Introduction
Obesity has become a severe public health problem
worldwide and it is closely associated with specific chronic
diseases, including type 2 diabetes, metabolic syndrome and certain
cancers. However, the risk of developing these disorders is
different in two obesity types. Fat distribution in subcutaneous
and visceral adipose tissues have different influences on
developing these disorders (1).
Individuals who exhibit peripheral obesity (fat mainly accumulated
in the gluteofemoral region) are at little or no risk of
obesity-related diseases, whereas individuals who exhibit central
obesity (fat mainly distributed in visceral depots) are prone to
developing these complications. However, depot-specific regulation
of adipocyte genes and the mechanisms underlying depot-differences
in the metabolism and function of subcutaneous and visceral fat
depots thus far remain to be investigated.
Glypican 4 (Gpc4), a member of the heparan sulfate
proteoglycan family, plays an important role in the regulation of
cell growth, differentiation and morphogenesis (2). Gpc4 also acted as an essential
modulator of key regulatory proteins, including Wnt, bone
morphogenetic proteins, fibroblast growth factor and sonic hedgehog
(3). The expression level of Gpc4
may contribute to the regulation of preadipocyte differentiation by
means of regulating the binding of growth and differentiation
factors to their cognate high affinity, signal-transducing
receptors (4). The expression
level of Gpc4 mRNA presents a clear difference in
subcutaneous and visceral adipose tissues. There were strong
correlations of Gpc4 expression with body mass index (BMI)
and waist/hip ratio (WHR) in human adipose, which indicated that
Gpc4 may play an important role in obesity and body fat
distribution (5). Our recent study
showed that the filial generation mice had higher epididymal
adipose tissue weight and Gpc4 mRNA expression when the
pregnant mice were exposed to low-dose di-2-ethylhexylphthalate
(6), indicating that the
Gpc4 gene may be involved in fat accumulation. However, the
mechanism of how Gpc4 regulates fat distribution is still not
understood.
Peroxisome proliferators-activated receptor γ
(PPARγ), a major regulator of adipocyte differentiation, exerts an
important role in fat accumulation. A study showed that the PPARγ
agonist in vivo induces adipose tissue redistribution from
visceral to subcutaneous fat (7).
The mechanisms that have been proposed include the variation of the
differentiation of preadipocytes from subcutaneous and visceral
regions (8) and the depot-specific
regulation of lipid uptake, storage and energy expenditure genes in
visceral and subcutaneous fat (9–11).
Another hypothesized mechanism is that PPARγ activation may affect
the expression of the Gpc4 gene in subcutaneous and visceral
adipose tissues that are involved in the regulation of fat
distribution. Therefore, to verify the hypothesis, in the present
study high-fat feeding (HF) C57BL/6J mice were treated with a PPARγ
agonist rosiglitazone (RSG) and the effects of PPARγ activation
in vivo on HF mice and the expression of Gpc4 mRNA
and protein in epididymal and inguinal depots, which were used as
representative of visceral and subcutaneous fat respectively, were
assessed. The mice were also evaluated in vivo for two
probable regulators of the Gpc4 gene, specificity protein 1
(Sp1) and Sp3 to explore the hypothesis that they,
perhaps, to a certain degree, are involved in fat distribution
regulation of Gpc4.
Materials and methods
Animals and treatment
Twenty-one male C57BL/6J mice (weight, 18–22 g) were
purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai,
China). The animals were divided into three groups. Mice in the
control (CON) group (n=7) were administered a standard diet
containing 10% kcal from fat. Mice in the HF group (n=7) were fed
with a home-made high-fat diet that contained 58% kcal from fat,
27% kcal from carbohydrate and 15% kcal from protein (12) for 12 weeks. The remaining mice were
fed with a high-fat diet for eight weeks and then supplemented with
the PPARγ agonist RSG (Sigma-Aldrich, St. Louis, MO, USA) at a dose
of 10 mg/kg high-fat diet for an additional four weeks as the RSG
group (n=7). The dose of RSG was selected based on a previously
published study (13). RSG was
mixed with the powder diet. Food and water were available ad
libitum. All the mice were maintained with a control
temperature of 20±2°C, a relative humidity of 50±10% and a 12-h
light/dark cycle. Animal experimental procedures were conducted
under an animal protocol approved by the Institutional Animal Care
and Use Committee of China Medical University (Shenyang, Liaoning,
China).
Serum biochemical and hormonal
measurements
Blood samples were collected from the abdominal
aorta. Serum glucose concentration was measured by the glucose
oxidase method. Serum triglycerides and total cholesterol were
determined using commercial kits (Sigma-Aldrich, St. Louis, MO,
USA). The levels of serum adiponectin, leptin and insulin were
determined by the ELISA kit (Boster Biotechnology, Wuhan, Hubei,
China).
Adipose tissue samples
At the end of the 12 weeks, all the mice were
sacrificed. The weights of the inguinal and epididymal fat pad
samples were determined immediately, and subsequently stored at
−80°C for reverse transcription quantitative polymerase chain
reaction (RT-qPCR) and western blot analysis.
RT-qPCR analysis
The total RNA of the subcutaneous and epididymal
adipose tissues was extracted using RNAiso plus (Takara, Dalian,
Liaoning, China) according to the manufacturer’s instructions. The
reverse transcription and PCR reactions were performed using the
ABI Prism Real-time PCR 7500 system (Applied Biosystems, Inc.,
Foster City, CA, USA) as described previously (14). β-actin was used as the
endogenous control gene. RT-qPCR data were analyzed using the
2−ΔΔCT method (15).
The sequences of the forward and reverse primers are listed in
Table I.
 | Table IOligonucleotide sequences and product
sizes of polymerase chain reaction primers. |
Table I
Oligonucleotide sequences and product
sizes of polymerase chain reaction primers.
| Gene | Sense (5′-3′) | Antisense
(5′-3′) | Size, bp |
|---|
| β-actin |
CATCCGTAAAGACCTCTATGCCAAC |
ATGGAGCCACCGATCCACA | 171 |
| Glypican 4 |
AGAGCAACGCCCAACCAC |
GCCATTCCAGCAGTCATC | 169 |
| Sp1 |
GGCCTCCAGACCATTAACCTCA |
TCATGTATCCCATCACCACCAGA | 149 |
| Sp3 |
AGATGATGCCTTGATTACTG |
ATGTCTTGATTGCTGGTG | 114 |
Western blot analysis
Briefly, epididymal and inguinal fat pads were
homogenized in lysis buffer (50 mM Tris-HCl, pH 7.5, 0.1 mM
Na3VO4, 1% Nonidet P-40, 25 mM NaF, 2 mM
EDTA, 2 mM EGTA, 1 mM DTT and 1% protease inhibitor mixture;
Sigma-Aldrich, St. Louis, MO, USA). Protein samples were boiled for
5 min in 1 × SDS sample buffer (50 mM Tris-HCl, pH 6.8, 20%
glycerol, 2% SDS and 0.02% bromophenol blue) containing
2%-mercaptoethanol. The proteins on the gels that were separated by
SDS-PAGE were transferred onto a polyvinylidene difluoride membrane
for 3 h at 4°C. The membrane was blocked with 5% skimmed milk for 1
h at room temperature and incubated with goat anti-mouse
Gpc4 polyclonal antibodies (1:300 dilution; Santa Cruz
Biotechnology, Santa Cruz, CA, USA) overnight at 4 °C, followed by
horseradish peroxidase-conjugated secondary antibodies (1:5000
dilution; Pierce, Rockford, IL, USA) for 45 min at room
temperature. Enhanced chemiluminescence reagent (Amersham
Biosciences, Buckinghamshire, UK) was used to obtain signals. The
blots were quantified using Scion Image 4.0 software (16).
Statistical analysis
All the data were expressed as the mean ± standard
deviation. Statistical analysis was conducted by the Student’s
t-test within the same group and one-way analysis of
variance among different groups using SPSS 13.0 software (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Body weight, energy balance and fat
distribution
After 12 weeks of feeding, mice in the RSG and HF
groups had a significantly higher body weight, body weight gain,
food efficiency, subcutaneous fat and epididymal mass than that of
mice in CON group. RSG treatment had no influence on the amount of
food intake (4.0±0.3 g/d in the CON group vs. 4.1±0.3 g/d in the HF
group vs. 4.1±0.3 g/d in the RSG group, P>0.05). After 4 weeks
of RSG treatment, mice in the RSG group showed a significant
increase in subcutaneous fat weight compared with the mice in the
HF group (0.8±0.2 g in the RSG group vs. 0.3±0.1 g in the HF group,
P<0.05). However, there was no difference in the epididymal fat
weight between the RSG-treated and untreated HF mice (Table II).
 | Table IIData of body weight, energy balance
and fat distribution in standard diet, HF and RSG-treated C57BL/6J
mice. |
Table II
Data of body weight, energy balance
and fat distribution in standard diet, HF and RSG-treated C57BL/6J
mice.
| Variable | CON (n=7) | HF (n=7) | RSG (n=7) |
|---|
| Initial body
weight, g | 19.9±1.0 | 20.2±0.6 | 20.5±0.7 |
| Final body weight,
g | 27.9±1.8 | 31.2±2.9a | 32.5±1.9a |
| Body weight gain,
g | 7.7±1.0 | 11.1±2.7a | 11.9±1.6a |
| Food intake, g | 4.0±0.3 | 4.1±0.3 | 4.1±0.3 |
| Food efficiency,
%b | 192.5 | 268.3a | 290.2a |
| Subcutaneous fat,
g | 0.07±0.02 | 0.3±0.1a | 0.8±0.2a,c |
| Visceral fat,
g | 0.4±0.08 | 1.2±0.4a | 1.1±0.4a |
Plasma glucose, serum hormones and
lipids
As shown in Table
III, plasma fasting glucose had significantly decreased in the
RSG-treated mice compared with the untreated HF mice (P<0.05).
Fasting insulin in the RSG-treated HF mice maintained the same
concentration as that in the CON mice, but was markedly lower than
that in the untreated HF mice (P<0.05). Serum leptin had
significantly increased in the RSG-treated mice compared with the
CON mice (P<0.05), but significantly decreased compared with the
untreated HF mice (P<0.05). Serum adiponectin in the RSG-treated
mice was higher than that in the untreated HF mice (P<0.05). The
concentration of serum triglyceride had significantly decreased in
the RSG-treated mice compared with the untreated HF mice
(P<0.05).
 | Table IIISerum biochemical and hormonal data
in standard diet, HF and RSG-treated C57BL/6J mice. |
Table III
Serum biochemical and hormonal data
in standard diet, HF and RSG-treated C57BL/6J mice.
| Variable | CON (n=7) | HF (n=7) | RSG (n=7) |
|---|
| Fasting glucose,
mmol/l | 0.4±0.06 | 2.0±0.5a | 0.6±0.2b |
| Fasting insulin,
ng/ml | 0.2±0.04 | 0.4±0.1a | 0.2±0.05b |
| Leptin, ng/ml | 10.7±2.7 | 33.3±3.7a | 25.5±2.9a,b |
| Adiponectin,
ng/ml | 24.5±3.1 | 20.1±2.0a | 32.1±4.8a,b |
| Triglyceride,
mmol/l | 1.6±0.1 | 1.9±0.4a | 1.6±0.3b |
| Cholesterol,
mmol/l | 2.3±0.3 | 3.4±0.7c | 3.8±0.6c |
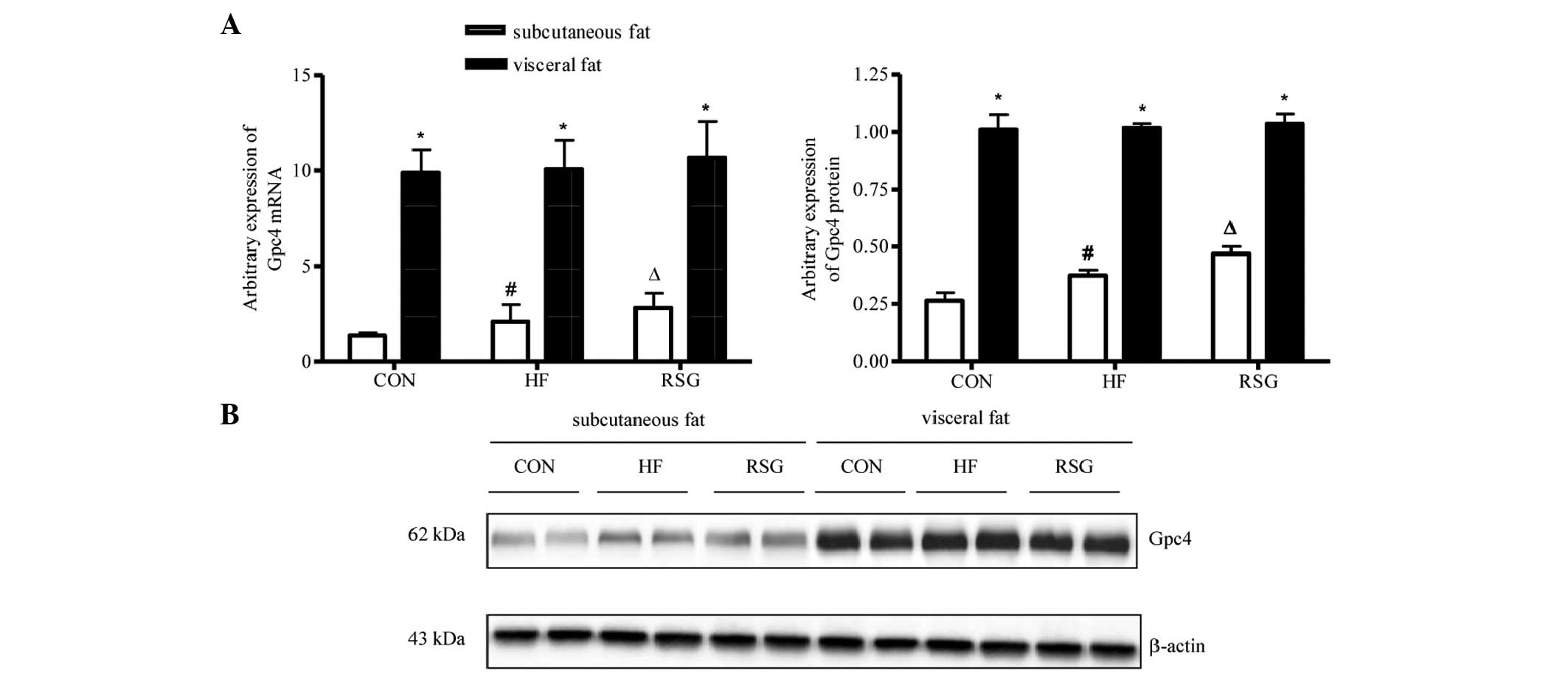
Expression of Gpc4 mRNA and protein in
subcutaneous and visceral fat
As shown in Fig. 1A and
B, the expression of Gpc4 mRNA and protein was
significantly higher in visceral than in subcutaneous fat in all
three groups (P<0.05). The mice in the HF group had increased
Gpc4 mRNA and protein expression levels in subcutaneous fat
compared with mice in the CON group. After 4 weeks of RSG treatment
the expression of Gpc4 mRNA and protein in subcutaneous fat
increased significantly compared with that of the untreated HF mice
(P<0.05). However, no significant difference was detected for
the expression of Gpc4 mRNA and protein in visceral fat
among all three groups.
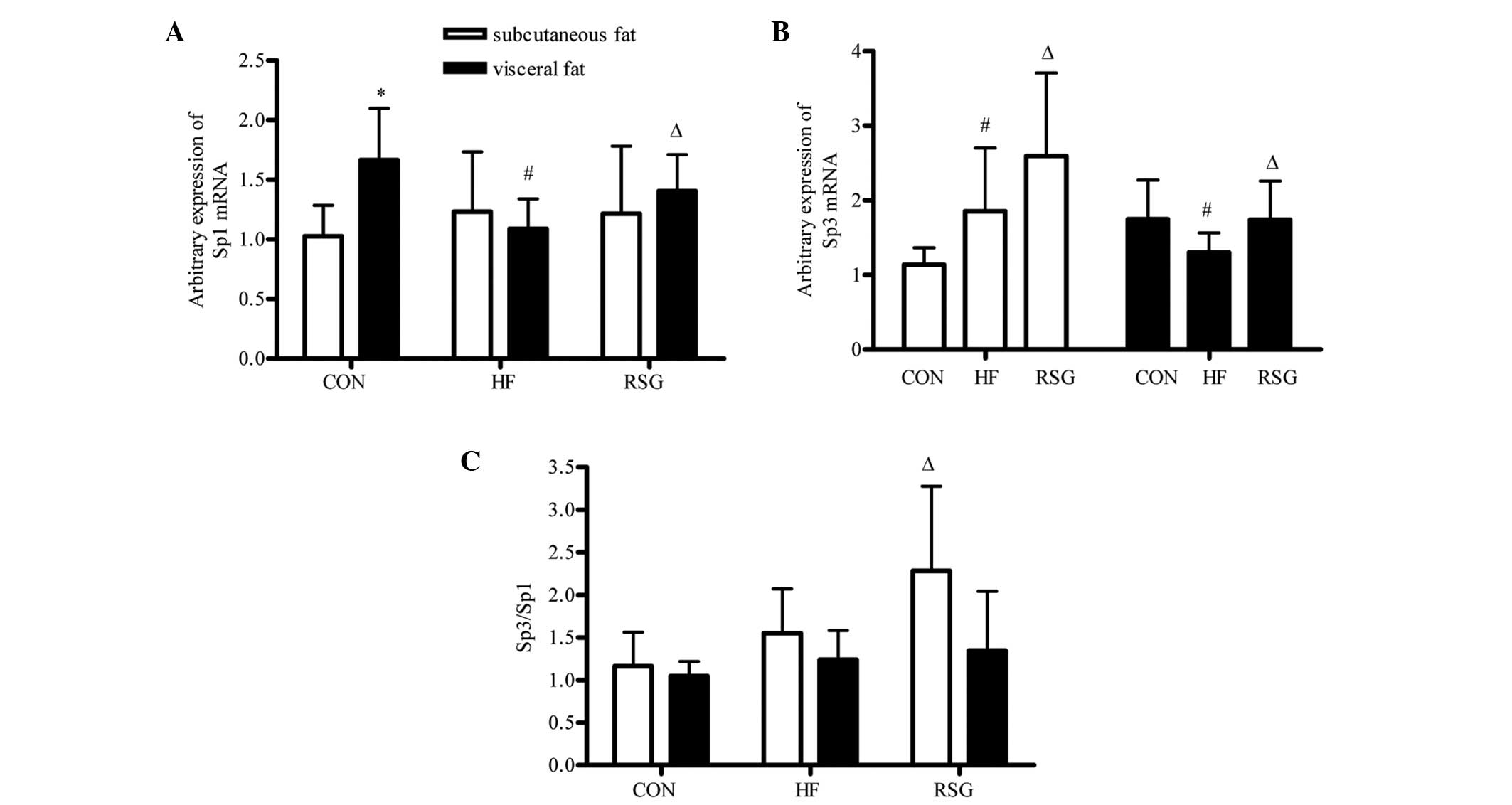
Expression of Sp1 and Sp3 mRNA and
Sp3/Sp1 ratio in subcutaneous and visceral fat
As shown in Fig.
2A, the expression of Sp1 mRNA in visceral fat was
significantly higher than that in subcutaneous fat within the CON
group (P<0.05), but no difference was detected in the HF and RSG
groups. Compared with the CON and RSG groups the expression of
Sp1 mRNA in visceral fat was markedly decreased in the HF
group (P<0.05). As shown in Fig.
2B, the expression of Sp3 mRNA in subcutaneous fat in
the HF group was significantly higher than that in the CON group,
but lower than that in the RSG group (P<0.05). The
Sp3/Sp1 ratio in subcutaneous fat in the RSG group
was significantly higher than that in the CON and HF groups
(P<0.05), as shown in Fig.
2C.
Discussion
RSG, an agonist of PPARγ, exerts multifunction via
the stimulation of PPARγ on a number of cell types (17,18)
and is used clinically to treat type 2 diabetes mellitus. In the
present study, a section of the results obtained were similar to
previous studies (19,20). RSG decreased serum glucose and
insulin, decreased the synthesis of leptin and triglyceride, and
increased the synthesis of adiponectin (21). The results also showed that RSG
promoted subcutaneous fat gain and had little effect on visceral
fat accumulation, which implied that PPARγ activation in
subcutaneous fat tissues exerts a higher ability to increase fat
accumulation than that in visceral fat tissues in mice feeding with
a high-fat diet. The possible mechanisms leading to the effects of
RSG treatment include the differentiation of preadipocytes from
subcutaneous regions being markedly augmented compared with that
from visceral regions (8).
However, another study showed that there was no difference in
preadipocytes between subcutaneous and visceral sites and they were
enhanced to the same extent by treating with RSG in the two depots
(22). The second possible
mechanism is that RSG treatment may be associated with the
depot-specific regulation of lipid uptake, storage and energy
expenditure genes in visceral and subcutaneous fat (9–11).
These studies demonstrated that RSG treatment resulted in the
increase in the subcutaneous fat accumulation and reduction in
visceral fat accretion by stimulating lipid uptake in subcutaneous
fat and increasing energy expenditure greatly in visceral fat.
Another hypothesized mechanism is that certain developmental genes
are differentially expressed in adipocytes derived from
subcutaneous and visceral depots, which may play an important role
in body fat distribution. Gpc4 is a gene known to exert a
role in early development and pattern specification. However, a
recent study identified Gpc4 as a novel adipokine capable of
enhancing insulin signaling and modulating adipocyte
differentiation (23), indicating
a potentially important role of body fat regulation. Gesta et
al (5) demonstrated that
different adipocyte precursors are responsible for a specific fat
depot development and may be involved later in the functional
differences observed between visceral and subcutaneous adipose
depots. The present study showed that in all groups the expression
of Gpc4 mRNA and protein was significantly higher in
visceral than in subcutaneous fat, which was similar to a previous
study that indicated a difference in Gpc4 expression in
subcutaneous and visceral adipose tissues (5). The mice treated with RSG were also
found to have increased Gpc4 mRNA and protein expression
levels in subcutaneous fat compared with untreated HF mice, but no
difference was measured in the visceral depot between HF and
RSG-treated mice. Thus, the present results indicated that the
levels of Gpc4 mRNA and protein expression in subcutaneous
adipose tissue were associated with PPARγ activation, and may play
an important role in fat distribution. Indeed, the expression of
Gpc4 in subcutaneous fat has a strong correlation with BMI
in humans, and high levels of Gpc4 expression in visceral
adipose tissues and low levels in subcutaneous adipose tissues
appear to be linked with high WHR (5). In addition, circulating Gpc4 levels
had a significant positive correlation with the WHR and the ratio
of visceral to subcutaneous fat area, indicating the association of
Gpc4 with body fat distribution and insulin resistance (24).
Sp1 and Sp3 have been reported as major activators
of the Gpc4 promoter. They play a significant role in the
regulation of the Gpc4 gene expression (4). The levels of Gpc4 expression
changed with the Sp3 protein and the ratio of Sp3 to Sp1 in the
process of differentiation of 3T3-F442A cells from adipoblasts to
differentiated adipocytes. Therefore, it was hypothesized that Sp1
and Sp3 are perhaps involved in regulating Gpc4 expression
in the process of PPARγ activation in subcutaneous and visceral fat
tissues. The results showed that the expression of Sp3 mRNA,
the ratio of Sp3/Sp1 in subcutaneous fat and the
expression of Sp3 and Sp1 mRNA in visceral fat in
RSG-treated mice had significantly increased compared with the mice
in the HF group, while no statistical difference was observed in
the expression of Sp1 mRNA in the subcutaneous fat in the HF
and RSG groups. The results indicated that PPARγ activation
affected the expression of Sp1 and Sp3 either in
subcutaneous and/or visceral fat. A possible explanation included
that the activation of PPARγ induces an unknown gene that is
involved in the Sp1 or Sp3 transcriptional activity. In addition,
it is possible that PPARγ directly regulated the expression of a
corepressor that interacts with Sp1 or Sp3. Several studies have
indicated that there is a physical interaction between Sp1 and
PPARγ (25,26). Activation of PPARγ decreased Sp1
activity by modulation of glycosylation and the GlcNAcylation
status of Sp1, but no direct interaction was detected between Sp1
and PPARγ (27,28). Notably, in the present study, the
ratio of Sp3 to Sp1 was consistent with the
expression of Gpc4 not only in subcutaneous fat but also in
visceral fat indicating that the ratio of Sp3 to Sp1 perhaps
regulated Gpc4 expression in the process of PPARγ
activation. However, the direct effect among PPARγ, Sp3, Sp1 and
Gpc4 expression was not detected, therefore further study is
required to resolve how the activation of PPARγ affects the
expression of Sp1 and Sp3, and how the change of Sp1/Sp3 affects
the expression of the Gpc4 gene.
In conclusion, PPARγ activation was demonstrated to
increase the Gpc4 mRNA and protein expression in
subcutaneous adipose tissues but had no effect on visceral adipose
tissues, which was consistent with the change of the ratio of
Sp3/Sp1 in these depots. The present study indicated that
Gpc4 may play an important role in fat distribution, and
this effect is possibly regulated by the ratio of Sp3/Sp1 in
the subcutaneous and visceral fat tissues.
Acknowledgements
The present study was supported by the Liaoning
Educational Foundation (grant no. L2011138) and the Liaoning
Natural Scientific Foundation (grant no. 201102285), China.
References
|
1
|
Pischon T, Boeing H, Hoffmann K, et al:
General and abdominal adiposity and risk of death in Europe. N Engl
J Med. 359:2105–2120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tumova S, Woods A and Couchman JR: Heparan
sulfate proteoglycans on the cell surface: versatile coordinators
of cellular functions. Int J Biochem Cell Biol. 32:269–288. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fico A, Maina F and Dono R: Fine-tuning of
cell signaling by glypicans. Cell Mol Life Sci. 68:923–929. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li H, Melford K, Judson A and Bensadoun A:
Murine glypican-4 gene structure and expression; Sp1 and Sp3 play a
major role in glypican-4 expression in 3T3-F442A cells. Biochim
Biophys Acta. 1679:141–155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gesta S, Blüher M, Yamamoto Y, et al:
Evidence for a role of developmental genes in the origin of obesity
and body fat distribution. Proc Natl Acad Sci USA. 103:6676–6681.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu L, Liu HM, Gu HL, et al: Effect of low
dose di-2-ethylhexylphthalate exposure on fat distribution of
offspring mice in pregnant mice. J Southeast Univ (Med Sci Edi).
33:147–150. 2014.(In Chinese).
|
|
7
|
Smith SR, De Jonge L, Volaufova J, et al:
Effect of pioglitazone on body composition and energy expenditure:
a randomized controlled trial. Metabolism. 54:24–32. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Adams M, Montague CT, Prins JB, et al:
Activators of peroxisome proliferator-activated receptor gamma have
depot-specific effects on human preadipocyte differentiation. J
Clin Invest. 100:3149–3153. 1997. View Article : Google Scholar
|
|
9
|
Kang JG, Park CY, Ihm SH, et al:
Mechanisms of adipose tissue redistribution with rosiglitazone
treatment in various adipose depots. Metabolism. 59:46–53. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Laplante M, Festuccia WT, Soucy G, et al:
Mechanisms of the depot specificity of peroxisome
proliferator-activated receptor gamma action on adipose tissue
metabolism. Diabetes. 55:2771–2778. 2006. View Article : Google Scholar
|
|
11
|
Laplante M, Sell H, MacNaul KL, et al:
PPAR-gamma activation mediates adipose depot-specific effects on
gene expression and lipoprotein lipase activity: mechanisms for
modulation of postprandial lipemia and differential adipose
accretion. Diabetes. 52:291–299. 2003. View Article : Google Scholar
|
|
12
|
Ma LJ, Mao SL, Taylor KL, et al:
Prevention of obesity and insulin resistance in mice lacking
plasminogen activator inhibitor 1. Diabetes. 53:336–346. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuda O, Stankova B, Tvrzicka E, et al:
Prominent role of liver in elevated plasma palmitoleate levels in
response to rosiglitazone in mice fed high-fat diet. J Physiol
Pharmacol. 60:135–140. 2009.PubMed/NCBI
|
|
14
|
Liu L, Gu H, Yang J, et al: Adipogenic
differentiation is not influenced by lentivirus-mediated shRNA
targeting the SOCS3 gene in adipose-derived stromal cells. Mol Biol
Rep. 37:2455–2462. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
|
|
16
|
Scion image software version 4.0.
http://www.bbioo.com/download/58-196-1.htmluri.
Accessed March 26, 2008
|
|
17
|
Hsu WH, Lee BH, Hsu YW and Pan TM:
Peroxisome proliferator-activated receptor-γ activators monascin
and rosiglitazone attenuate carboxymethyllysine-induced fibrosis in
hepatic stellate cells through regulating the oxidative stress
pathway but independent of the receptor for advanced glycation end
products signaling. J Agric Food Chem. 61:6873–6879. 2013.
|
|
18
|
Chappard D, Marchand-Libouban H, Moreau MF
and Baslé MF: Thiazolidinediones cause compaction of nuclear
heterochromatin in the pluripotent mesenchymal cell line C3H10T1/2
when inducing an adipogenic phenotype. Anal Quant Cytopathol
Histopathol. 35:85–94. 2013.PubMed/NCBI
|
|
19
|
Day C: Thiazolidinediones: a new class of
antidiabetic drugs. Diabet Med. 16:179–192. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fonseca V: Effect of thiazolidinediones on
body weight in patients with diabetes mellitus. Am J Med. 115 Suppl
8A:42S–48S. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takasawa K, Kubota N, Terauchi Y and
Kadowaki T: Impact of increased PPARgamma activity in adipocytes in
vivo on adiposity, insulin sensitivity and the effects of
rosiglitazone treatment. Endocr J. 55:767–776. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Harmelen V, Dicker A, Rydén M, et al:
Increased lipolysis and decreased leptin production by human
omental as compared with subcutaneous preadipocytes. Diabetes.
51:2029–2036. 2002.PubMed/NCBI
|
|
23
|
Ussar S, Bezy O, Blüher M and Kahn CR:
Glypican-4 enhances insulin signaling via interaction with the
insulin receptor and serves as a novel adipokine. Diabetes.
61:2289–2298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoo HJ, Hwang SY, Cho GJ, et al:
Association of glypican-4 with body fat distribution, insulin
resistance, and nonalcoholic fatty liver disease. J Clin Endocrinol
Metab. 98:2897–2901. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sassa Y, Hata Y, Aiello LP, et al:
Bifunctional properties of peroxisome proliferator-activated
receptor gamma1 in KDR gene regulation mediated via interaction
with both Sp1 and Sp3. Diabetes. 53:1222–1229. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sugawara A, Uruno A, Kudo M, et al:
Transcription suppression of thromboxane receptor gene by
peroxisome proliferator-activated receptor-gamma via an interaction
with Sp1 in vascular smooth muscle cells. J Biol Chem.
277:9676–9683. 2002. View Article : Google Scholar
|
|
27
|
Chung SS, Choi HH, Cho YM, Lee HK and Park
KS: Sp1 mediates repression of the resistin gene by PPARgamma
agonists in 3T3-L1 adipocytes. Biochem Biophys Res Commun.
348:253–258. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chung SS, Kim JH, Park HS, et al:
Activation of PPARgamma negatively regulates O-GlcNAcylation of
Sp1. Biochem Biophys Res Commun. 372:713–718. 2008. View Article : Google Scholar : PubMed/NCBI
|