a
pronounced effect on the differentiation of cartilage precursor
cells into chondroblasts and chondrocytes (8), and promote collagen II and
proteoglycan synthesis in chondrocytes (9,10).
Researchers also found that the disturbance of glucose metabolism
induced by an insulin disorder may negatively affect articular
cartilage (11), while insulin
treatment ameliorated cartilage degeneration in an animal model of
osteoarthritis (12). In addition,
recent studies have shown that the insulin receptor can be detected
on human cartilage and chondrocytes (13). These results indicate that insulin
exerts a direct effect on articular cartilage metabolism.
The aim of the present study was to elucidate the
effects of type 1 diabetes on the condylar response during
treatment with a functional appliance by analyzing the expression
levels of matrix metalloproteinase (MMP)-8, MMP-9 and tissue
inhibitor of metalloproteinase-1 (TIMP-1).
Materials and methods
Animals and the experimental diabetes and
mandibular advancement models
Seventy-five male Sprague-Dawley rats (age, 3 weeks;
weight, 60–70 g) were purchased from the Experimental Animal Center
of the Third Xiangya Hospital (Changsha, China) and randomly
divided into 3 groups: normal (NG), diabetes (DG) and diabetes with
insulin-treatment (TG) (n=25 per group). All animals were treated
according to the ethical regulations for animal experiments defined
by the Ethics Committee of the Central South University (Changsha,
China). Type 1 diabetes was induced in rats (DG and TG) by
intraperitoneal injection of 85 mg kg−1 streptozotocin
(STZ; Sigma, St. Louis, MO, USA) freshly dissolved in citrate
buffer (0.1 mol/l; pH 4.5), while the NG was injected with an
equivalent volume of citrate buffer. The rats were fasted for 12 h
prior to STZ injection. Three days following induction, blood
samples were collected from the tail vein and the plasma glucose
levels were evaluated using a glucose-oxidase enzymatic method
(Accu-Chek Performa; Roche Diagnostics GmbH, Mannheim, Germany).
STZ-injected rats were considered to have diabetes if glucose
levels were >300 mg/dl after 8 h of fasting. The injection of
STZ was repeated when glucose levels of <300 mg/dl were
detected. Determination of glucose levels was repeated once a week
in the non-insulin treated groups. The rats in the TG received
daily hypodermic injections of premixed insulin consisting of
insulin N and R (Novolin 30R; Novo Nordisk, Bagsvaerd, Denmark)
following the successful establishment of the diabetic animal
model. Plasma glucose levels were measured twice a day. The dose of
insulin was individually adjusted to maintain random blood glucose
levels of 90–190 mg/dl.
A functional appliance to advance mandible growth
was applied to the rats in the experimental groups under anesthetic
(10% chloral hydrate, 0.33 ml/100 g of body weight) on the day 1 of
week 3 of the experiment. No appliance was fitted to the control
animals. The bite-jumping appliance used in this study was
constructed according to the method used by Xiong et al
(14) (Fig. 1). The appliances were worn 24 h per
day and resulted in an anterior advancement of 3–4 mm and a
vertical displacement of 1–2 mm of the mandible.
Rats were anesthetized and decapitated at 0, 7, 14,
21 and 28 days following mandibular advancement. Condyles were
fixed in the 4% paraformaldehyde for 24 h at 4°C and decalcified
for 1 week in EDTA at 4°C. The demineralized tissues were
dehydrated and embedded in paraffin and the tissue blocks were cut
into 5-μm thick sagittal serial sections.
Histology and histomorphometry
The morphological conditions of the condyles were
observed with hematoxylin and eosin staining. The condylar
cartilage was divided into four layers for measurement (Fig. 2), which have been previously
characterized and described (15).
Layer thickness was measured using the method by Ghafari and
Degroote (16).
Immunohistochemical assay
Immunohistochemical reagents were obtained from
Wuhan Boster Biological Technology, Ltd. (Wuhan, China). Following
deparaffinization, sections were treated with antigen retrieval
buffers (compound enzyme digestive juice) for 10 min at room
temperature, washed twice in 0.01 M phosphate-buffered saline (PBS)
and immersed in 3.0% H2O2 for 5 min to block
endogenous peroxidase activity. Sections were washed twice in 0.01
M PBS, exposed to blocking buffer with 3% goat serum/PBS for 60 min
and reacted with rabbit anti-MMP-8, -MMP-9 and -TIMP-1 antibodies
overnight at room temperature. The dilution ratio was 1:75, 1:150
and 1:150, respectively. Following rinsing with PBS three times,
sections were incubated with goat anti-rabbit secondary antibody
conjugated to horseradish peroxidase (dilution, 1:400) for 2 h.
Sections were then rinsed with PBS three times and stained with
3,3-diaminobenzidine for 3–5 min at room temperature. PBS was as a
negative control.
Statistical analysis
Results are presented as the mean ± standard
deviation. The statistical analysis of the results was performed
using one-way analysis of variance followed by Scheffe’s test for
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
Histological observations
Histological analyses of the samples revealed a
difference in condylar layer thickness between the DG and NG
following mandibular forward positioning (Figs. 3 and 4). Total thickness of the cartilage in
the DG was less than that of the NG at day 14 (P<0.05), which
was largely indicated by the decreased thickness of the
proliferative layer and chondrogenic layer. However, in the DG, the
cartilage was thicker than that of the NG at days 21 and 28
(P<0.05), which was largely determined by an increased thickness
of the hypertrophic layer, and thinner proliferative and
chondrogenic layers than that of the NG. There were no significant
differences in histological morphous between the NG and the TG.
Immunoreactivity of MMP-8 and 9 and
TIMP-1
Positive immunohistochemical staining for MMP-8 was
observed in cells in all condylar layers (Fig. 5A). Following mandibular forward
positioning, the expression levels of MMP-8 increased during the
experiment and reached a peak on day 28 in the DG and NG
(P<0.05). However, expression was significantly decreased in the
DG compared with that of the NG (P<0.05) (Fig. 5A and B). Positive
immunohistochemical staining for MMP-9 was observed mainly in the
proliferation and chondrogenic layers in the DG and NG prior to
mandibular advancement (Fig. 6A).
Following mandibular forward positioning, the expression levels of
MMP-9 increased throughout the experimental period and peaked at
day 21 in the NG and DG (P<0.05). However, expression was
significantly decreased in the DG compared with that of the NG
(P<0.05). Positive immunohistochemical staining of MMP-9 was
observed predominantly in the proliferation and chondrogenic layers
at days 7 and 14, and in the proliferation, chondrogenic and
hypertrophic layers at days 21 and 28 in the NG (Fig. 6A and B).
Positive immunohistochemical staining for TIMP-1 was
observed mainly in the proliferation, chondrogenic and hypertrophic
layers (Fig. 7A). Following
mandibular forward positioning, TIMP-1 expression levels were
relatively stable in the NG and DG at days 7 and 14, whereas an
increase was observed at days 21 and 28 in the two groups
(P<0.05). There was a significant difference in the expression
levels of TIMP-1 between the NG and DG at days 21 and 28. Diabetes
significantly increased the expression levels of TIMP-1 (P<0.05)
(Fig. 7A and B).
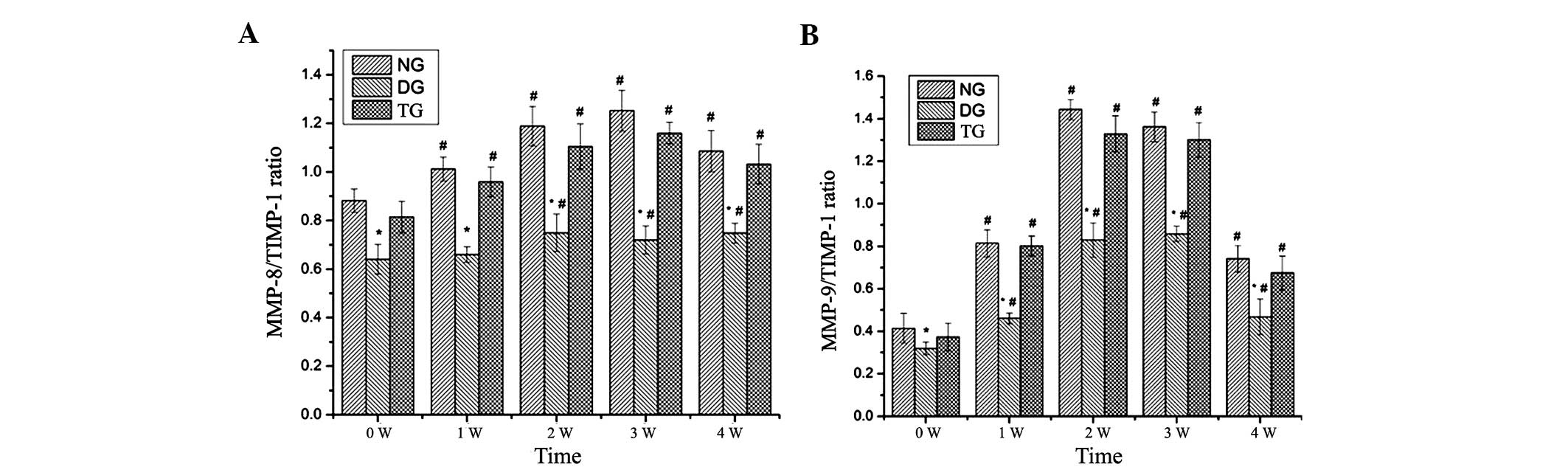
The ratio of MMP-8 to TIMP-1 increased and peaked at
day 21 in the NG, while the ratio of MMP-8 to TIMP-1 increased and
peaked on day 14 in the DG. The ratio of MMP-9 to TIMP-1 peaked on
day 14 and then gradually declined in the NG, while it reached a
peak on day 21 and then declined gradually in the DG. Experimental
diabetes significantly decreased the ratio of MMP-8 to TIMP-1 and
MMP-9 to TIMP-1 compared with that of the NG (P<0.05) (Fig. 8A and B).
Reversal of the diabetic state using insulin
treatment resulted in a recovery of the aforementioned parameters.
There were no significant differences in the parameters between the
NG and TG (P>0.05) (Figs.
5,6 and 7).
Discussion
Besides heredity, the growth and development of the
condylar cartilage is thought to be induced by external stimuli,
including mandibular advancement, hormones and growth factors
(17). Insulin can exert a direct
effect on chondrocytes and the articular cartilage (8–13),
therefore it has been hypothesized that type 1 diabetes mellitus
may affect the condylar response during functional appliance
treatment. However, the mechanisms involved have yet to be fully
elucidated. The results of the present study reveal a significant
alteration in the condylar cartilage response of diabetic animals
to mandibular advancement.
In the growth and development of the condylar
cartilage, hypertrophic condylar cartilage matures to a
nonhypertrophic stage. The sequence of this begins with
differentiation of mesenchyme cells into chondrocytes in the
proliferative layer followed by hypertrophy in the hypertrophic
layer and circumferential calcification of the cartilage matrix.
The calcified matrix is resorbed and the majority of the matrix in
the hypertrophic layer breaks down, which creates space for
vascular invasion. The invading capillaries are accompanied by
osteogenic progenitors and bone marrow stem cells that
differentiate into osteoblasts. Subsequently, bone is formed under
the hypertrophic layer (16,18).
Histological observations of the present study show that the
proliferative layer is thinner in diabetic rats than that of normal
rats during experimental mandibular advancement. These result
suggest that diabetes decreases chondrogenesis induced by
mandibular advancement. In addition, transition from chondrogenesis
to osteogenesis was also inhibited and subsequently decreased bone
formation under the hypertrophic layer in the DG, as the
hypertrophic layer was thicker at days 21 and 28 compared with that
of the NG.
To further validate our hypothesis, the expression
levels of MMP-8, MMP-9 and TIMP-1 were tested. Mandibular condylar
cartilage is mainly consisted of chondrocytes and extracellular
matrix (ECM), which is composed of proteoglycan aggregates and
collagen fibre that contains collagen types I and II. The
remodeling of condylar cartilage is characterized by the balance
between the synthesis and degradation of various components of the
ECM through the actions of MMPs. These actions may be inhibited by
TIMP (19). MMP-8 (collagenases)
and MMP-9 (gelatinases) are members of the MMP family, which cleave
the major structural components of the ECM of condylar cartilage
and may be inhibited by TIMP-1 (20). MMP-8 is expressed in chondrocytes
of condylar cartilage throughout the cartilaginous cell layers
during the growth period and ageing process, suggesting that it
participates in the remodeling of the ECM during the
differentiation of chondrocytes and degradation of cartilaginous
ECM, accompanying endochondral bone formation (21). The results of the present study
support this data and identified that mandibular advancement
increases MMP-8 expression levels and the ratio of MMP-8 to TIMP-1
in condylar cartilage, while diabetes downregulates this
augmentation. These results indicate that mandibular advancement
enhances the remodeling of ECM and facilitates the proliferation of
condylar chondrocytes and subsequent bone formation. Diabetes
significantly impairs the remodeling of ECM and the proliferation
of condylar chondrocytes caused by mandibular advancement.
Cartilage is usually resistant to vascular invasion
(22). At the time of
ossification, a specific process occurs to allow ingrowth of
vessels into the matrix. MMP-9 is an important candidate for
regulating these functions and is crucial for regulating
hypertrophic cartilage angiogenesis by degrading the matrix and
thus allowing penetration of endothelial cells and the release of
specific factors that accelerate angiogenesis, for example vascular
endothelial growth factor and fibroblast growth factor (23–25).
In the present study, the mechanisms by which mandibular
advancement increases the expression levels of MMP-9 in the
proliferation and chondrogenic layers in the DG and NG, remain
unknown, particularly as MMP-9 plays a crucial role in
ossification. To understand this process, further analysis must be
performed, taking into account whether MMP-9 participates in
proliferation of condylar chondrocytes. Positive
immunohistochemical staining for MMP-9 was observed in the
hypertrophic layer at days 21 and 28 in the NG suggesting
ossification occurred due to angiogenesis induced by MMP-9.
Diabetes downregulated MMP-9 expression, the ratio of MMP-9 to
TIMP-1 and the total layer thickness of the cartilage, which
implies that diabetes may alter the condylar response to mandibular
forward positioning. In addition, diabetes downregulated MMP-9
expression in the hypertrophic layer, indicating that diabetes may
have impaired condylar ossification, which supports the
histological findings that the hypertrophic layer was thicker than
that of the NG at days 21 and 28.
According to our results, proliferation and
ossification of condylar chondrocytes and subsequent bone formation
in diabetic animals in response to mandibular advancement, was
lower than that observed in healthy animals subjected to the same
stimulus. Insulin deficiency-altering condylar growth
modifications, which were induced by mandibular advancement, may
account for these observations, since insulin exerts a direct
effect on the chondrocytes and articular cartilage. Previous
studies have shown that extracellular glucose concentrations may
directly affect specific chondrocyte functions. For example, high
glucose concentrations have been found to decrease proteoglycan
synthesis and dehydroascorbate uptake, which is essential for
collagen synthesis (26,27). High glucose levels also increased
the expression of MMP-1 and MMP-13 in osteoarthritic human
chondrocytes (28). Therefore, we
hypothesize that hyperglycemia may also be associated with the
alteration of condylar growth modification induced by mandibular
advancement in diabetic animals. This hypothesis is consistent with
the observations of the current study in which recovery of the
condylar growth modification was observed in the TG, and subsequent
blood glucose levels were well controlled.
In conclusion, the results of the present study
suggest that young patients with type 1 diabetes mellitus with
class II malocclusion who are not strictly monitored should not
receive treatment with functional appliances until after
hyperglycemia is controlled with insulin treatment.
References
|
1
|
McNamara JA Jr: Components of class II
malocclusion in children 8–10 years of age. Angle Orthod.
51:177–202. 1981.
|
|
2
|
Paulsen HU: Morphological changes of the
TMJ condyles of 100 patients treated with the Herbst appliance in
the period of puberty to adulthood: a long-term radiographic study.
Eur J Orthod. 19:657–668. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Serbesis-Tsarudis C and Pancherz H:
‘Effective’ TMJ and chin position changes in Class II treatment.
Angle Orthod. 78:813–818. 2008.
|
|
4
|
Bensch L, Braem M, Van Acker K and Willems
G: Orthodontic treatment considerations in patients with diabetes
mellitus. Am J Orthod Dentofacial Orthop. 123:74–78. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burden D, Mullally B and Sandler J:
Orthodontic treatment of patients with medical disorders. Eur J
Orthod. 23:363–372. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Braga SM, Taddei SR, Andrade I Jr, et al:
Effect of diabetes on orthodontic tooth movement in a mouse model.
Eur J Oral Sci. 119:7–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Villarino ME, Lewicki M and Ubios AM: Bone
response to orthodontic forces in diabetic Wistar rats. Am J Orthod
Dentofacial Orthop. 139:S76–S82. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maor G, Silbermann M, von der Mark K,
Heingard D and Laron Z: Insulin enhances the growth of cartilage in
organ and tissue cultures of mouse neonatal mandibular condyle.
Calcif Tissue Int. 52:291–299. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kellner K, Schulz MB, Göpferich A and
Blunk T: Insulin in tissue engineering of cartilage: a potential
model system for growth factor application. J Drug Target.
9:439–448. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Claassen H, Schlüter M, Schünke M and Kurz
B: Influence of 17beta-estradiol and insulin on type II collagen
and protein synthesis of articular chondrocytes. Bone. 39:310–317.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mobasheri A, Vannucci SJ, Bondy CA, et al:
Glucose transport and metabolism in chondrocytes: a key to
understanding chondrogenesis, skeletal development and cartilage
degradation in osteoarthritis. Histol Histopathol. 17:1239–1267.
2002.PubMed/NCBI
|
|
12
|
Cai L, Okumu FW, Cleland JL, et al: A slow
release formulation of insulin as a treatment for osteoarthritis.
Osteoarthritis Cartilage. 10:692–706. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rosa SC, Rufino AT, Judas F, Tenreiro C,
Lopes MC and Mendes AF: Expression and function of the insulin
receptor in normal and osteoarthritic human chondrocytes:
modulation of anabolic gene expression, glucose transport and
GLUT-1 content by insulin. Osteoarthritis Cartilage. 19:719–727.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiong H, Hägg U, Tang GH, Rabie AB and
Robinson W: The effect of continuous bite-jumping in adult rats: a
morphological study. Angle Orthod. 74:86–92. 2004.PubMed/NCBI
|
|
15
|
Proff P, Gedrange T, Franke R, et al:
Histological and histomorphometric investigation of the condylar
cartilage of juvenile pigs after anterior mandibular displacement.
Ann Anat. 189:269–275. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ghafari J and Degroote C: Condylar
cartilage response to continuous mandibular displacement in the
rat. Angle Orthod. 56:49–57. 1986.PubMed/NCBI
|
|
17
|
Shen G and Darendeliler MA: The adaptive
remodeling of condylar cartilage - a transition from chondrogenesis
to osteogenesis. J Dent Res. 84:691–699. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cancedda R, Castagnola P, Cancedda FD,
Dozin B and Quarto R: Developmental control of chondrogenesis and
osteogenesis. Int J Dev Biol. 44:707–714. 2000.PubMed/NCBI
|
|
19
|
Birkedal-Hansen H, Moore WG, Bodden MK, et
al: Matrix metalloproteinases: a review. Crit Rev Oral Biol Med.
4:197–250. 1993.PubMed/NCBI
|
|
20
|
Kerrigan JJ, Mansell JP and Sandy JR:
Matrix Turnover. J Orthod. 27:227–233. 2000. View Article : Google Scholar
|
|
21
|
Bae JW, Takahashi I, Sasano Y, et al:
Age-related changes in gene expression patterns of matrix
metalloproteinases and their collagenous substrates in mandibular
condylar cartilage in rats. J Anat. 203:235–241. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carlevaro MF, Albini A, Ribatti D, et al:
Transferrin promotes endothelial cell migration and invasion:
implication in cartilage neovascularization. J Cell Biol.
136:1375–1384. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Colnot C, Thompson Z, Miclau T, Werb Z and
Helms JA: Altered fracture repair in the absence of MMP9.
Development. 130:4123–4133. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Engsig MT, Chen QJ, Vu TH, Pedersen AC, et
al: Matrix metalloproteinase 9 and vascular endothelial growth
factor are essential for osteoclast recruitment into developing
long bones. J Cell Biol. 151:879–889. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Melton JT, Clarke NM and Roach HI: Matrix
metalloproteinase-9 induces the formation of cartilage canals in
the chondroepiphysis of the neonatal rabbit. J Bone Joint Surg Am.
88:155–161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kelley KM, Johnson TR, Ilan J and
Moskowitz RW: Glucose regulation of the IGF response system in
chondrocytes: induction of an IGF-I-resistant state. Am J Physiol.
276:R1164–R1171. 1999.PubMed/NCBI
|
|
27
|
McNulty AL, Stabler TV, Vail TP, McDaniel
GE and Kraus VB: Dehydroascorbate transport in human chondrocytes
is regulated by hypoxia and is a physiologically relevant source of
ascorbic acid in the joint. Arthritis Rheum. 52:2676–2685.
2005.PubMed/NCBI
|
|
28
|
Rosa SC, Rufino AT, Judas FM, Tenreiro CM,
Lopes MC and Mendes AF: Role of glucose as a modulator of anabolic
and catabolic gene expression in normal and osteoarthritic human
chondrocytes. J Cell Biochem. 112:2813–2824. 2011. View Article : Google Scholar : PubMed/NCBI
|