Introduction
Nasopharyngeal carcinoma (NPC) is derived from the
epithelial cells that cover the surface of and line the nasopharynx
(1). Worldwide, NPC is most
prevalent in China, with an age-standardized incidence rate of
>20 per 100,000 individuals (2). NPC, often accompanied by early lymph
node metastasis, is hard to detect due to the hidden location and
lack of clinical manifestations at the early stages (3). The present clinical screening method
for NPC is mainly dependent on detecting Epstein-Barr virus (EBV)
infection in the serum, but not all NPC carcinogenesis is
associated with EBV; furthermore, EBV infection can be observed in
a number of other diseases (4).
The use of EBV-related detection alone for NPC screening cannot
meet the requirements as a diagnostic marker; therefore, finding
the molecular biomarkers for NPC is necessary to improve the
treatment regimes and predict the prognosis for patients with
NPC.
Amplification of 1q21 is a frequent genetic
alteration in a number of solid tumors (5). In the last decade, a novel oncogene,
chromodomain helicase/ATPase DNA binding protein 1-like (CHD1L)
gene, has been isolated from the 1q21 amplicon. CHD1L belongs to
the SNF2 ATPase superfamily with a carboxy-terminal macrodomain,
which is involved in DNA damage repair and cell cycle progression
(6,7). In a previous study amplification of
CHD1L at the protein level was detected in most hepatocellular
carcinomas (HCCs); and CHD1L-transfected cells were shown to
possess a strong oncogenic ability (8). Furthermore, studies have shown that
CHD1L expression was significantly associated with venous
infiltration, microsatellite tumor nodule formation, an advanced
tumor stage and poor survival in HCC (9,10).
Although CHD1L is implicated in the pathogenesis of several types
of cancer, the expression of CHD1L and its significance in NPC have
not been well documented. In this study, the expression of CHD1L
was investigated to evaluate the prognostic role in patients with
NPC with long-term follow-up.
Materials and methods
Patient samples
NPC biopsies were collected from 30 fresh samples as
well as 133 primary NPC and 133 paired adjacent nasopharyngeal
tissues at the Department of Otorhinolaryngology and Head and Neck
Surgery, Shandong People’s Armed Police Corps Hospital (Jinan,
China) between December 1, 2005 and December 1, 2009. No patients
received treatment prior to surgery. The cause of mortality was
determined according to medical records.
Fresh and formalin-fixed paraffin-embedded tissues
were collected from the hospital at the time surgical resections
were performed. All biopsies were histologically confirmed by two
pathologists in a blind manner. The clinical stage of NPC was
determined according to the tumor, node and metastasis (TNM)
classification system of the American Joint Committee on
Cancer/Union for International Cancer Control, and the histological
type was designated according to the World Health Organization
(WHO) criteria (11). Follow-up
data included survival status and disease status (disease-free,
recurrence or metastasis), along with dates of the events and cause
of mortality.
All the patients provided informed consent prior to
this study. This study was approved by the Ethics Committee of
Shandong People’s Armed Police Corps Hospital and performed in line
with the Declaration of Helsinki.
Western blot analysis
Frozen NPC or non-tumor tissue samples were
homogenized in radioimmunoprecipitation assay buffer (Qiagen,
Shanghai, China). Following centrifugation at 15000 × g, 4°C for 20
min, 70 μg protein samples were run on a 12.5% SDS-PAGE gel and
transferred to polyvinylidene difluoride membranes (Millipore, St.
Charles, MO, USA). Subsequent to blocking non-specific binding
sites for 60 min with 5% fat milk, the membranes were incubated
with rabbit monoclonal antibodies against CHD1L (1:1,000;
Millipore) and GAPDH (1:1,000; Millipore) at 4°C overnight,
respectively. The membranes were then washed with Tris buffered
saline with Tween 20 (TBST) three times, for 15 min each time, and
incubated with horseradish peroxidase-conjugated anti-rabbit
secondary antibodies (1:10,000; Millipore) for 60 min at room
temperature. The membrane was developed by an enhanced
chemiluminescence system (Millipore) following washing with TBST
three times. The intensity of the protein bands was determined by
densitometry using Image J software (National Institutes of Health,
Bethesda, MD, USA).
Histological assessment
Paraffin-embedded sections of NPC samples were
analyzed for the localization of CHD1L protein using anti-CHD1L
antibody (1:50; Millipore) as described previously (10). The absence of primary antibody
served as a negative control. The degree of immunohistochemical
staining was evaluated independently by two pathologists blinded to
the study, and a consensus was reached. CHD1L nuclear
immunoreactivity was scored using a semiquantitative scoring system
as follows: 0, no staining; 1, weak staining; 2, moderate staining;
3, strong staining. For statistical analysis, 0 and 1 were
classified as CHD1L-negative; 2 and 3 were classified as
CHD1L-positive.
Statistical analysis
Statistical analyses were processed with SPSS
version 17.0 (SPSS, Inc., Chicago, IL, USA). The χ2 test
was performed for categorical data. The Kaplan-Meier method and
log-rank test were used for survival analyses. Univariate and
multivariate Cox regression models were used to assess the
correlations between CHD1L status and the risk of mortality.
P<0.05 was considered to denote a statistically significant
difference.
Results
Upregulation of CHD1L in human NPC
The expression of CHD1L protein was detected by
western blotting in the 30 paired NPC cancerous tissues and their
corresponding adjacent non-cancerous tissues. Western blot analyses
showed that CHD1L expression was markedly elevated in NPC cancerous
tissues in comparison with their corresponding non-cancerous
tissues (P<0.001, Fig. 1).
Following the western blotting, CHD1L expression in
133 NPC specimens was evaluated by immunohistochemistry. Among the
133 NPC biopsies, 66.2% (88/133) of the NPC samples were classified
as positive for CHD1L expression. Representative images are shown
in Fig. 2. Immunohistochemical
staining of CHD1L was predominantly located in the cytoplasm. By
contrast, the 133 adjacent tissue specimens were negative for CHD1L
expression. These data suggest that CHD1L protein was highly
expressed in NPC tissues.
 | Figure 2Immunohistochemical staining of CHD1L
in NPC tissues. (A and B) CHD1L-negative staining in normal
nasopharyngeal epithelium tissue (negative control): (A)
magnification, ×200; (B) magnification, ×400. (C and D)
CHD1L-negative staining in NPC tissue: (C) magnification, ×200; (D)
magnification, ×400. (E and F) Weak staining of CHD1L in the
cytoplasm: (E) magnification, ×200; (F) magnification, ×400. (G and
H) Moderate staining of CHD1L in the cytoplasm: (G) magnification,
×200; (H) magnification, ×400. (I and J) Strong staining of CHD1L
in the cytoplasm (I) magnification, ×200; (J) magnification, ×400.
CHD1L, chromodomain helicase/ATPase DNA binding protein 1-like;
NPC, nasopharyngeal carcinoma. |
Association of CHD1L protein expression
with the clinicopathological characteristics of human NPC
Table I summarizes
the association of CHD1L protein expression, detected by
immunohistochemical staining, with clinicopathological parameters
in 133 patients with NPC. High CHD1L expression was closely
associated with an advanced clinical stage of NPC (P=0.016). In
addition, a significant difference was observed in CHD1L expression
in patients categorized according to recurrence status (P=0.002).
The expression levels of CHD1L protein in patients with NPC with
positive recurrence were significantly higher than those in
patients without recurrence. Positive metastasis also correlated
with higher CHD1L expression (P=0.031). No significant association
was observed between CHD1L expression and age, gender, T-stage,
N-stage or WHO histological type (Table I).
 | Table IAssociation of CHD1L expression with
clinicopathological parameters in 133 patients with nasopharyngeal
carcinoma. |
Table I
Association of CHD1L expression with
clinicopathological parameters in 133 patients with nasopharyngeal
carcinoma.
| | CHD1L expression
(n) | |
|---|
| |
| |
|---|
| Parameters | N | Positive | Negative | P-value |
|---|
| Age (years) |
| <48 | 50 | 31 | 19 | NS |
| ≥48 | 83 | 57 | 26 | |
| Gender |
| Male | 83 | 53 | 30 | NS |
| Female | 50 | 35 | 15 | |
| Clinical stage |
| I–II | 34 | 15 | 19 | 0.016 |
| III–IV | 99 | 73 | 26 | |
| T-stage |
| T1–T2 | 45 | 28 | 17 | NS |
| T3–T4 | 88 | 60 | 28 | |
| N-stage |
| N0 | 39 | 25 | 14 | NS |
| N1–N3 | 94 | 63 | 31 | |
| WHO histology |
| II | 39 | 26 | 13 | NS |
| III | 94 | 62 | 32 | |
| Recurrence |
| No | 98 | 58 | 40 | 0.002 |
| Yes | 35 | 30 | 5 | |
| Metastasis |
| No | 100 | 61 | 39 | 0.031 |
| Yes | 33 | 27 | 6 | |
Association of CHD1L protein expression
with the prognosis of human NPC
The association of CHD1L protein expression with the
prognosis of human NPC was also evaluated. The log-rank test and
Kaplan-Meier analysis were used to determine the effect of classic
clinicopathological characteristics, including age, gender,
clinical stage, T-stage, N-stage, WHO histological type, recurrence
and metastasis, and CHD1L expression on survival. The log-rank test
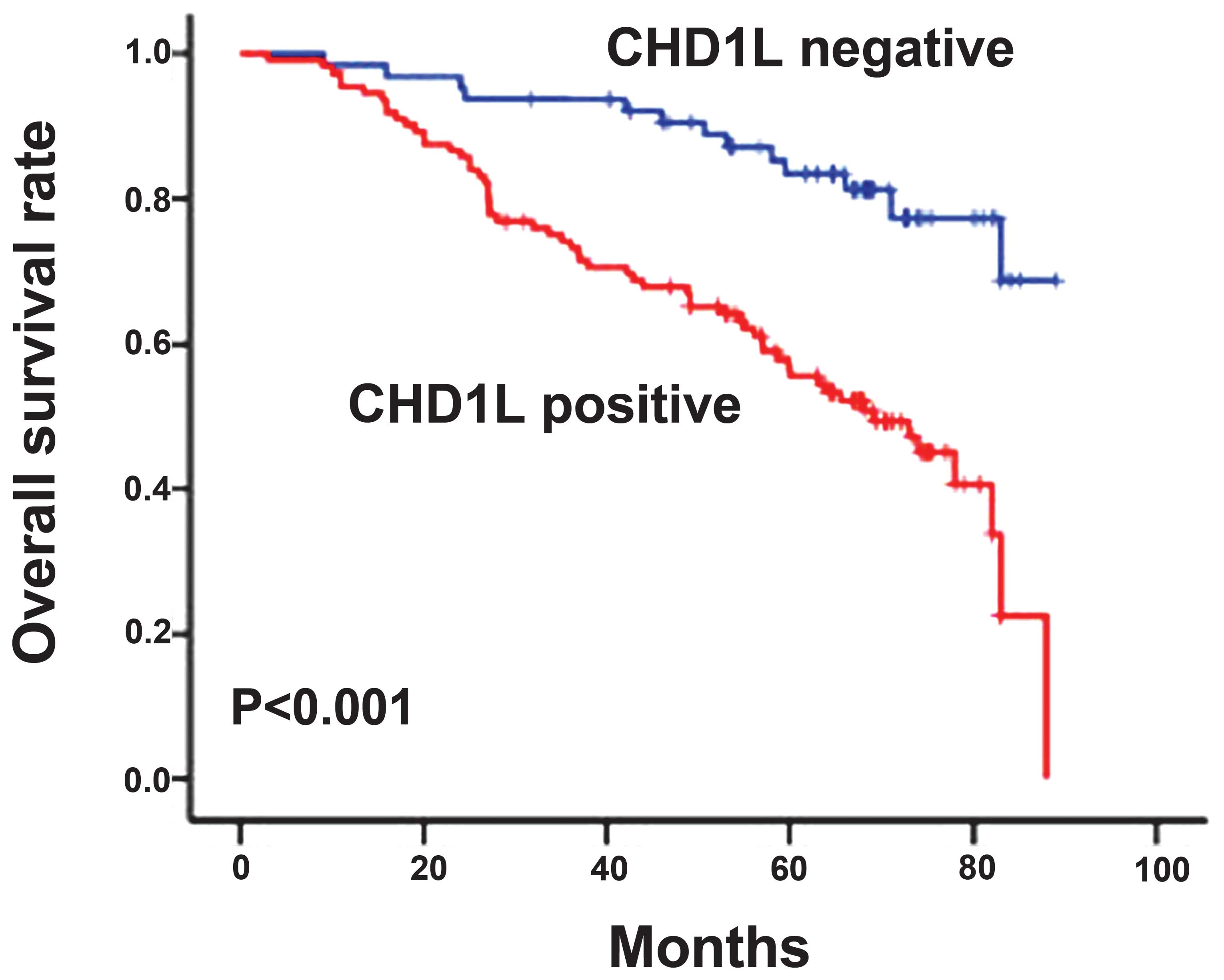
showed that the low CHD1L expression group had a significantly
improved survival time, whereas the high CHD1L expression group had
a shorter survival time (P<0.001, Fig. 3). In addition, clinical stage,
recurrence and metastasis showed strong correlations with survival
in Kaplan-Meier analysis and log-rank tests (for clinical stage,
P=0.002; for recurrence and metastasis, P<0.001; Table II).
 | Table IIUnivariate analysis of different
prognostic variables in 133 patients with nasopharyngeal
carcinoma. |
Table II
Univariate analysis of different
prognostic variables in 133 patients with nasopharyngeal
carcinoma.
| Variables | Subset | HR | 95% CI | P-value |
|---|
| Patient gender | Male vs.
female | 1.987 | 0.608–4.092 | NS |
| Patient age | <48 vs.
≥48 | 1.566 | 0.465–3.853 | NS |
| Clinical stage | I–II vs.
III–IV | 6.814 | 1.031–14.613 | 0.002 |
| T-stage | T1–T2 vs.
T3–T4 | 2.380 | 0.738–5.046 | NS |
| N-stage | N0 vs.
N1–N3 | 1.778 | 0.732–4.028 | NS |
| WHO histological
type | II vs.
III | 1.458 | 0.689–3.076 | NS |
| Recurrence | No vs.
yes | 8.916 | 1.021–19.656 | <0.001 |
| Metastasis | No vs.
yes | 8.421 | 1.042–17.975 | <0.001 |
| CHD1L | Negative vs.
positive | 10.204 | 1.833–30.225 | <0.001 |
Multivariate survival analysis, which included CHD1L
expression level, clinical stage, recurrence and metastasis, was
used to determine whether CHD1L expression level was an independent
prognostic factor. In this analysis, as well as clinical stage,
recurrence and metastasis, CHD1L expression (hazard ratio, 7.916;
95% confidence level, 2.067–16.034; P=0.003) was an independent
prognostic factor for patients with NPC (Table III).
 | Table IIIMultivariate analysis of different
prognostic variables in 133 patients with nasopharyngeal
carcinoma. |
Table III
Multivariate analysis of different
prognostic variables in 133 patients with nasopharyngeal
carcinoma.
| Variables | Subset | HR | 95% CI | P-value |
|---|
| Clinical stage | I–II vs.
III–IV | 6.193 | 1.011–13.392 | 0.006 |
| Recurrence | No vs.
yes | 6.928 | 0.922–13.556 | 0.005 |
| Metastasis | No vs.
yes | 6.893 | 1.023–13.528 | 0.005 |
| CHD1L | Negative vs.
positive | 7.916 | 2.067–16.034 | 0.003 |
Discussion
At present, the prognosis of NPC remains
unsatisfactory, and NPC represents an invasive, rapidly
proliferating tumor; therefore, it is necessary to identify
prognostic biomarkers that are independently correlated with tumor
prognosis and aggressiveness. In the present study, the CHD1L
expression status was examined by immunohistochemistry in 133
patients with NPC in relation to survival as well as clinical and
pathological features. The data showed that CHD1L was upregulated
in NPC and found in 66.2% of cases. Significant correlations were
also observed between CHD1L positivity and poor clinical outcome,
independent of other characteristics. The results indicate that
CHD1L positivity may be considered as a good prognostic marker for
NPC.
According to a recent study, CHD1L can be considered
to be a novel independent biomarker for progression, prognosis and
survival in several types of solid tumor (12). This accumulated knowledge about the
functions of CHD1L could facilitate a search for targeted
treatments in specific subtypes of tumors. Data have shown that
positive expression of the CHD1L protein is significantly
associated with the metastasis of ovarian carcinoma; therefore,
CHD1L protein expression, as examined by immunohistochemistry, may
act as a novel prognostic biomarker for patients with ovarian
carcinoma (13). In mice, CHD1L
activates the expression of Sparc/osteonectin, cwcv and kazal-like
domains proteoglycan 1 (SPOCK1), which activates Akt signaling to
block apoptosis and invasion by HCC cells, and levels of SPOCK1
increase with the progression of human HCC (14). Furthermore, overexpression of CHD1L
in patients with colorectal carcinoma has been shown to correlate
with a large tumor size, deep tumor invasion, a high histological
grade and poor disease-free survival (15). CHD1L-transfected cells have been
observed to exhibit a strong oncogenic ability, increasing the
tumorigenicity in nude mice, which could be effectively suppressed
by small interfering RNA targeting CHD1L. In addition, a functional
study by Ji et al (15)
showed that overexpression of CHD1L could promote
G1/S-phase cells and inhibit apoptosis. It was recently
demonstrated that, in bladder cancer, CHD1L overexpression was
significantly correlated with the histological grade and stage of
the tumor (16). Multivariate
analysis further demonstrated that CHD1L was an independent
prognostic factor for patients with bladder cancer (16). These data suggest that CHD1L may
play an important role in promoting tumorigenesis or progression;
however, to date, the expression and clinical significance of CHD1L
in NPC have not been explored. In this present study, therefore,
the aim was to analyze CHD1L protein expression in tumor tissue and
to assess its prognostic significance for NPC.
In the present study, CHD1L expression in NPC
specimens was investigated using western blot analysis. The results
showed that the CHD1L protein levels were significantly increased
in tumor tissue samples compared with those in the adjacent
non-tumor tissue samples. Furthermore, immunohistochemical analysis
demonstrated high CHD1L expression in 66.2% of cases of NPC. It was
additionally found that a high expression of CHD1L correlated
significantly with an advanced clinical stage, recurrence and the
metastasis status of NPC, indicating that an increase in CHD1L
expression could promote tumor growth and invasion. These results
suggest that CHD1L may play an important role in the tumorigenesis
or progression of NPC.
In the Kaplan-Meier survival analysis, patients with
positive CHD1L expression had a significantly shorter overall
survival time than patients who were negative for CHD1L expression.
The univariate analysis showed that increased expression of CHD1L
in NPC tissues correlated significantly with overall survival. Cox
hazard ratio regression analyses further demonstrated that CHD1L
expression was an independent prognostic factor in patients with
NPC. These results indicate that CHD1L could serve as a valuable
prognostic biomarker for patients with NPC.
Overall, it has been demonstrated that CHD1L is an
independent prognostic factor for survival in patients with NPC and
its positive expression could be a potential prognostic biomarker
for clinical application in these patients. Prospective clinical
studies of CHD1L as a novel biomarker in NPC are warranted.
References
|
1
|
Wei KR, Xu Y, Liu J, Zhang WJ and Liang
ZH: Histopathological classification of nasopharyngeal carcinoma
(Review). Asian Pac J Cancer Prev. 12:1141–1147. 2011.
|
|
2
|
Zhen Y, Ye Y, Yu X, Mai C, Zhou Y, Chen Y,
Yang H, Lyu X, Song Y, Wu Q, Fu Q, Zhao M, Hua S, Wang H, Liu Z,
Zhang Y and Fang W: Reduced CTGF expression promotes cell growth,
migration, and invasion in nasopharyngeal carcinoma. PLoS One.
8:e649762013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ho FC, Tham IW, Earnest A, Lee KM and Lu
JJ: Patterns of regional lymph node metastasis of nasopharyngeal
carcinoma: a meta-analysis of clinical evidence. BMC Cancer.
12:982012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jia WH and Qin HD: Non-viral environmental
risk factors for nasopharyngeal carcinoma: a systematic review.
Semin Cancer Biol. 22:117–126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Isinger-Ekstrand A, Johansson J, Ohlsson
M, Francis P, Staaf J, Jönsson M, Borg A and Nilbert M: Genetic
profiles of gastroesophageal cancer: combined analysis using
expression array and tiling array - comparative genomic
hybridization. Cancer Genet Cytogenet. 200:120–126. 2010.
View Article : Google Scholar
|
|
6
|
Gottschalk AJ, Timinszky G, Kong SE, Jin
J, Cai Y, Swanson SK, Washburn MP, Florens L, Ladurner AG, Conaway
JW and Conaway RC: Poly(ADP-ribosyl)ation directs recruitment and
activation of an ATP-dependent chromatin remodeler. Proc Natl Acad
Sci USA. 106:13770–13774. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kokavec J, Podskocova J, Zavadil J and
Stopka T: Chromatin remodeling and SWI/SNF2 factors in human
disease. Front Biosci. 13:6126–6134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma NF, Hu L, Fung JM, Xie D, Zheng BJ,
Chen L, Tang DJ, Fu L, Wu Z, Chen M, Fang Y and Guan XY: Isolation
and characterization of a novel oncogene, amplified in liver cancer
1, within a commonly amplified region at 1q21 in hepatocellular
carcinoma. Hepatology. 47:503–510. 2008.
|
|
9
|
Chen L, Chan TH, Yuan YF, Hu L, Huang J,
Ma S, Wang J, Dong SS, Tang KH, Xie D, Li Y and Guan XY: CHD1L
promotes hepatocellular carcinoma progression and metastasis in
mice and is associated with these processes in human patients. J
Clin Invest. 120:1178–1191. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen L, Yuan YF, Li Y, Chan TH, Zheng BJ,
Huang J and Guan XY: Clinical significance of CHD1L in
hepatocellular carcinoma and therapeutic potentials of
virus-mediated CHD1L depletion. Gut. 60:534–543. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chung MS, Lee SH, Lee DH and Chung BH:
Evaluation of the 7th American Joint Committee on cancer TNM
staging system for prostate cancer in point of classification of
bladder neck invasion. Jpn J Clin Oncol. 43:184–188. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng W, Su Y and Xu F: CHD1L: a novel
oncogene. Mol Cancer. 12:1702013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He WP, Zhou J, Cai MY, Xiao XS, Liao YJ,
Kung HF, Guan XY, Xie D and Yang GF: CHD1L protein is overexpressed
in human ovarian carcinomas and is a novel predictive biomarker for
patients survival. BMC Cancer. 12:4372012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Y, Chen L, Chan TH, Liu M, Kong KL, Qiu
JL, Li Y, Yuan YF and Guan XY: SPOCK1 is regulated by CHD1L and
blocks apoptosis and promotes HCC cell invasiveness and metastasis
in mice. Gastroenterology. 144:179–191.e4. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ji X, Li J, Zhu L, Cai J, Zhang J, Qu Y,
Zhang H, Liu B, Zhao R and Zhu Z: CHD1L promotes tumor progression
and predicts survival in colorectal carcinoma. J Surg Res.
185:84–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tian F, Xu F, Zhang ZY, Ge JP, Wei ZF, Xu
XF and Cheng W: Expression of CHD1L in bladder cancer and its
influence on prognosis and survival. Tumour Biol. 34:3687–3690.
2013. View Article : Google Scholar : PubMed/NCBI
|