Introduction
Cerebral hemorrhage, a common and frequently
occurring disease with extremely high mortality and morbidity,
accounts for 10–15% of all cerebrovascular strokes, causing a
mortality rate that is >50% (1). Different treatment options exhibit
different efficacies following cerebral hemorrhage. Minimally
invasive hematoma evacuation following cerebral hemorrhage can
reduce the hematoma-induced oppression of the surrounding tissues,
release the ischemia and hydrocephalus caused by hematoma and
extenuate perihematoma brain tissue damage aggravated by hematoma
decomposition products, thus improving the brain function. In
addition, mild hypothermia therapy exerts substantial protective
effects on the brain (2,3), and has attracted considerable
attention. This therapy can suppress the inflammatory response,
reduce hydrocephalus and protect the brain. In recent years, the
protection of the perihematoma brain tissue function has become a
particular focus of studies of cerebral hemorrhage (4).
It is widely believed that the inflammatory response
is involved in the pathological process of cerebral hemorrhage. In
the early stage of cerebral hemorrhage, the local inflammatory
response already exists in the tissues surrounding the hematoma, in
which the inflammatory cytokine tumor necrosis factor-α (TNF-α)
plays an important role. The aim of the present study was to
enhance the understanding of the nuclear factor-κB (NF-κB)
pathway-mediated inflammatory injury in perihematoma tissues. This
was investigated using hematoxylin and eosin (HE) staining of
perihematoma brain tissue slices and immunohistochemistry to
examine the expression and distribution of NF-κB and peripheral
vascular TNF-α following mild hypothermia in combination with
minimally invasive evacuation of hematoma or minimally invasive
evacuation of hematoma alone.
Materials and methods
Clinical data
In this study, 76 patients exhibiting the first
onset of acute spontaneous intracerebral hemorrhage who were
treated within 48 h of occurrence between September 2009 and
September 2011 were selected (Table
I). The study was approved by the Ethics Review Board of
Shandong University (Jinan, China). Prior written informed consent
was obtained from all the patients. The 76 patients were randomly
assigned into two groups: The minimally invasive hematoma
evacuation (MIHE) group, which contained 39 patients, and the mild
hypothermia and minimally invasive evacuation of hematoma (MHMIHE)
group, which contained 37 patients. All patients were confirmed for
cerebral hemorrhage by computed tomography (CT) or magnetic
resonance imaging. The volume of hemorrhage was >30 ml, as
determined by CT film measurement and Tada formula (Volume = π ×
length × width × thickness/6) calculation (5). Prior and subsequent to treatment, the
patients were all scored according to the National Institutes of
Health Stroke Scale (NIHSS) with confirmation by the same
neurologist prior and subsequent to scoring. All patients of the
two groups were treated by minimally invasive hematoma evacuation
on same or next day of the occurrence (<48 h). Patients of the
MHMIHE group were additionally treated with mild hypothermia
immediately following the surgery. Conventional treatments,
including dehydrating agents and brain protection agents, were
equally applied to the two groups. No statistically significant
difference was identified between the two groups in gender, age,
average initial NIHSS scores, position of hemorrhage and volume of
hemorrhage. In order to avoid confounding factors for the
inflammatory markers, the following situations were excluded: i)
Inflammation from infections or nosocomial infections within the
past two weeks; ii) severe diseases of the major organs, such as
the heart, liver, lungs and kidney, as well as endocrine, blood,
neoplastic and immune system diseases; iii) surgery, trauma, heart
and brain strokes, and pregnancy within six months or at ages
<18 years; iv) being treated with glucocorticoids,
anticoagulants or β-receptor inhibitors; and v) reluctance
exhibited by the patients or their family members for the
surgery.
 | Table IPatient information. |
Table I
Patient information.
| Group MIHE, n=39 | Group MHMIHE,
n=37 | P-value |
|---|
| Age (years) | 52.70±16.24 | 50.38±11.32 | 0.09 |
| Admission delay
(h) | 5.42±1.85 | 4.63±2.46 | 0.11 |
| NIHSS score | 16.98±3.02 | 17.22±2.91 | 0.08 |
| Bleeding (ml) | 39.39±8.12 | 39.48±10.56 | 0.07 |
| Systolic pressure
(mmHg) | 149.92±14.08 | 145.72±19.11 | 0.91 |
| Diastolic pressure
(mmHg) | 95.58±12.25 | 87.25±10.65 | 0.89 |
| Body temperature
(°C) | 37.28±0.48 | 37.03±0.42 | 0.31 |
| Leukocytes
(×109) | 12.05±2.74 | 11.86±1.81 | 0.29 |
| Mononuclear cells
(×109) | 0.78±0.34 | 0.74±0.48 | 0.23 |
| Lymphocytes
(×109) | 4.44±0.51 | 4.05±0.17 | 0.30 |
| Neutrophils
(×109) | 7.98±2.88 | 7.85±1.42 | 0.33 |
| Blood sugar
(mmol/l) | 8.07±1.77 | 8.55±4.68 | 0.56 |
Minimally invasive hematoma
evacuation
Minimally invasive hematoma evacuation was performed
under local anesthesia using the YL-1 disposable intracranial
hematoma crushed puncture needle (Beijing WanTeFu Medical Apparatus
Co., Ltd., Beijing, China).
Mild hypothermia therapy
For body cooling, three patients were treated using
an intravascular cooling instrument (CoolGard 3000®;
Alsius, Chelmsford, MA, USA), while the remaining 34 patients were
treated using body water circulation cooling blankets (Model
P&C-A; Beijing Hengbang Kaijie Heating Radiator Co., Ltd.,
Beijing, China). For brain local mild hypothermia therapy, all the
patients were treated using ice hats between −4 and +2°C (HGT-200;
Beijing Dawei Tongchuang Medical Treatment Equipment Co., Ltd,
Beijing, China). Patients with shivering were administered sedative
drugs. Brain temperatures were reduced to 32.5–34.5°C, and were
obtained by measuring the tympanic membrane temperatures on the
same side of the nidus (brain temperature = tympanic membrane
temperature ± 0.5°C) using an infrared ear thermometer (Omron
Corp., Kyoto, Japan) twice a day and recording the higher
temperature.
NIHSS scoring
Prior and subsequent to treatment, the patients were
scored according to the NIHSS (6,7),
with the full score being 22. The degree of neurological deficit
was classed as low, medium or high. NIHSS scores were defined as
follows: Low, <7; medium, 7–15; and high, >15.
HE staining
Samples of perihematoma brain tissue were fixed,
embedded in paraffin and cut into tissue sections. Next, the tissue
sections were dewaxed using xylene and rehydrated using graded
alcohols. After washing with running water and distilled water, the
sections were stained with hematoxylin for 3–5 min. Following
further washing with running water, the sections were
differentiated using 1% HCl in 70% alcohol. Subsequently, the
sections were stained with eosin for 1–4 min, after washing with
running water. Following dehydration and differentiation in
alcohol, the sections were mounted and observed under an Olympus
CX41 inverted fluorescence microscope (Olympus Optical Co., Ltd.,
Tokyo, Japan).
Immunohistochemistry
Perihematoma brain tissue fragments from all
patients were washed out during the surgery and at one, three and
seven days after the surgery, fixed by formalin, and
paraffin-embedded for slicing. Following slicing, the brain tissue
slices were dewaxed by xylene, processed by ethanol and fixed by
acetone. Endogenous peroxidase was then deactivated by 3 ml/l
H2O2 for 10 min, and the slices were washed
with double-distilled H2O and soaked with
phosphate-buffered saline (PBS) for 5 min. Normal goat serum was
subsequently added to block the tissue for 40 min. Following
blocking, rabbit anti-human NF-κB (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) was added, and the slices were incubated
overnight at 4°C and washed for 5 min with PBS three times. An
equal amount of biotinylated goat anti-rabbit IgG (Abcam,
Cambridge, MA, USA) was subsequently added, and the sections were
further incubated for 30 min at 37°C and washed for 5 min with PBS
four times. Following washing, avidin-biotin complex was added, and
the slices were incubated for 30 min at 37°C and washed for 5 min
with PBS four times. The samples were then dyed for 3 min by
diaminobenzidine and the reaction was terminated by soaking with
PBS, prior to the samples being prepared for optical inspections.
Positive cells were dyed dark brown in the cytoplasm and nucleus.
The number of positive cells was counted under the microscope
(Olympus CX41 inverted fluorescence microscope), and the density of
positive cells was calculated.
Statistical analysis
The results were analyzed using SPSS 10.0 software
(SPSS, Inc., Chicago, IL, USA). The counting and measurement data
are presented as the mean ± standard deviation, while two groups of
mean values were compared using an independent sample Student’s
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
General patient data
In this study, 76 patients with the first onset of
acute spontaneous intracerebral hemorrhage were enrolled. The 76
patients were randomly assigned into the MIHE (39 patients) and
MHMIHE (37 patients) groups. Basic patient data on admission are
provided in Table I. To evaluate
the changes in neurological function damage, NIHSS scoring of
patients in the two groups was performed. As shown in Table II, the scores of the two groups
were the highest on admission and on the first day after treatment,
and decreased gradually with the progression of treatment. No
statistically significant differences were identified between the
two groups (P>0.05) on admission and the first day after
treatment. The scores of the MHMIHE group on the third and seventh
day after treatment were lower than those of the MIHE group, with
statistically significant difference (P<0.05). These results
suggested that mild hypothermia and minimally invasive evacuation
of hematoma reduces the damage to the neurological function of
perihematoma brain tissues and protects the brain tissues.
 | Table IINational Institutes of Health Stroke
Scale scores of the two groups. |
Table II
National Institutes of Health Stroke
Scale scores of the two groups.
| Group | n | Admission | Day 1 | Day 3 | Day 7 |
|---|
| MIHE | 39 | 16.98±3.02 | 16.48±3.02 | 15.98±2.69 | 14.42±1.23 |
| MHMIHE | 37 | 17.22±2.91 | 16.32±2.91 | 15.02±1.81 | 12.14±2.02 |
Dynamic changes in TNF-α levels in the
serum of the two groups of patients
To assess whether mild hypothermia and minimally
invasive evacuation of hematoma could alleviate inflammatory
responses in the perihematoma brain tissues, the content of TNF-α
in the serum of the two groups of patients was tested by ELISA
assay. As shown in Table III,
the content of TNF-α in serum of the two groups of patients was
elevated on day 1 (MIHE, 3.0223±0.4799 ng/ml; MHMIHE, 2.9492±0.6069
ng/ml), reached peak value on day 3 (MIHE, 3.4363±0.6374 ng/ml;
MHMIHE, 2.8180±0.2178 ng/ml) and showed the lowest value on day 7
(MIHE, 2.7354±0.8083 ng/ml; MHMIHE, 1.1560±0.7074 ng/ml). Of note,
the content of TNF-α in the MHMIHE group at each time-point was
lower than that of TNF-α in the MIHE group (P<0.05). The time
course of these changes concurred with that observed in the HE
staining and NIHSS scoring, as well as with that observed for the
NF-κB analysis. These results suggested that mild hypothermia and
minimally invasive evacuation of hematoma alleviated the
inflammatory responses in the perihematoma brain tissues.
 | Table IIIDynamic changes in tumor necrosis
factor-α levels in the serum of the two groups. |
Table III
Dynamic changes in tumor necrosis
factor-α levels in the serum of the two groups.
| Group | n | Day 1 (ng/ml) | Day 3 (ng/ml) | Day 7 (ng/ml) |
|---|
| MIHE | 39 | 3.0223±0.4799 | 3.4363±0.6374 | 2.7354±0.8083 |
| MHMIHE | 37 | 2.9492±0.6069 | 2.8180±0.2178 | 1.1560±0.7074 |
Pathological observations of perihematoma
brain tissues
To investigate the pathological changes in the
perihematoma brain tissues, fragments of perihematoma brain tissues
were fixed, paraffin-embedded and sliced for HE staining. The
histological analysis (Fig. 1)
revealed that the perihematoma tissues were loose and the
extravascular space was expanded in patients treated with minimally
invasive evacuation of hematoma (Fig.
1A). However, space had appeared around the nerve and glial
cells and the size of nerve cells was reduced, and karyopyknosis
was observed in patients treated with mild hypothermia and
minimally invasive evacuation of hematoma (Fig. 1B). Furthermore, in patients treated
with mild hypothermia and minimally invasive evacuation of
hematoma, Nissl substance had disappeared and the cytoplasm was
observed to have developed eosinophilic changes. Large numbers of
neutrophils and lymphocytes appeared around the hematoma. These
results suggested that edema, necrosis and inflammatory responses
occurred in the perihematoma brain tissues.
Changes in NF-κB in the perihematoma
brain tissues of the two groups of patients
To assess whether mild hypothermia and minimally
invasive evacuation of hematoma effectively reduced NF-κB levels in
perihematoma brain tissues, immunohistochemical assays were
performed. In the immunohistochemical staining (Fig. 2), NF-κB was expressed in the 76
patients with dynamic changes. NF-κB levels reached the peak on the
third day and were elevated on the first and seventh days. The
NF-κB expression was reduced for the MHMIHE group. The expression
was mainly localized in inflammatory tissues, microglia and nerve
cells. These results suggested that mild hypothermia and minimally
invasive evacuation of hematoma effectively reduced the expression
of NF-κB in perihematoma brain tissues and therefore alleviated
inflammatory damage.
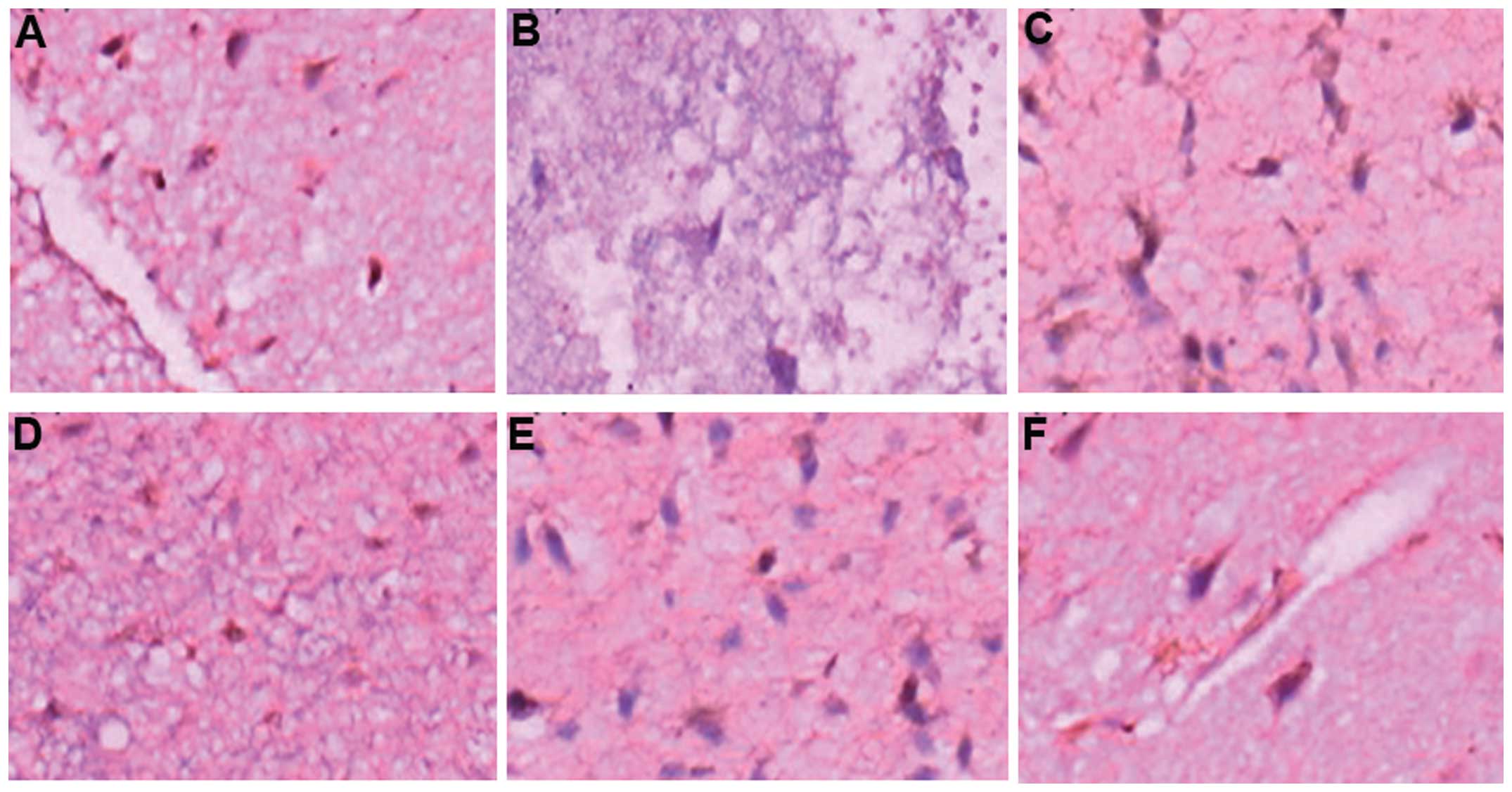 | Figure 2Immunohistochemical assays of nuclear
factor-κB expression. (A) Group MIHE, day 1 (magnification, ×400);
(B) Group MHMIHE, day 1 (magnification, ×200); (C) Group MIHE, day
3 (magnification, ×400); (D) Group MHMIHE, day 3 (magnification,
×200); (E) Group MIHE, day 7 (magnification, ×400); (F) Group
MHMIHE, day 7 (magnification, ×400). MIHE, minimally invasive
evacuation of hematoma; MHMIHE, mild hypothermia and minimally
invasive evacuation of hematoma. |
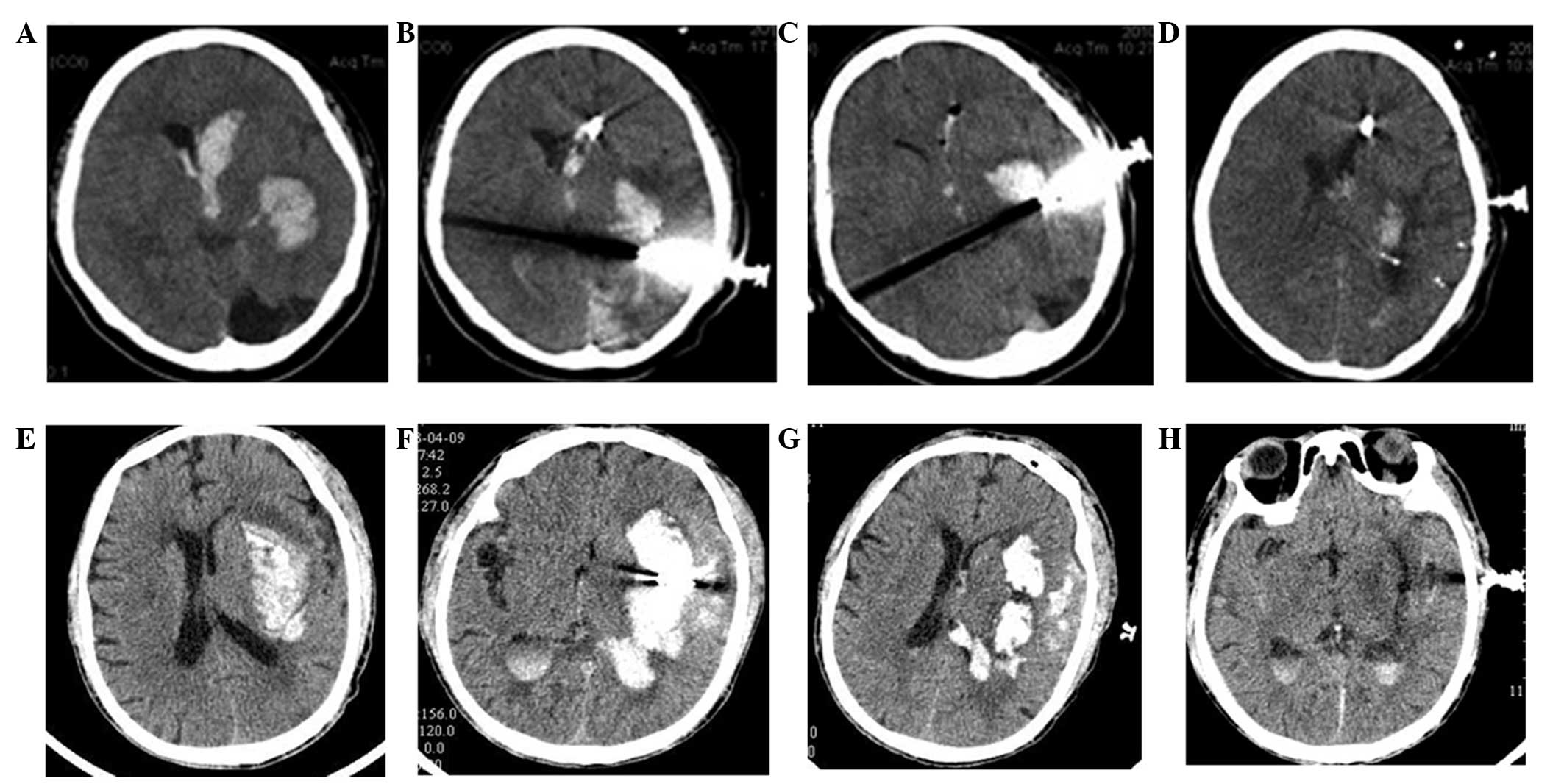
CT of patient brains
To compare the effects of the two types of
treatments on the two groups of patients with cerebral hemorrhage,
the brains of the patients were examined by CT on days 1, 3 and 7.
As shown in the brain CT film (Fig.
3) on admission and following surgery, lateral ventricle and
local hematoma was significantly reduced on the first day after
surgery, and this change became more significant on the seventh day
after surgery. These results suggested that mild hypothermia and
minimally invasive evacuation of hematoma effectively alleviated
cerebral hemorrhage and improved brain function.
Discussion
The brain protection effect of mild hypothermia
therapy was first applied by Busto in 1987 and has become a focus
in brain protection studies in recent years (8). Studies have suggested that mild
hypothermia therapy >48 h can effectively reduce the damage to
the brain (6,7). However, the therapy should be kept
<196 h at a temperature of 32–35°C. Cardiopulmonary
resuscitation research found that patients could reach the target
temperature (34°C) 2 h earlier if the temperature began to decrease
at the beginning of resuscitation, with higher safety and
feasibility, as well as improved prognosis of the nervous system
(6,7,9). In
addition, no additional arrhythmia, infection, coagulopathy or
hypotension was observed. Therefore, whole body cooling and brain
local mild hypothermia therapy was selected to reduce the brain
temperature to 32.5–34.5°C. The results showed that the NIHSS
scores of the degree of neurological deficit, the expression of
NF-κB in perihematoma brain tissue and the concentration of TNF-α
in serum in the MHMIHE group of patients were improved compared
with those in the MIHE group. Therefore, patients may increasingly
benefit from this advance in medical technology.
In the acute phase of cerebral hemorrhage, hematoma
exerts oppression to its surrounding tissues, and blood, hemoglobin
and their decomposition products enter the brain tissue, causing
cerebral ischemia, hypoxia or even necrosis. This induces damage
from the immune and inflammatory response and therefore generates a
high levels of inflammatory factors (10). A positive correlation exists
between the amount of bleeding and this damage (11,12).
Minimally invasive evacuation of hematoma can reduce the size of
the hematoma, release the hematoma oppression to the surrounding
tissues, ease cerebral edema, lower the intracranial pressure,
reduce the inflammatory factor production and inflammatory damage,
and protect the brain function (13–16).
The present results showed that, following cerebral
hemorrhage, the TNF-α concentration and NF-κB expression in the
blood followed the same trend when the patient condition changed.
The concentration of TNF-α in serum began to rise on the first day
and reached the peak on the third day; the concentration on the
seventh day was the lowest. Similar changes were observed for NF-κB
expression. At each time-point, the TNF-α concentration and NF-κB
expression of the MHMIHE group were lower than those of the MIHE
group (P<0.05). The NIHSS scores of the neurological deficit
degree of the two groups were the highest on admission and on the
first day after treatment, with no statistically significant
difference between the two groups (P>0.05), and decreased
gradually with the progression of treatment. The score on the third
day was higher than that on the seventh day, which was consistent
with the changes in TNF-α and NF-κB expression. The degree of
neurological deficit in the MHMIHE group on the third and seventh
day was improved compared with that in the MIHE group, with a
statistically significant difference (P<0.05). The NIHSS scores
of the neurological deficit degree were lower in the MHMIHE group
than those in the MIHE group, and no other complications, such as
arrhythmia and infection, were observed. This result suggests that
the NF-κB-mediated inflammatory response participated in the
pathological process of brain tissue damage following cerebral
hemorrhage. Mild hypothermia and minimally invasive evacuation of
hematoma can alleviate the pathological damage of the brain tissue
caused by the NF-κB-mediated inflammatory response. Similarly, the
NF-κB expression, TNF-α level and NIHSS scores of the MHMIHE group
were improved compared with those of the MIHE group
(P<0.05).
NF-κB is a type of important transcription factor
that participates in the regulation of mRNA transcription of
multiple target genes, including TNF-α, and affects their protein
synthesis (17). TNF-α is a key
cytokine for the inflammatory response following brain damage, and
plays an important role in the inflammatory damage caused by
cerebral hemorrhage (18). Mild
hypothermia can reduce vascular permeability, stabilize ion pumps,
inhibit nerve excitability cascade reactions and lower the brain
metabolism to suppress inflammation and immune responses, and
therefore protects the brain function following cerebral hemorrhage
(19).
The present study used minimally invasive evacuation
of hematoma to alleviate the damage of the hematoma to the
surrounding brain tissues. At the same time, in order to improve
the brain function, mild hypothermia therapy was used to reduce the
inflammation and immune responses that could cause damage to the
brain tissues. The results demonstrated the effectiveness of the
mild hypothermia and minimally invasive evacuation of hematoma
procedure.
References
|
1
|
Broderick JP, Brott T, Tomsick T, Miller R
and Huster G: Intracerebral hemorrhage more than twice as common as
subarachnoid hemorrhage. J Neurosurg. 78:188–191. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu L and Yenari MA: Clinical application
of therapeutic hypothermia in stroke. Neurol Res. 31:331–335. 2009.
View Article : Google Scholar
|
|
3
|
Kollmar R, Staykov D, Dörfler A,
Schellinger PD, Schwab S and Bardutzky J: Hypothermia reduces
perihemorrhagic edema after intracerebral hemorrhage. Stroke.
41:1684–1689. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qin J, Song B, Zhang H, et al:
Transplantation of human neuro-epithelial-like stem cells derived
from induced pluripotent stem cells improves neurological function
in rats with experimental intracerebral hemorrhage. Neurosci Lett.
548:95–100. 2013. View Article : Google Scholar
|
|
5
|
Broderick JP, Brott TG, Duldner JE,
Tomsick T and Huster G: Volume of intracerebral hemorrhage. A
powerful and easy-to-use predictor of 30-day mortality. Stroke.
24:987–993. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsai MS, Barbut D, Wang H, et al:
Intra-arrest rapid head cooling improves postresuscitation
myocardial function in comparison with delayed postresuscitation
surface cooling. Crit Care Med. 36:S434–S439. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guan J, Barbut D, Wang H, et al: A
comparision between head cooling begun during cardiopulmonary
resuscitation and surface cooling after resuscitation in a pig
model of cardiac arrest. Crit Care Med. 36:S428–S433. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Busto R, Dietrich WD, Globus MY, Valdés I,
Scheinberg P and Ginsberg MD: Small differences in intraischemic
brain temperature critically determine the extent of ischemic
neuronal injury. J Cereb Blood Flow Metab. 6:729–738. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wan Z and Tang WC: 2015 CPR Guidelines
Prospects. Zhonghua Ji Zhen Yi Xue Za Zhi. 20:7–10. 2011.(In
Chinese).
|
|
10
|
Zazulia AR, Diringer MN, Derdeyn CP and
Powers WJ: Progression of mass effect after intracerebral
hemorrhage. Stroke. 30:1167–1173. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mayer SA and Rincon F: Treatment of
intracerebral haemorrhage. Lancet Neurol. 4:662–672. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Anderson CS, Huang Y, Wang JG, et al:
INTERACT Investigators: Intensive blood pressure reduction in acute
cerebral haemorrhage trial (INTERACT): a randomised pilot trial.
Lancet Neurol. 7:391–399. 2008. View Article : Google Scholar
|
|
13
|
Zhang T, Zou HS and Chen WQ: A comparative
study of therapeutic effects of small bone flap craniotomy and
traditional craniotomy in patients with hypertensive cerebral
hemorrhage. Zhonghua Shen Jing Yi Xue Za Zhi. 10:953–955. 2011.(In
Chinese).
|
|
14
|
Dunn KL, Espino PS, Drobic B, He S and
Davie JR: The Ras-MAPK signal transduction pathway, cancer and
chromatin remodeling. Biochem Cell Biol. 83:1–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gong Y, Xue B, Jiao J, Jing L and Wang X:
Triptolide inhibits COX-2 expression and PGE2 release by
suppressing the activity of NF-kappaB and JNK in LPS-treated
microglia. J Neurochem. 107:779–788. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aoki E, Yano R, Yokoyama H, Kato H and
Araki T: Role of nuclear transcription factor kappa B (NF-kappaB)
for MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahyropyridine)-induced
apoptosis in nigral neurons of mice. Exp Mol Pathol. 86:57–64.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chandel NS, Trzyna WC, McClintock DS and
Schumacker PT: Role of oxidants in NF-kappa B activation and
TNF-alpha gene transcription induced by hypoxia and endotoxin. J
Immunol. 165:1013–1021. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Waterhouse RM, Kriventseva EV, Meister S,
et al: Evolutionary dynamics of immune-related genes and pathways
in disease-vector mosquitoes. Science. 316:1738–1743. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baud’huin M, Lamoureux F, Duplomb L,
Rédini F and Heymann D: RANKL, RANK, osteoprotegerin: key partners
of osteoimmunology and vascular diseases. Cell Mol Life Sci.
64:2334–2350. 2007.PubMed/NCBI
|