Introduction
Clinically, atrial fibrillation (AF), which is of
one of the most common types of arrhythmia, shows high disability
and mortality rates in patients (1,2). In
recent years, angiotensin II (AngII) and AF occurrence and
maintenance has experienced increasing attention. The AngII levels
in AF increase and eventually induce atrial fibrosis (3). Atrial fibrosis plays dual roles in
inducing and maintaining AF (4–6). A
previous study (7) showed that the
expression level of hypoxia-inducible factor-1α (HIF-1α) is
associated with AngII, which is involved in renal fibrosis.
However, no associated study has been conducted for myocardial
fibrosis. The present study refers to the method by Zhang et
al (8), which used a
subcutaneous bolus injection of isoproterenol (ISO) to induce AngII
expression to establish an atrial fibrosis rat model. The HIF-1α
inhibitor (9) [sirolimus, also
known as rapamycin (Rapa)] was administered to examine the protein
expression and mRNA levels of AngII, HIF-1α, transforming growth
factor-beta1 (TGF-β1) and matrix
metalloproteinase-9 (MMP-9) in myocardial tissue in the atrial
fibrosis rat model, and thus the present study investigated their
relevance and the possible mechanism of how HIF-1α following the
ISO injection would induce atrial fibrosis.
Materials and methods
Animal model
Thirty healthy male Wistar rats, 180±20 g body
weight, were purchased from the Lanzhou School of Medicine Animal
Center in Lanzhou University (Lanzhou, China), and were maintained
at 20–25°C with lighting-controlled circadian rhythms (8:00
am-10:00 pm) under normal feeding with free food and water. The
rats were randomly divided into three groups of 10 rats: Control,
ISO and ISO plus sirolimus (Rapa). The animal experiment was
approved by the Animal Ethics Committee. The study referred to the
method by Zhang et al (8)
to establish an atrial fibrosis rat model. The ISO group rats were
administered multipoint subcutaneous bolus injections of
hydrochloric acid ISO (batch no. 080705; Shanghai Hefeng
Pharmaceutical Co., Ltd., Shanghai, China), 5 mg/g/day, and once
per day for seven days. The Rapa-intervention group rats were
provided sirolimus oral solution (batch no. 110901; Hangzhou
Zhongmei Huadong Pharmaceutical Co., Ltd., Hangzhou, China),
specification 50 ml:50 mg, initiated on the second of the same ISO
treatment as in the ISO group, 3 mg/kg/day (10), once per day and gavage for 14 days,
with the interval between gavage and subcutaneous injection being
4–6 h. Simultaneously, the control and ISO groups were separately
administered an equal amount of double-distilled water for stomach
gavage as for the Rapa group. All the rats were sacrificed by
cervical dislocation after 15 days.
Sample collection and preservation
Along the coronary plane maximum transverse
diameter, partial myocardial tissue was cut and placed in 10%
formaldehyde solution for 24 h fixation. Following this, the tissue
was paraffin-embedded and five serial slices (4-μm) were cut from
it. Two slices were used for hematoxylin and eosin (HE) and Masson
staining to observe the extent of myocardial fibrosis, which used
collagen volume fraction (CVF) as the atrial fibrosis index.
Immunohistochemistry (IHC) was performed on the remaining three
slices to detect the expression of HIF-1α, TGF-β1 and
MMP-9. The samples were obtained from the remaining cardiac tissue
for detection of AngII by radioimmunoassay, and HIF-1α,
TGF-β1 and MMP-9 expression levels by western blot (WB)
analysis and reverse transcription quantitative polymerase chain
reaction (RT-qPCR). The remaining cardiac tissue was cryopreserved
in liquid nitrogen.
Detection of AngII level in the
myocardium in rats
A radioimmunoassay kit (Beijing North Institute of
Biotechnology, Beijing, China) was used to detect the concentration
of AngII.
Observation of myocardial fibrosis
Myocardial tissue underwent formaldehyde fixation,
dehydration, transparency, embedding in paraffin and slicing into
two 4-μm sections. HE and Masson staining were subsequently
performed, and the sections were mounted and observed by light
microscope and radiography.
The CVF [CVF = (collagen area/total area of view
field) × 100%] calculation was as follows: Three non-vascular
vision images (magnification, ×400) were selected from each Masson
staining slice. The image scanning software, Image-Pro Plus 6.0
(Media Cybernetics, Inc., Rockville, MD, USA), was used for image
analysis and myocardial CVF calculation.
IHC
Myocardial tissue paraffin sections underwent a
number of steps, including dewaxing, antigen hot fix, blocking
solution incubation, first and secondary antibody incubation,
diaminobenzidine coloration, counterstaining, transparency and
mounting. Phosphate-buffered saline (PBS) was used to replace the
first antibody as the negative control, and the positive control
was provided in the IHC kit (Boster Biological Tech Ltd., Wuhan,
China). Rabbit anti-rat antibodies of HIF-1α, TGF-β1 and
MMP-9 were all purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). The first antibody was diluted by 1:50, and the
secondary goat anti-rabbit antibody was provided by Jackson
ImmunoResearch Laboratories, Inc. (West Grove, PA, USA) under the
dilution of 1:500.
WB analysis
Myocardial tissue was placed in liquid nitrogen
pre-cooling mortars, and a proper amount of protein lysate was
added and centrifuged. Subsequent to removal of the supernatant, a
small portion of the precipitation was used for the determination
of protein concentration. Protein (100 μg) was mixed with 5×
protein electrophoresis loading buffer, placed into a boiling water
bath for 5 min, centrifuged at 12,000 × g for 1 min and fully
loaded along with the protein marker (Fermentas, Waltham, MA, USA).
In Tris-glycine buffer (pH 8.0) and under an 80 V voltage, a 1.5–2
h electrophoresis was performed, followed by a 20 V constant
voltage and 1.5 h transferring to nitrocellulose film (Millipore,
Billerica, MA, USA). The film was removed and 5% skimmed milk
powder-sealed liquid/PBS with Tween (PBST) was added under room
temperature and slow agitation was performed for 1.5 h. First
antibody incubation was as follows: HIF-1α, TGF-β1 and
MMP-9 antibodies were purchased from Santa Cruz Biotechnology,
Inc., and diluted 1:300 in 5% skimmed milk powder/PBST at 4°C
overnight; and anti-GAPDH (Santa Cruz Biotechnology, Inc.)
monoclonal antibody was 1:10,000 diluted in 5% skimmed milk
powder/PBST at 4°C overnight. PBST was used to wash the membrane
three times for 10 min each. Secondary antibody incubation was
performed as follows: Goat anti-rabbit antibody was 1:2,000 diluted
in 5% skimmed milk powder/PBST and sheep anti-mouse (second for
GAPDH) was 1:2,000 diluted in 5% skimmed milk powder/PBST,
incubated at room temperature for 1 h, followed by washing of the
membrane three times with PBST for 15 min each. The film was placed
in the SuperSignal™ West Pico (Pierce, Rockford, IL,
USA) for 2 min, tableted and developed to detect specific protein
bands. The gel imaging system was photographed and the strip area
and gray analysis of the protein zone was expressed by the integral
gray value (D).
RT-qPCR
TRIzol® was added to 0.1 g myocardial
tissue to extract RNA; 1–4 μg RNA was obtained for RT-qPCR. CFX-96
(Bio-Rad, Hercules, CA, USA) was used for fluorescence qPCR and the
20 μl PCR amplification reaction system was performed. Primer
sequences were as follows: HIF-1α forward,
5′-ATCTCGGCGAAGCAAAGAGT-3′; reverse, 5′-TGACCA
TCATCTGTTAGCACCAT-3′; TGF-β1 forward,
5′-CTAATG GTGGACCGCAACAAC-3′; reverse, 5′-TAACGCCAGGAA
TTGTTGCTAT-3′; MMP-9 forward, 5′-CAAACCCTGCGT ATTTCCATT-3′,
reverse, 5′-ACATCTCTCCTGCCGAGT TGC-3′; and GAPDH forward,
5′-AGTGCCAGCCTCGTC TCATAG-3′, reverse, 5′-CGTTGAACTTGC
CGTGGGTAG-3′. GAPDH was used as the internal control. Each
sample was loaded in three replicates. HIF-1α,
TGF-β1, MMP-9 and GAPDH
RT-qPCR amplification conditions were as follows: Annealing
temperature at 95°C for 15 sec, extension temperature at 60°C for
20 sec and melting curve temperature at 72°C for 20 sec, in a total
of 45 cycles. At the end of the reaction, fluorescence quantitative
data were collected including the amplification curve, working
curve, melting curve and the corresponding Ct value, according to
the 2−ΔmRNAΔCt method to determine the relative mRNA
expression level.
Statistical methods
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis. All data are shown as
mean±standard deviation. Single factor analysis of variance was
used for inter-group comparison, least significant difference
method was used for pairwise comparison, and the Pearson method was
used for product-moment correlation analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Myocardial interstitial fibrosis
HE staining showed that the control group exhibited
normal space between the myocardial nuclei, regular shape of the
nuclei along the heart muscle and structured alignment of
myocardial interstitial fibrosis (Fig.
1A). The ISO group had increased myocardial interstitial
components and widened nuclear space. Fibrosis and cardiac muscle
fiber shapes were disordered (Fig.
1B). The Rapa group showed a degree of reduction compared with
the ISO group in the shape of the nuclei, and myocardial
interstitial fibrous and interstitial fiber arrangements were
disordered (Fig. 1C).
The Masson staining and CVF results showed that
under light microscopy, normal myocardial interstitial collagen
components appeared green (light green counterstained), the nuclei
appeared blue, and myocardial fibers, cytoplasm and red blood cells
appeared red (Fig. 1D-F). The
image was analyzed to calculate the level of atrial fibrosis (CVF
index), taking the average value as the measurement value. The
control group (15.482±0.837%) did not show atrial fibrosis, and the
Rapa group (16.730±1.052%) showed a greatly reduced atrial fibrosis
level compared with the ISO group (86.704±1.982%) (P<0.01). The
difference between the Rapa and control groups did not show a
statistically significant difference (P>0.05).
Ang II levels in the myocardium of rats
by radioimmunoassay
The result showed that the ISO (139.402±4.431 ng/l)
and Rapa (132.712±5.316 ng/l) groups had significantly increased
Ang II levels (P<0.01) compared with the control group
(31.172±7.271 ng/l).
IHC
Immunohistochemical detection of HIF-1α,
TGF-β1 and MMP-9 expression showed claybank color for
positive staining in myocardial cells of the rats under microscopy,
distributed throughout the myocardial cytoplasm. The ISO group
showed stronger expression levels than the control group, while the
Rapa group showed markedly reduced levels of expression compared
with the ISO group (Fig. 2).
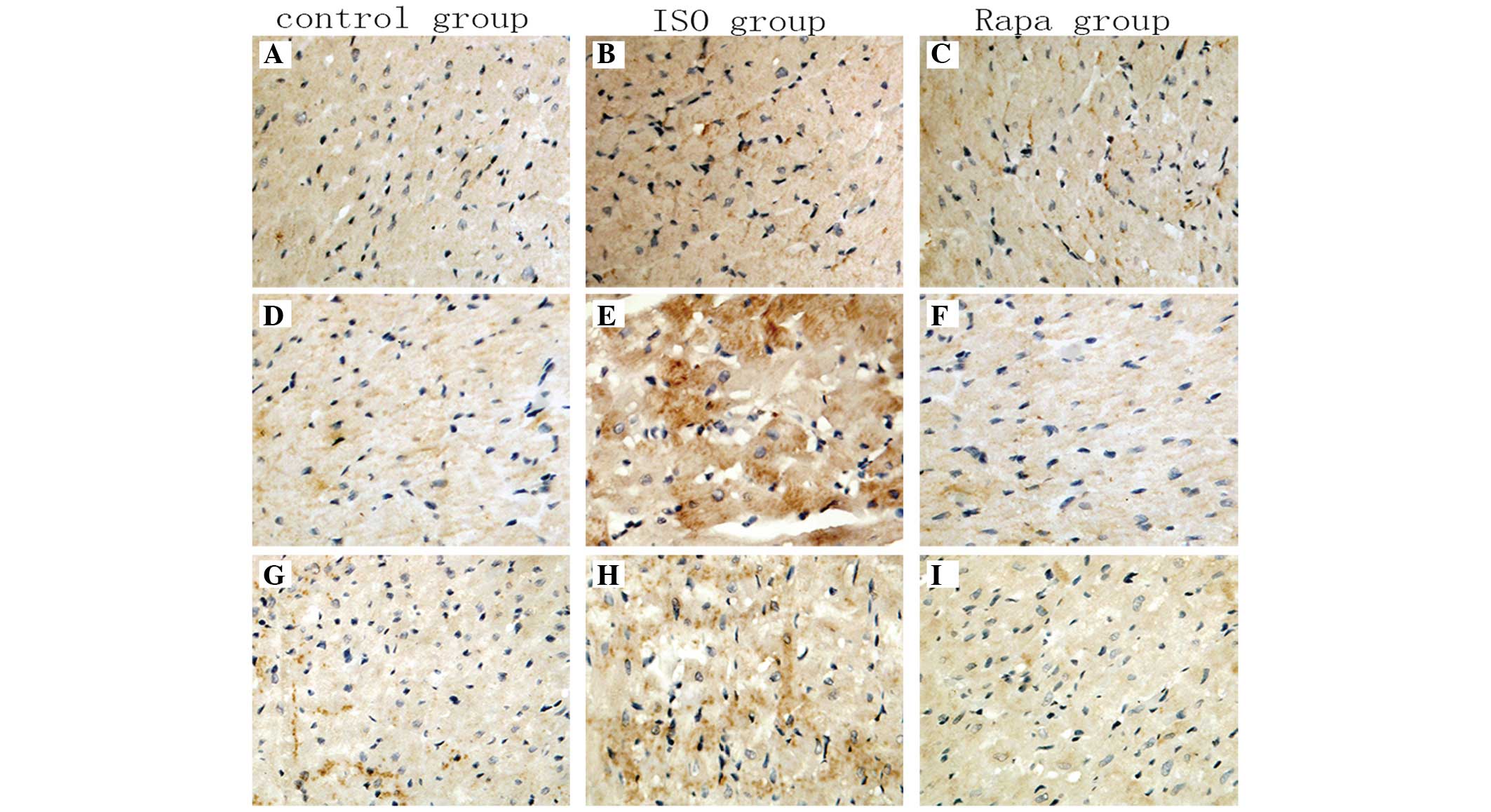 | Figure 2Immunohistochemistry results of
HIF-1α, TGF-β1 and MMP-9 expression. Comparison of
HIF-1α, TGF-β1 and MMP-9 expression in myocardial cells
(claybank spots) in the cytoplasm represents positive staining. (A,
D and G) Control; (B, E and H) ISO; and (C, F and I) Rapa groups
(magnification, ×400). ISO, isoproterenol; Rapa, ISO plus
sirolimus; HIF-1α, hypoxia-inducible factor-1α; TGF-β1,
transforming growth factor-β1; MMP-9, matrix
metalloproteinase-9. |
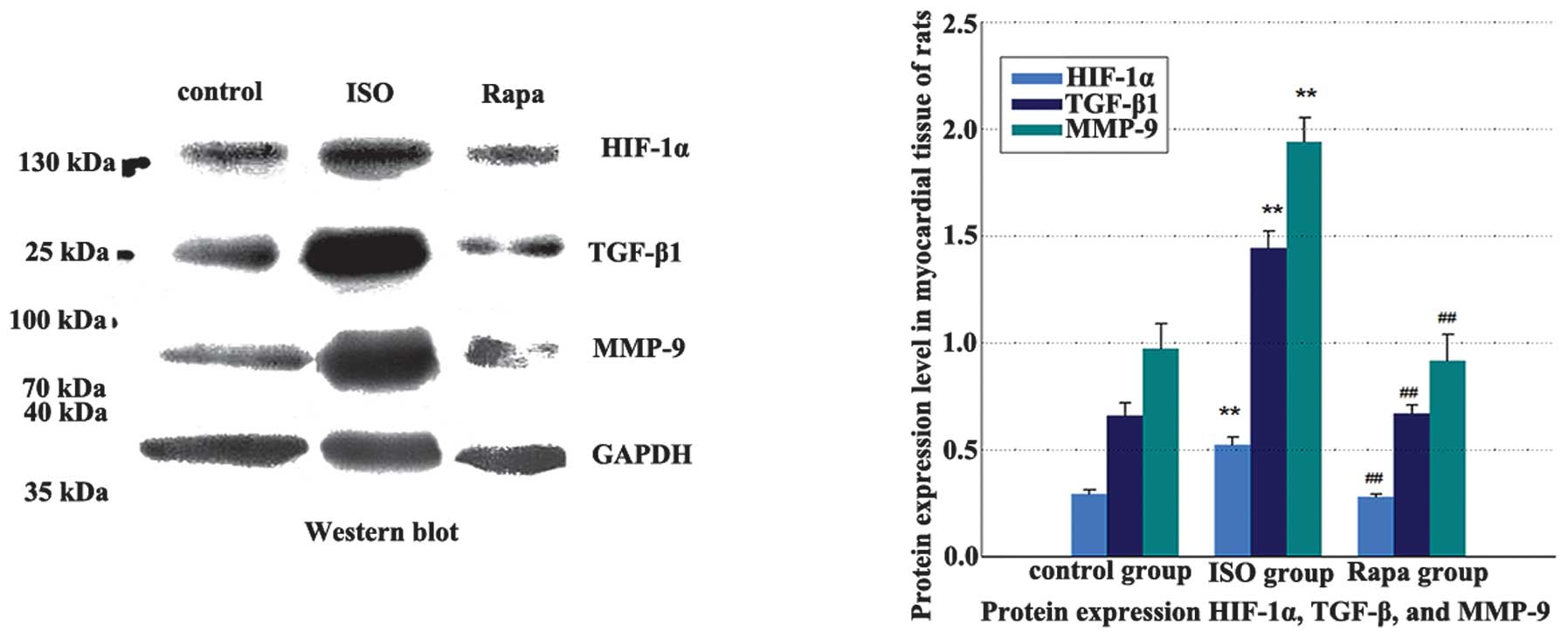
WB analysis and RT-qPCR
The HIF-1α, TGF-β1
and MMP-9 at the mRNA and protein levels were higher in the
ISO group than those in the control group. The mRNA and protein
expression in the Rapa group were significantly lower than those in
the ISO group (Table I and
Fig. 3).
 | Table IWestern blot analysis and RT-qPCR
results of HIF-1α, TGF-β1 and MMP-9. |
Table I
Western blot analysis and RT-qPCR
results of HIF-1α, TGF-β1 and MMP-9.
| HIF-1α |
TGF-β1 | MMP-9 |
|---|
|
|
|
|
|---|
| Group | Protein | mRNA | Protein | mRNA | Protein | mRNA |
|---|
| Control | 0.294±0.021 |
(5.795±0.822)×10−5 | 0.660±0.059 |
(1.223±0.085)×10−2 | 0.974±0.116 |
(2.596±0.193)×10−6 |
| ISO | 0.522±0.039a |
(2.103±0.492)×10−4a | 1.444±0.081a |
(7.511±0.156)×10−2a | 1.939±0.113a |
(4.854±0.210)×10−6a |
| Rapa | 0.279±0.016b |
(6.374±0.993)×10−5b | 0.669±0.040b |
(1.205±0.090)×10−2b | 0.916±0.125b |
(2.789±0.180)×10−6b |
Associations among HIF-1α,
TGF-β1 and MMP-9 expression levels in muscle
fibrosis
In myocardial tissue, HIF-1α, TGF-
β1 and MMP-9 mRNA expression levels
were positively correlated (r=0.919, 0.997 and 0.985; P<0.01)
with atrial fibrosis (CVF index). HIF-1α,
TGF-β1 and MMP-9 mRNA expression
levels themselves also exhibited a significant positive correlation
(r=0.936 and 0.888; P<0.01). MMP-9 and
TGF-β1 mRNA expression levels were
positively correlated (r=0.981, P<0.01).
Discussion
The majority of studies show that the
renin-angiotensin system (RAS) is activated by AF, and
simultaneously, as a major effecter molecule of the RAS in
circulating and certain tissues (11), AngII levels increase and eventually
induce atrial fibrosis (3). The
present study identified that AngII levels in the ISO and Rapa
groups in myocardial tissue were significantly higher than the
control group after seven days of subcutaneous multiple high-dose
continuous injection of ISO in rats, which implies that following
ISO injection, RAS was activated and caused increased expression
levels of AngII in myocardial tissue. According to the
morphological observation, no atrial fibrosis was identified in the
control group, while the ISO group had significant myocardial
interstitial fibrosis, which indicates that high expression of
AngII in myocardial tissues may be involved in atrial fibrosis
formation, and this has been confirmed by a previous study
(12).
The level of HIF-1α expression, as a tissue hypoxia
index product, will increase during tissue hypoxia. Hypoxia has
been linked to fibrosis (13),
including in the liver (14,15),
lung (16) and kidney (17). Although studies have shown that the
increase in HIF-1α gene expression in the myocardium may be
involved in its structural changes, including atrial fibrosis
(18,19), no study has been conducted for the
elevated AngII-induced myocardial fibrosis. The present study, from
the aspects of pathology and protein and mRNA expression levels,
found that the expression of HIF-1α in the ISO group was
significantly higher than that in the control group, while when
administering the HIF-1α inhibitor rapamycin intervention in the
Rapa group, HIF-1α expression decreased significantly, and was
positively correlated with myocardial fibrosis degree (CVF), which
proves that AngII is involved in atrial fibrosis by regulating the
expression of HIF-1α.
TGF-β1, as one of the AngII downstream
factors (20), is associated with
myocardial fibrosis occurrence (21). The present study also provides
evidence for this. HIF-1α has a close association with
TGF-β1 and can regulate its expression (22,23).
The present study shows that in the ISO group,
TGF-β1 mRNA expression levels were much
higher than those in the control group, and its expression in the
Rapa group was significantly reduced. The expression of HIF-1α and
the extent of myocardial fibrosis was positively correlated, which
implies that HIF-1α can facilitate the expression level of
TGF-β1 and thus induce atrial fibrosis.
In patients with AF, it has been reported that the
atrial HIF-1α level rises with the increasing expression level of
MMP-9 (24). MMP-9, as an
important protease in the MMP family, is associated with myocardial
matrix remodeling (25), and its
increasing activity can result in acute myocardial fibrosis
(26,27). The present study shows that in the
ISO group, HIF-1α and MMP-9 mRNA expression levels
were significantly increased. They were positively correlated
between themselves and also positively correlated with atrial
fibrosis. Furthermore, HIF-1α can be involved in myocardial
fibrosis formation by regulating the MMP-9 expression level.
TGF-β1 is closely associated with MMP-9 and can regulate
its gene level expression (28)
and thus induce its synthesis (29). In the present study, it was also
observed that in the ISO group, the MMP-9 mRNA expression
level was markedly higher than in the control group, while in the
Rapa group this was significantly decreased. The expression level
of TGF-β1 was also found to be positively associated
with the degree of myocardial fibrosis. These results imply that
TGF-β1 expression levels increase and cause the high
expression of MMP-9, and thus aggravates myocardial fibrosis, which
could be a possible mechanism in the AngII-induced atrial fibrosis
model.
In conclusion, the present study shows that in the
ISO-induced atrial fibrosis model, AngII, HIF-1α, TGF-β1
and MMP-9 were all highly expressed. By inhibiting the expression
of HIF-1α, the expression levels of TGF-β1 and MMP-9
decreased accordingly, and the extent of myocardial fibrosis was
also reduced. Considering the association among these factors, we
can infer that, during the atrial fibrosis formation, HIF-1α
promotes the expression of TGF-β1 and MMP-9 protein. A
possible signal transduction pathway among HIF-1α,
TGF-β1 and MMP-9 may exist, which could contribute
significantly to the further study of the pathogenesis of AF and a
new direction of drug research and development in AF therapy.
References
|
1
|
Meiltz A, Zimmermann M, Urban P and Bloch
A: Association of Cardiologists of the Canton of Geneva: Atrial
fibrillation management by practice cardiologists: a prospective
survey on the adherence to guidelines in the real world. Europace.
10:674–680. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Benjamin EJ, Levy D, Vaziri SM, D’Agostino
RB, Belanger AJ and Wolf PA: Independent risk factors for atrial
fibrillation in a population-based cohort. The Framingham Heart
Study. JAMA. 271:840–844. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pan CH, Lin JL, Lai LP, Chen CL, Stephen
Huang SK and Lin CS: Downregulation of angiotensin converting
enzyme II is associated with pacing-induced sustained atrial
fibrillation. FEBS Lett. 581:526–534. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Frustaci A, Chimenti C, Bellocci F,
Morgante E, Russo MA and Maseri A: Histological substrate of atrial
biopsies in patients with lone atrial fibrillation. Circulation.
96:1180–1184. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Everett TH IV, Li H, Mangrum JM, et al:
Electrical, morphological, and ultrastructural remodeling and
reverse remodeling in a canine model of chronic atrial
fibrillation. Circulation. 102:1454–1460. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li D, Shinagawa K, Pang L, et al: Effects
of angiotensin-converting enzyme inhibition on the development of
the atrial fibrillation substrate in dogs with ventricular
tachypacing-induced congestive heart failure. Circulation.
104:2608–2614. 2001. View Article : Google Scholar
|
|
7
|
Wang Z, Tang L, Zhu Q, et al:
Hypoxia-inducible factor-1α contributes to the profibrotic action
of angiotensin II in renal medullary interstitial cells. Kidney
Int. 79:300–310. 2011.
|
|
8
|
Zhang YG, Li YG, Liu BG, et al: Urotensin
II accelerates cardiac fibrosis and hypertrophy of rats induced by
isoproterenol. Acta Pharmacol Sin. 28:36–43. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hudson CC, Liu M, Chiang GG, et al:
Regulation of hypoxia-inducible factor 1alpha expression and
function by the mammalian target of rapamycin. Mol Cell Biol.
22:7004–7014. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dong Y, Huang YF, Wang LQ and Chen B:
Experimental study on the effects of rapamycin in prevention of rat
corneal allograft rejection. Zhonghua Yan Ke Za Zhi. 41:930–935.
2005.(In Chinese).
|
|
11
|
Matsushita K, Wu Y, Okamoto Y, Pratt RE
and Dzau VJ: Local renin angiotensin expression regulates human
mesenchymal stem cell differentiation to adipocytes. Hypertension.
48:1095–1102. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang WZ, Wang ZG, Chen YQ, et al: Effects
of valsartan and U0126 on atrial fibrosis and connexin40 remodeling
in rats. Zhonghua Xin Xue Guan Bing Za Zhi. 39:1129–1134. 2011.(In
Chinese).
|
|
13
|
Fine LG and Norman JT: Chronic hypoxia as
a mechanism of progression of chronic kidney diseases: from
hypothesis to novel therapeutics. Kidney Int. 74:867–872. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Copple BL, Kaska S and Wentling C:
Hypoxia-inducible factor activation in myeloid cells contributes to
the development of liver fibrosis in cholestatic mice. J Pharmacol
Exp Ther. 341:307–316. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rosmorduc O and Housset C: Hypoxia: a link
between fibrogenesis, angiogenesis, and carcinogenesis in liver
disease. Semin Liver Dis. 30:258–270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ueno M, Maeno T, Nomura M, et al:
Hypoxia-inducible factor-1α mediates TGF-β-induced PAI-1 production
in alveolar macrophages in pulmonary fibrosis. Am J Physiol Lung
Cell Mol Physiol. 300:L740–L752. 2011.
|
|
17
|
Haase VH: Hypoxia-inducible factor
signaling in the development of kidney fibrosis. Fibrogenesis
Tissue Repair. 5(Suppl 1): S162012.PubMed/NCBI
|
|
18
|
Thijssen VL, van der Velden HM, van
Ankeren EP, et al: Analysis of altered gene expression during
sustained atrial fibrillation in the goat. Cardiovasc Res.
54:427–437. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gramley F, Lorenzen J, Jedamzik B, et al:
Atrial fibrillation is associated with cardiac hypoxia. Cardiovasc
Pathol. 19:102–111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rosenkranz S, Flesch M, Amann K, et al:
Alterations of beta-adrenergic signaling and cardiac hypertrophy in
transgenic mice overexpressing TGF-beta(1). Am J Physiol Heart Circ
Physiol. 283:H1253–H1262. 2002.PubMed/NCBI
|
|
21
|
Lim JY, Park SJ, Hwang HY, et al:
TGF-beta1 induces cardiac hypertrophic responses via PKC-dependent
ATF-2 activation. J Mol Cell Cardiol. 39:627–636. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mihout F, Shweke N, Bigé N, et al:
Asymmetric dimethylarginine (ADMA) induces chronic kidney disease
through a mechanism involving collagen and TGF-β1 synthesis. J
Pathol. 223:37–45. 2011.PubMed/NCBI
|
|
23
|
Higgins DF, Kimura K, Bernhardt WM, et al:
Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of
epithelial-to-mesenchymal transition. J Clin Invest. 117:3810–3820.
2007.PubMed/NCBI
|
|
24
|
Ogi H, Nakano Y, Niida S, et al: Is
structural remodeling of fibrillated atria the consequence of
tissue hypoxia? Circ J. 74:1815–1821. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Isobe K, Kuba K, Maejima Y, et al:
Inhibition of endostatin/collagen XVIII deteriorates left
ventricular remodeling and heart failure in rat myocardial
infarction model. Circ J. 74:109–119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu J, Cui G, Esmailian F, et al: Atrial
extracellular matrix remodeling and the maintenance of atrial
fibrillation. Circulation. 109:363–368. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakano Y, Niida S, Dote K, et al: Matrix
metalloproteinase-9 contributes to human atrial remodeling during
atrial fibrillation. J Am Coll Cardiol. 43:818–825. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Santibañez JF, Guerrero J, Quintanilla M,
Fabra A and Martínez J: Transforming growth factor-beta1 modulates
matrix metalloproteinase-9 production through the Ras/MAPK
signaling pathway in transformed keratinocytes. Biochem Biophys Res
Commun. 296:267–273. 2002.PubMed/NCBI
|
|
29
|
Zhang BB, Cai WM, Weng HL, et al:
Diagnostic value of platelet derived growth factor-BB, transforming
growth factor-beta1, matrix metalloproteinase-1, and tissue
inhibitor of matrix metalloproteinase-1 in serum and peripheral
blood mononuclear cells for hepatic fibrosis. World J
Gastroenterol. 9:2490–2496. 2003.
|