Introduction
Tuberculosis (TB) is a major public health issue in
Turkey, and worldwide. Preventive treatment (TB-PT) is essential in
the fight against TB, and the determination and treatment of index
cases. TB-PT aims to prevent the occurrence of active TB in
patients with latent TB infection (1). Prior to TB-PT, the tuberculosis skin
test (TST), also known as the purified protein derivative (PPD)
test, is used to determine whether an individual has developed an
immune response to the bacterium that causes tuberculosis. The TST
is based on the fact that infection with Mycobacterium
tuberculosis bacterium causes a delayed-type hypersensitivity
skin reaction to certain components of the bacterial reaction with
the skin. PPD is initiated when T cells, which have been sensitized
by prior infection, are recruited to the skin site. Here, they
release lymphokines which induce induration (a hard, raised area
with clearly defined margins at and around the injection site)
through local vasodilation leading to edema, fibrin deposition and
the recruitment of other types of inflammatory cells to the area.
TB-PT has been part of the TB control program in Turkey for
decades. Since 2003, with the publication of the new TB Guidelines
in Turkey, contact tracing and the use TB-PT has increased
(2). TB cases that develop from
contact with other active TB cases have decreased following this
practice.
The Turkish national guidelines (3) on the administration of preventative
treatment identify the following groups at a high risk for TB
infection: Children <15 years-old showing a positive response to
the TST; patients with TST conversion, patients <35 years-old
who have come into close contact with active pulmonary TB cases;
and patients with other conditions that are vulnerable to
developing active TB, including HIV, diabetes mellitus, scalp,
neck, blood and lymph system cancers, low weight, silicosis or
apical fibrotic lesions, patients using tumor necrosis factor
(TNF)-α inhibitors or corticosteroids, and patients that have
undergone mastectomy, jejunoileal bypass and organ transplantations
(2,3).
The aim of the present study was to evaluate the
application of TB-PT in a single medical center between 2008 and
2011.
Materials and methods
Methodology
Demographic data, indications for treatment, BCG
vaccine scarring, TST values and therapy results of patients who
received TB-PT between 2008 and 2011 at the Ankara Tuberculosis
Control Dispensary No. 7 (Ankara, Turkey) were evaluated
retrospectively. The ‘Prevention with Drugs’ registry in the
dispensary was used. Prior to the initiation of TB-PT, 0.1 ml 5
tuberculin units (0.1 ml) of tuberculin were intradermally injected
on the inner forearm of the patients, in accordance with the
Mantoux technique. The induration size was measured after 72 h and
assessed according to the Turkish national guidelines (3). For BCG-vaccinated patients, an
induration size of ≤5 mm was defined as negative, 6–14 mm was
considered to be associated with the vaccine or suspicious, and an
induration of >15 mm was considered positive. For non-vaccinated
subjects, an induration of 6–9 mm was regarded as suspicious, while
>10 mm was considered positive. A chest X-ray was performed in
all cases prior to treatment, and TB-PT was administered to all
cases without active TB disease (3). This retrospective study was approved
by the Department of Tuberculosis Control at the Ministry of Health
(no. 26475; Ankara, Turkey), and written informed consent was
provided by the patients.
Results
Patients
A TST was performed in 5,855 cases and TB-PT was
administered to 463 cases between 2008 and 2011. The indications
for TB-PT included close contact with an active TB case (44%), a
positive TST in a child <15 years-old (25%) and the
administration of immunosuppressants (31%). Table I demonstrates the yearly
indications for TB-PT within the 2008–2011 period. Immunosupression
in 144 patients was caused by the use of steroids (10%) and TNF-α
inhibitors (90%), administered to treat conditions, such as
rheumatoid arthritis, ankylosing spondylitis, psoriasis, Behçet’s
disease and Crohn’s disease. TNF-α inhibitors were most commonly
used to treat rheumatoid arthritis (52%). TB-PT was not
administered to cases with TST conversion and sequela lesions. The
male/female patient ratios were 106/98 for cases of close contact
with active TB, 61/54 for TST-positive cases and 85/59 for
immunosuppressed cases. The mean age was 9±5.7 years (age range,
1–35 years) for cases with close contact with active TB, 9.5±3.8
years (age range, 1–15 years) for TST-positive cases and 38±14.9
years (age range, 1–77 years) for immunosuppression cases. The
gender and age distributions of TB-PT cases are reported in
Table II.
 | Table INumber and percentage of TB-PT
indications in study subjects between 2008 and 2011. |
Table I
Number and percentage of TB-PT
indications in study subjects between 2008 and 2011.
| Year | |
|---|
|
| |
|---|
| Indication | 2008 | 2009 | 2010 | 2011 | Total |
|---|
| TB close contact, n
(%) | 59 (53) | 86 (56) | 30 (28) | 29 (31) | 204 (44) |
| TST-positive, n
(%) | 37 (34) | 34 (22) | 24 (23) | 20 (22) | 115 (25) |
| Immunosuppression, n
(%) | 14 (13) | 34 (22) | 52 (49) | 44 (47) | 144 (31) |
| Total, n | 110 | 154 | 106 | 93 | 463 |
 | Table IISubject characteristics according to
the indications of TB-PT. |
Table II
Subject characteristics according to
the indications of TB-PT.
| Parameter | TB close contact | TST-positive |
Immunosuppression |
|---|
| Female, n (%) | 98 (48) | 54 (47) | 59 (41) |
| Male, n (%) | 106 (52) | 61 (53) | 85 (59) |
| Mean age ± SD,
years | 9.0±5.7 | 9.5±3.8 | 38.0±14.9 |
| Age range, years | 1–35 | 1–15 | 1–77 |
Preventive therapy
The completion rate of TB-PT was 78.6% (n=364), with
65 patients not completing the therapy (14.0%) and 13 cases
transferred to a different center. The results of TB-PT are
summarized in Table III.
 | Table IIIResults of TB-PT according to
indications (number and percentage). |
Table III
Results of TB-PT according to
indications (number and percentage).
| Parameter | Therapy
completion | Default | TB diagnosis | Mortality | Center transfer | Other* [adverse effects]** | Total |
|---|
| TB close contact, n
(%) | 158 (77) | 38 (19) | 1 (1) | 0 (0) | 3 (1) | 4 [1]** | 204 (100) |
| TST-positive, n
(%) | 102 (89) | 10 (8) | 1 (1) | 0 (0) | 0 (0) | 2 [2]** | 115 (100) |
| Immunosuppression, n
(%) | 104 (72) | 17 (12) | 0 (0) | 1 (1) | 10 (7) | 12 [5]** | 144 (100) |
| Total, n (%) | 364 (78.6) | 65 (14.0) | 2 (0.4) | 1 (0.2) | 13 (2.8) | 18 (3.9)* [2.3]** | 463 (100) |
TB development
Active TB developed in two cases during the course
of the TB-PT, despite showing normal chest X-rays prior to
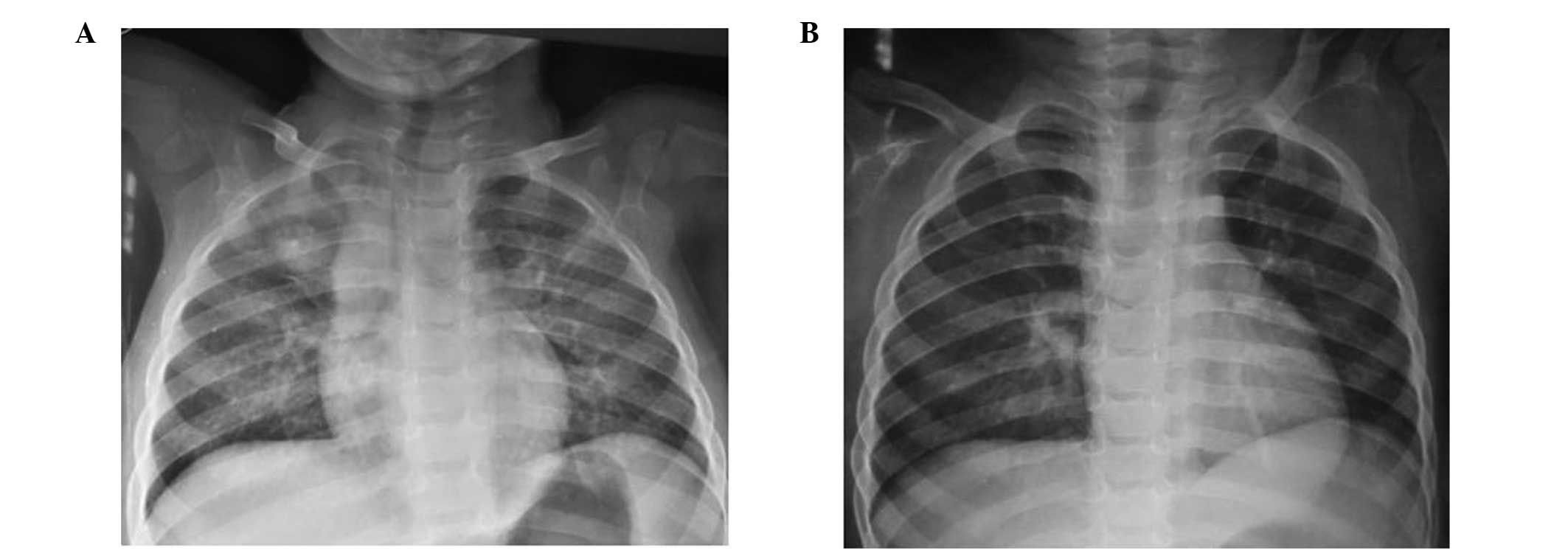
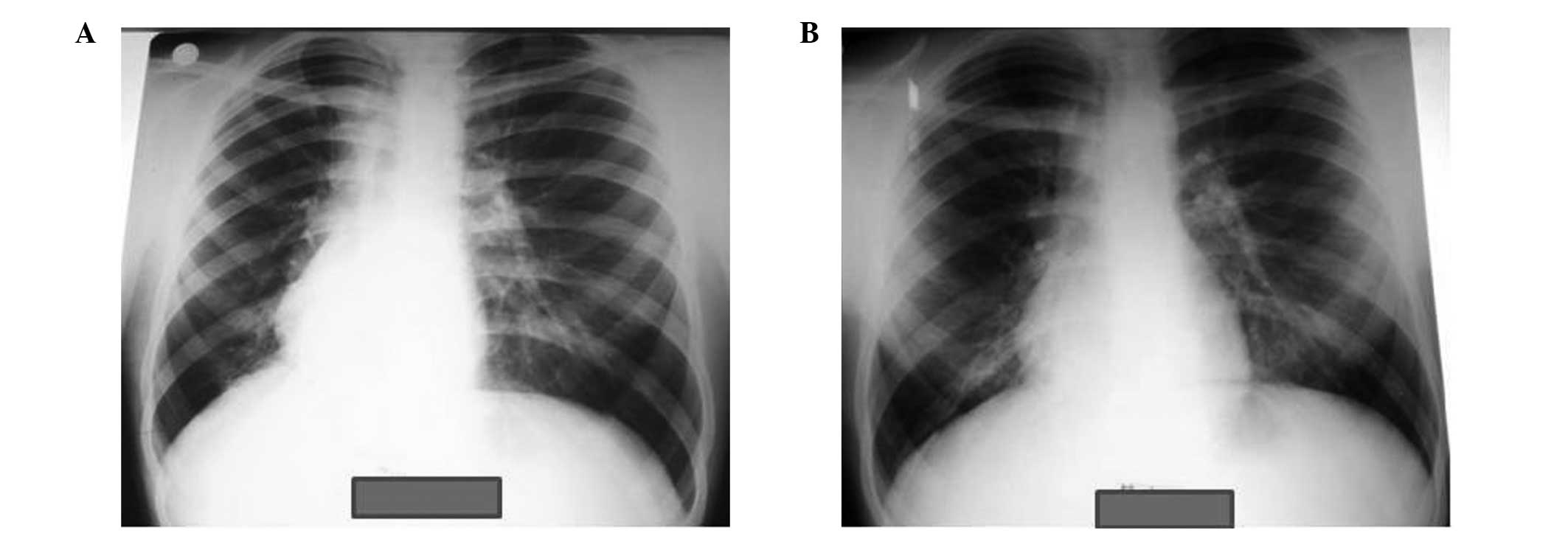
isoniazid (INH) therapy. The first case involved a BCG-vaccinated
female aged 1.5 years, who exhibited a TST induration of 10 mm. The
father was identified as a smear-positive case of pulmonary TB, and
the child was diagnosed with active pulmonary TB after 120 days of
TB-PT. The second case involved a 14-year-old TST-positive male (16
mm induration; BCG-vaccinated) with dextrocardia, who developed
active pulmonary TB after 50 days of treatment. Chest X-rays of the
two cases with developing active TB are presented in Figs. 1 and 2. Standardized active TB treatment for
children (two months of INH, rifampin and pyrazinamide plus four to
seven months of INH and rifampin) was administered to these cases
and completed according to the national guidelines.
Therapeutic outcomes
The rate of TB-PT discontinuation was 1% for
patients with close contact to active TB, 2% for TST-positive
patients receiving INH prophylaxis for six months, and 5% for
immunosuppressed patients receiving INH prophylaxis for nine
months. Between 2008 and 2011, the percentage of TB-PT indications
in patients with TB close contact decreased, while TB-PT in
immunosuppressed patients increased.
Discussion
Alongside early diagnosis and treatment, BCG
vaccination and TB-PT are crucial in the fight against TB. Since
2006, single-dose BCG vaccinations have been applied to the general
population under the Vaccination Program of the Turkish Ministry of
Health, as opposed to the two-dose vaccinations applied prior to
2006. Children receive BCG vaccinations within the first three
months of their life, unless any contraindications exist. After
this age, a BCG vaccination is only applied according to the TST
reaction, while after six years of age, BCG vaccinations are not
administered. Active TB should be excluded prior to TB-PT, and
periodical liver function tests (LFTs) should be performed
following the initiation of treatment.
TB-PT should be discontinued if LFTs reveal a
five-fold elevation of the basal value or three-fold elevation of
the upper limit of aspartate aminotransferase, symptoms of nausea
and vomiting or elevated levels of bilirubin and transaminase,
unless the elevation is associated with other causes, such as viral
hepatitis (2). The Tuberculosis
Control Society of the Turkish Ministry of Health recommends TB-PT
with INH for six months at 10 mg/kg/day (maximum 300 mg/day). In
cases of HIV-positive patients or immunosuppression, the duration
of the TB-PT is nine months (2,3).
Latent tuberculosis infection (LTBI) can be detected
with immune-based tests, including the TST and interferon-γ release
assay. Therapy in TST-positive patients can reduce the subsequent
risk of disease reactivation and development of active TB. The
effectiveness of INH treatment for LTBI, as measured in randomized
controlled trials, varied between 25 and 92% (4–8). The
current standard therapy using INH reduces the risk of active TB by
up to 90%, if administered daily for nine months. However, the long
therapy duration is disapproved by patients, and the risk of
serious adverse effects, including hepatotoxicity, discourages
patients and clinicians. As a result, therapy completion is <50%
in a number of programs, and the problems associated with INH have
stimulated the development and evaluation of several shorter
regimens. An alternative therapy is the daily administration of
rifampin and pyrazinamide for two months; however, this regimen is
not currently used due to unacceptably high rates of hepatotoxicity
and poor tolerability. A combination of INH and rifampin,
administered for three or four months, has demonstrated an efficacy
equivalent to six months of INH therapy, albeit with somewhat
increased hepatotoxicity. Four months of rifampin treatment has an
efficacy that is at least equivalent to six months of INH therapy;
however, there are inadequate trial data available on efficacy. The
safety of a four-month regimen of rifampin has been demonstrated in
numerous studies. Recently, a large-scale trial evaluated a
three-month INH regimen with weekly administration of rifapentine,
under direct observation. This regimen may be promising in the
treatment of LTBI if found as effective as INH treatment; however,
the study results are yet to be published (4–8).
Chronic viral hepatitis (CVH) was not established as
a risk factor for INH hepatotoxicity during TB-PT of CVH patients
(9). Dobler and Marks reviewed the
clinical files of patients that received TB-PT between 2000 and
2010. Out of 216 patients who commenced INH treatment for TB-PT,
163 (75%) completed the six-month treatment. Of the patients who
did not complete the treatment, 53% dropped out after three months
of treatment (10). A prospective
cohort study demonstrated that only 53% of patients that began LTBI
treatment completed the therapy (11). Horsburgh et al performed a
retrospective trial at 68 clinics providing LTBI treatment, and
observed that less than half the patients beginning the nine month
LTBI treatment completed the therapy (12). According to the retrospective
analysis performed by Kwara et al at a TB clinic in the USA
in 2003, of the 845 patients with LTBI, 690 patients (81.6%)
initiated a nine-month INH therapy. Only 426 patients (61.7%)
completed the therapy, and follow-up was not possible for 246
patients (35.6%). Treatment was discontinued in 18 patients (2.6%),
and it was primarily younger patients that failed to complete the
therapy (13). In an additional
study, adults that were not infected with HIV began treatment for
LTBI at two specialist TB units in Spain. Of the 599 individuals
that initiated treatment, 484 patients (80.8%) completed the
treatment course. In patients under <36 years-old, short
treatment regimens were not associated with improved treatment
completion rates, when compared with the six-nine-month INH therapy
(14). A systematic review of two
databases (MEDLINE and EMBASE), stratifying patients according to
age, aimed to determine the age-related risk of INH and rifampin
hepatotoxicity under the recommended LTBI treatment regimens.
Hepatotoxicity rates were low; however, the rates were higher among
patients aged ≥35 years (1.7%) when compared with patients aged
<35 years (0.2%) (15).
A survey of 217 LTBI patients in the USA revealed
that only 28.6% of the patients finished at least six months of INH
therapy under usual clinical conditions (16). In addition, a previous study
performed on 1–18 year-old Mexican immigrant children revealed that
out of 150 children with LTBI, 111 individuals (74%) completed INH
treatment, 13 (9%) were transferred to a different center, while
four children (3%) did not begin the treatment. One of the patients
receiving treatment developed INH hepatitis (17).
Furthermore, a previous study reviewed the medical
records of 474 LTBI patients placed on a four-month rifampin or
nine-month INH treatment course between 2000 and 2003. The rifampin
treatment was completed by 80.5% of the patients, while the INH
treatment was completed by 53.1%. Patients receiving rifampin
exhibited fewer reactions to the treatment and were significantly
more likely to complete the therapy when compared with the patients
receiving INH (18).
The present study revealed that the overall TB-PT
therapy completion rate was 78.6%. More specifically, the
completion rate was 77% for patients with close contact to active
TB, 89% for TST-positive patients and 72% for immunosuppressed
patients. The completion of long-term LTBI treatment was lowest in
patients with immunosuppression. The rates of TB-PT discontinuation
due to adverse effects were 1% for patients with TB close contact,
2% for TST-positive patients and 5% for patients with
immunosuppression. The percentage of cases with TB close contact
undergoing treatment decreased, while the immunosuppression cases
(particularly using TNF-α inhibitors) increased in the four-year
study period. The results of the present study, with regard to the
rate of chemoprophylaxis completion, are comparable with those of
previously published studies (10–13).
In conclusion, patients receiving TB-PT should be
monitored and/or followed-up carefully to control any side-effects
from the treatment and the development of active TB.
References
|
1
|
Migliori GB, Raviglione MC, Schaberg T, et
al: Tuberculosis management in Europe. Task Force of the European
Respiratory Society (ERS), the World Health Organisation (WHO) and
the International Union against Tuberculosis and Lung Disease
(IUATLD) Europe Region. Eur Respir J. 14:978–992. 1999. View Article : Google Scholar
|
|
2
|
Gokcay G: Tuberculin skin test. Thorax
Books: Tuberculosis. Ozkara S and Kilicaslan Z: 11. AVES
Publishing; Istanbul: pp. 206–217. 2010
|
|
3
|
Ozkara S, Aktas Z, Ozkan S and Ecevit H;
T.R. Ministry of Health, Department of Struggle Against
Tuberculosis. Reference Book for the Control of Tuberculosis in
Turkey. Rekmay Press; Ankara: pp. 55–58. 2003
|
|
4
|
Menzies D, Al Jahdali H and Al Otaibi B:
Recent developments in treatment of latent tuberculosis infection.
Indian J Med Res. 133:257–266. 2011.PubMed/NCBI
|
|
5
|
Lobue P and Menzies D: Treatment of latent
tuberculosis infection: An update. Respirology. 15:603–622. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chee CB, Sester M, Zhang W and Lange C:
Diagnosis and treatment of latent infection with Mycobacterium
tuberculosis. Respirology. 18:205–216. 2013. View Article : Google Scholar
|
|
7
|
No authors listed. Targeted tuberculin
testing and treatment of latent tuberculosis infection. American
Thoracic Society MMWR Recomm Rep. 49(RR-6): 1–51. 2000.PubMed/NCBI
|
|
8
|
Cohn DL: Treatment of latent tuberculosis
infection: renewed opportunity for tuberculosis control. Clin
Infect Dis. 31:120–124. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bliven EE and Podewils LJ: The role of
chronic hepatitis in isoniazid hepatotoxicity during treatment for
latent tuberculosis infection. Int J Tuberc Lung Dis. 13:1054–1060.
2009.PubMed/NCBI
|
|
10
|
Dobler CC and Marks GB: Completion of
treatment for latent tuberculosis infection with monthly drug
dispensation directly through the tuberculosis clinic. PLoS One.
7:e489002012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goswami ND, Gadkowski LB, Piedrahita C, et
al: Predictors of latent tuberculosis treatment initiation and
completion at a U.S. public health clinic: a prospective cohort
study. BMC Public Health. 12:4682012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Horsburgh CR Jr, Goldberg S, Bethel J, et
al: Tuberculosis Epidemiologic Studies Consortium: Latent TB
infection treatment acceptance and completion in the United States
and Canada. Chest. 137:401–409. 2010. View Article : Google Scholar
|
|
13
|
Kwara A, Herold JS, Machan JT and Carter
EJ: Factors associated with failure to complete isoniazid treatment
for latent tuberculosis infection in Rhode Island. Chest.
133:862–868. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Anibarro L, Casas S, Paz-Esquete J, et al:
Mycobacteria Study Group (GEIM) of Spanish Society of Clinical
Microbiology and Infections Diseases (SEIMC): Treatment completion
in latent tuberculosis infection at specialist tuberculosis units
in Spain. Int J Tuberc Lung Dis. 14:701–707. 2010.
|
|
15
|
Kunst H and Khan KS: Age-related risk of
hepatotoxicity in the treatment of latent tuberculosis infection: a
systematic review. Int J Tuberc Lung Dis. 14:1374–1381.
2010.PubMed/NCBI
|
|
16
|
Shieh FK, Snyder G, Horsburgh CR, et al:
Predicting non-completion of treatment for latent tuberculous
infection: a prospective survey. Am J Respir Crit Care Med.
174:717–721. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Young J, Edick T, Klee D and O’Connor ME:
Successful treatment of pediatric latent tuberculosis infection in
a community health center clinic. Pediatr Infect Dis J.
31:e147–e151. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lardizabal A, Passannante M, Kojakali F,
et al: Enhancement of treatment completion for latent tuberculosis
infection with 4 months of rifampin. Chest. 130:1712–1717. 2006.
View Article : Google Scholar : PubMed/NCBI
|