Introduction
Innate immunity is a major part of the immune
system, playing a significant role in the acute inflammation
induced by microbial infection or tissue damage (1). Innate immune cells, including
monocytes, dendritic cells and T cells, can be recognized by their
germ line-encoded pattern recognition receptors (PRRs), which are
responsible for sensing the structure of microbial species
(2). PRRs have also been shown to
recognize endogenous ligands released from damaged cells and
tissues (3).
The Toll-like receptor (TLR) family is a
well-characterized PRR family that is responsible for recognizing
invading pathogens (4). TLR
stimulation initiates a signal transduction pathway via the adaptor
protein, MyD88, which induces the expression of proinflammatory
cytokines, such as interleukin (IL)-6, IL-8 and tumor necrosis
factor (TNF)-α (5). In total, 11
members constitute the human TLR family. Among them, TLR4 is a
transmembrane protein specialized in the recognition of
lipopolysaccharide (LPS), which is a component of gram-negative
bacteria (6).
A previous study has demonstrated that the
activation of TLR4 induces the expression of numerous
proinflammatory molecules, which are essential in shaping the
immune status of immune cells (7).
However, the expression level variation of immune molecules within
immune cells upon TLR4 stimulation remains unclear. In the current
study, peripheral blood mononuclear cells (PBMCs) were isolated
from healthy volunteers and stimulated by the TLR4 agonist, LPS,
while the expression levels of various cytokines, chemokines,
growth factors and kinases were screened. The aim of the present
study was to confirm which types of immune molecules are involved
in the activation of the TLR4 signaling pathway.
Materials and methods
Isolation and stimulation of PBMCs
Heparinized venous blood samples were isolated from
three healthy male volunteers (aged 25–28 years) and PBMCs were
separated by density separation over Ficoll-Hypaque. After washing
twice with phosphate buffered-saline, the PBMCs were plated into
24-well plates with a total number of 2×106 cells/well.
LPS was added to the PBMCs at a concentration of 100 ng/ml.
Supernatants were collected at 4 h following stimulation for use in
the antibody chip. The present study was approved by the Ethics
Committee of West China Hospital, Sichuan University (Sichuan,
China) and written informed consent was obtained from the
patients.
RNA extraction and cDNA synthesis
This procedure was performed as previously described
(8). In brief, the total RNA from
the PBMCs was extracted using a RNeasy mini kit (74104; Qiagen,
Hilden, Germany) and quantified using a NanoDrop 3300
Fluorospectrometer (Thermo Fisher Scientific, Wilmington, DE, USA).
cDNA was synthesized using a ReverTra Ace qPCR kit (FSQ-101;
Toyobo, Kagoshima, Japan) and the reverse transcription (RT)
conditions were as follows: 65°C for 5 min, 37°C for 15 min and
98°C for 5 min.
Quantification polymerase chain reaction
(PCR)
This procedure was performed as previously described
(8). In brief, quantitative PCR
(qPCR) was performed to amplify the synthesized cDNA using the
RealMaster Mix Reagent (SYBR Green; FP202; Tiangen Biotech Co.,
Ltd., Beijing, China). An iCycler iQTM Optical Module (Beckman
Coulter, Fullerton, CA, USA) was used for RT-qPCR under the
following conditions: One cycle at 95°C for 30 sec, 40 cycles at
95°C for 30 sec, 58°C for 30 sec and 72°C for 30 sec, followed by a
melt curve between 55 and 95°C in 0.5°C-increments and 10-sec
intervals. All the tests were performed in triplicate and the
primers used are shown in Table
I.
 | Table IOligonucleotides used in quantitative
polymerase chain reaction analysis. |
Table I
Oligonucleotides used in quantitative
polymerase chain reaction analysis.
| Gene | Forward primer | Reverse primer | GenBank number |
|---|
| CCL5 |
GACACCACACCCTGCTGCT |
TACTCCTTGATGTGGGCACG | NM_002985 |
| CCL7 |
AGCAGAGGCTGGAGAGCTACA |
GGGTCAGCACAGATCTCCTTGT | NM_006273 |
| CCL8 |
GTTTCTGCAGCGCTTCTGTG |
TGGCTGAGCAAGTCCCTGA | Y10802 |
| CCL15 |
CCTCTCCTGCCTCATGCTTATT |
CTCTGTCTCTGCATCATTTGTGAA | U58914 |
| CCL17 |
CCATCGTTTTTGTAACTGTGCAG |
TGCATTCTTCACTCTCTTGTTGTTG | NM_002987 |
| CCL22 |
TGCGCGTGGTGAAACACT |
GGTTAGCAACACCACGCCA | NM_002990 |
| CCL24 |
AGCCTTCTGTTCCTTGGTGTCT |
GGGAGAGGGTATGACCACAGAG | NM_002991 |
| CCL26 |
CCAAGACCTGCTGCTTCCAA |
GAATTCATAGCTTCGCACCCA | NM_006072 |
| CCL28 |
CTCGCCATCGTGGCCTT |
GCAATGGGAAGTATGGCTTCTG | AF220210 |
| c-Myc |
CAAGACTCCAGCGCCTTCTC |
GTTGAGTAACGAGCTGACCCC | AM393287 |
| CTNNB |
CATCGTGAGGGCTTACTGGC |
GAGCAAGGCAACCATTTTCTG | XM_006712984 |
| CXCL2 |
AGGTGAAGTCCCCCGGAC |
GCCCATTCTTGAGTGTGGCT | NM_002089 |
| CXCL6 |
GCTGAGAGTAAACCCCAAAACG | GGAGCACTGCGGGCC | NM_002993 |
| GAPDH |
GAAGGTGAAGGTCGGAGTC |
GAAGATGGTGATGGGATTTC | J04038 |
| IFN-β |
CAGCAATTTTCAGTGTCAGAAGCT |
TCATCCTGTCCTTGAGGCAGT | M28622 |
| IFN-γ |
CCAACGCAAAGCAATACATGA |
CGCTTCCCTGTTTTAGCTGC | J00219 |
| IL-1β |
ACGAATCTCCGACCACCACT |
CCATGGCCACAACAACTGAC | M15330 |
| IL-2 |
CAAGAATCCCAAACTCACCAGG |
GACACTGAAGATGTTTCAGTTCTGT | J00264 |
| IL-6 |
GACCCAACCACAAATGCCA |
GTCATGTCCTGCAGCCACTG | M14584 |
| IL-7 |
ACCAGTAGAAGACAATTGCATC |
CCAGGTTTTCATCATCTTCAGCT | NM_001199888 |
| IL-8 |
CTGGCCGTGGCTCTCTTG |
CCTTGGCAAAACTGCACCTT | NM_000584 |
| IL-12 |
CGGTCATCTGCCGCAAA |
CAAGATGAGCTATAGTAGCGGTCCT | M65272 |
| IL-15 |
GACCCCACCAAAGCTGGAC |
TCACAGTGCTGCTGTCTGCTG | M90391 |
| IL-23 |
GAAGGCTCCGCTCTGCAAT |
TCTGGGTCTTCTCGATGGCA | L06801 |
| IP-10 |
TGAAATTATTCCTGCAAGCCAA |
CAGACATCTCTTCTCACCCTTCTTT | NM_001565 |
| JNK |
GCTAATTCTGTACCAATGTC |
GAAGAGTGCACGTCAGGAAC | NM_139049 |
| MAP3K |
CCTGCTCGGTGCACGATGCTG |
CTCTGTCTCTTCACGTGGCGG | NM_003954 |
| MAP3K1 |
CTTTTAAGTCAGAAGTTGCTG |
CTTCTCCATTTTCAACCTGC | AF042838 |
| MIP-3α |
TCCTGGCTGCTTTGATGTCA |
TCAAAGTTGCTTGCTGCTTCTG | NM_004591 |
| MIP-3β |
GGCACCAATGATGCTGAAGA |
GAAGTTCCTCACGATGTACCCAG | NM_006274 |
| NF-κB |
AGAGTGCTGGAGTTCAGGATA |
AAGGTGGATGATTGCTAAGTGT | AJ271718 |
| PTEN |
ACCATAACCCACCACAGC |
CAGTTCGTCCCTTTCCAG | NM_058074 |
| PI3K |
CCTGGGGGTTGGTGGCTGTTC |
GTCTGGCTGGAATGATGCTATC | NM_006219 |
| STAT3 |
CCTACAAAGGGGACCCCATTGTAC |
CAGGGAATTTGACCAGCAACC | NM_213662 |
| TGF-α |
TATCGACATGGAGCTGGTGAAG |
CAGCTTGGACAGGATCTGGC | X02812 |
| TNF-β |
GGTGCTTGTTCCTCAGCCTC |
CAGGCAGAAGAGCGTGGTG | M10988 |
| VEGF |
GACTTGAGTTGGGAGGGGAA |
GAGGCTCAGCGCCAGGGCTGGG | AF024710 |
Antibody chip
The supernatants collected from the TLR4-stimulated
and unstimulated PBMCs were arrayed for molecule secretion using
the RayBio® Human Antibody Array C-Series 1000
(RayBiotech, Inc., Norcross, GA, USA), according to the
manufacturer’s instructions. Blots were analyzed using ImageJ
software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data analysis was performed using the Bio-Rad iQ5
software (Bio-Rad Laboratories, Hercules, CA, USA), with
glyceraldehyde 3-phosphate dehydrogenase as the internal control
and normal PBMCs as the negative control. The results are expressed
as the mean ± standard error of the mean and were analyzed using
SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 and
P<0.001 were considered to indicate a statistically significant
difference when compared with the control group. The figures were
obtained using GraphPad Prism 5 software (GraphPad Software, Inc.,
La Jolla, CA, USA).
Results
TLR4 agonist increases the expression
levels of cytokines and chemokines
RT-qPCR was performed to screen the expression
levels of a number of cytokines and chemokines, in order to
identify candidate genes responsible for the LPS-mediated changes
in PBMCs. The expression level variation of a number of these genes
may significantly affect the biological function of the PBMCs,
induced by microenvironment or pathogen stimulation.
With regard to cytokine detection, the TLR4 agonist,
LPS, was found to significantly increase the expression levels of
several major cytokines (P<0.001), including IL-1β, IL-8, IL-15,
interferon (IFN)-β, IFN-γ, macrophage inflammatory protein (MIP)-3α
and MIP-β. In addition, LPS slightly increased the expression level
of IL-6 (P<0.05). IL-23 was the only downregulated cytokine
detected (P<0.001; Fig. 1A).
During the chemokine assay, only the chemokine (C-C motif) ligand
(CCL) 26, chemokine (C-X-C motif) ligand (CXCL) 2 and CXCL6 were
found to be evidently enhanced upon LPS stimulation (P<0.001),
although CCL22, CCL24 and CCL28 were also found to be significantly
different compared with the controls (P<0.05; Fig. 1B).
LPS stimulation enhances the expression
levels of growth factors
Growth factors were screened to obtain their
expression levels following LPS stimulation. The results indicated
an evident upregulation of nuclear factor (NF)-κB, phosphatase and
tensin homolog (PTEN), transforming growth factor (TGF)-β and TNF-α
in the PBMCs activated by the TLR ligand (P<0.001). The
expression of c-Myc remained unchanged, while the expression of
vascular endothelial growth factor (VEGF) was inhibited (P<0.05;
Fig. 2).
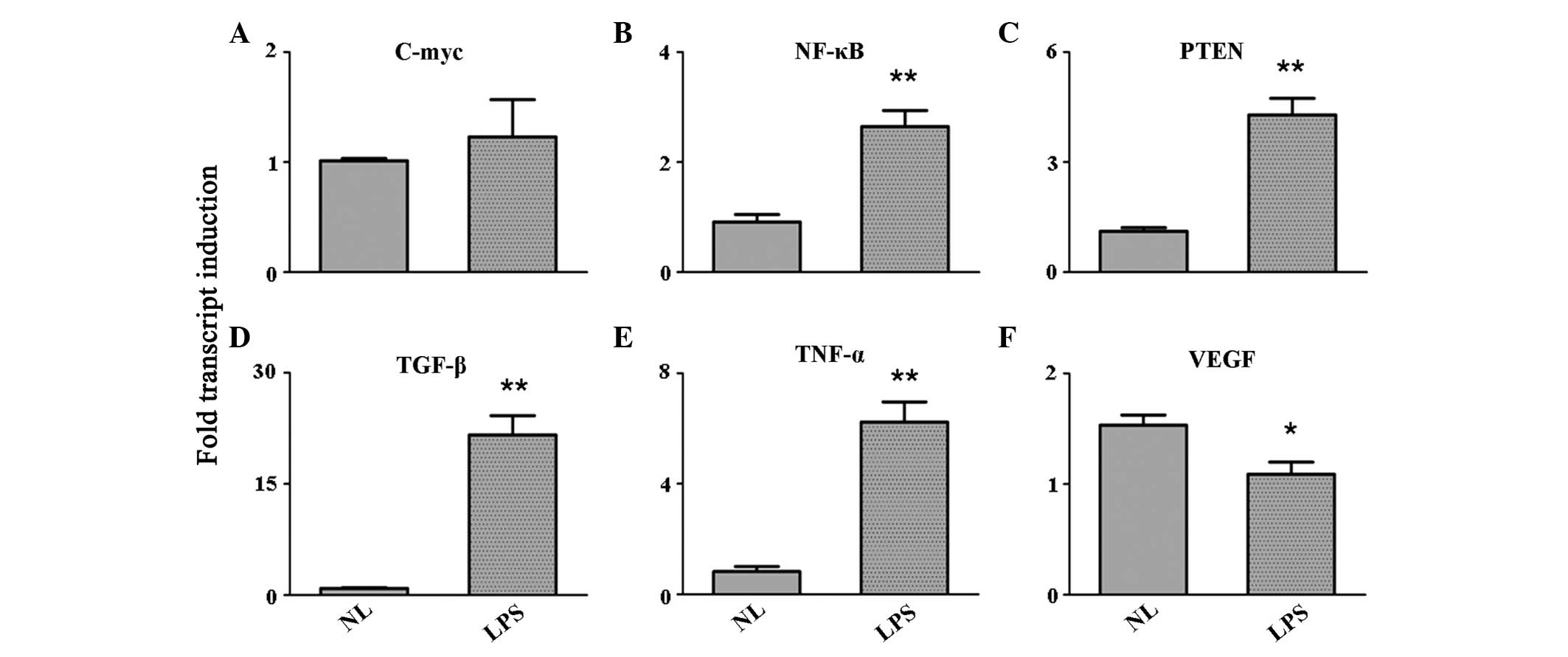 | Figure 2Expression level variation of the
growth factors, (A) c-Myc, (B) NF-κB, (C) PTEN, (D) TGF-β, (E)
TNF-α and (F) VEGF, as detected by quantitative reverse
transcription polymerase chain reaction. *P<0.05 and
**P<0.001, vs. control group. NF, nuclear factor;
PTEN, phosphatase and tensin homolog; TGF, transforming growth
factor; TNF, tumor necrosis factor; VEGF, vascular endothelial
growth factor; NL, normal PBMCs; LPS, lipopolysaccharide-treated
PBMCs; PBMCs, peripheral blood mononuclear cells. |
Activation of the TLR4 signaling pathway
has no evident effect on the expression levels of kinases
TLR4 agonist stimulation performed on PBMCs may
induce the activation of protein kinases. Therefore, a number of
protein kinase signaling pathways were analyzed in the study. The
results indicated that the expression of phosphorylated signal
transducer and activator of transcription 3 (pSTAT3) was
significantly enhanced in PBMCs following LPS stimulation
(P<0.001). However, cadherin-associated protein β (CTNNB),
mitogen-activated protein kinase kinase kinase 1 (MAP3K1) and
phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) expression
levels remained unchanged during the detection. Notably, the
expression levels of two important kinases, c-Jun N-terminal kinase
(JNK) and MAP3K, were inhibited following LPS treatment (P<0.05;
Fig. 3).
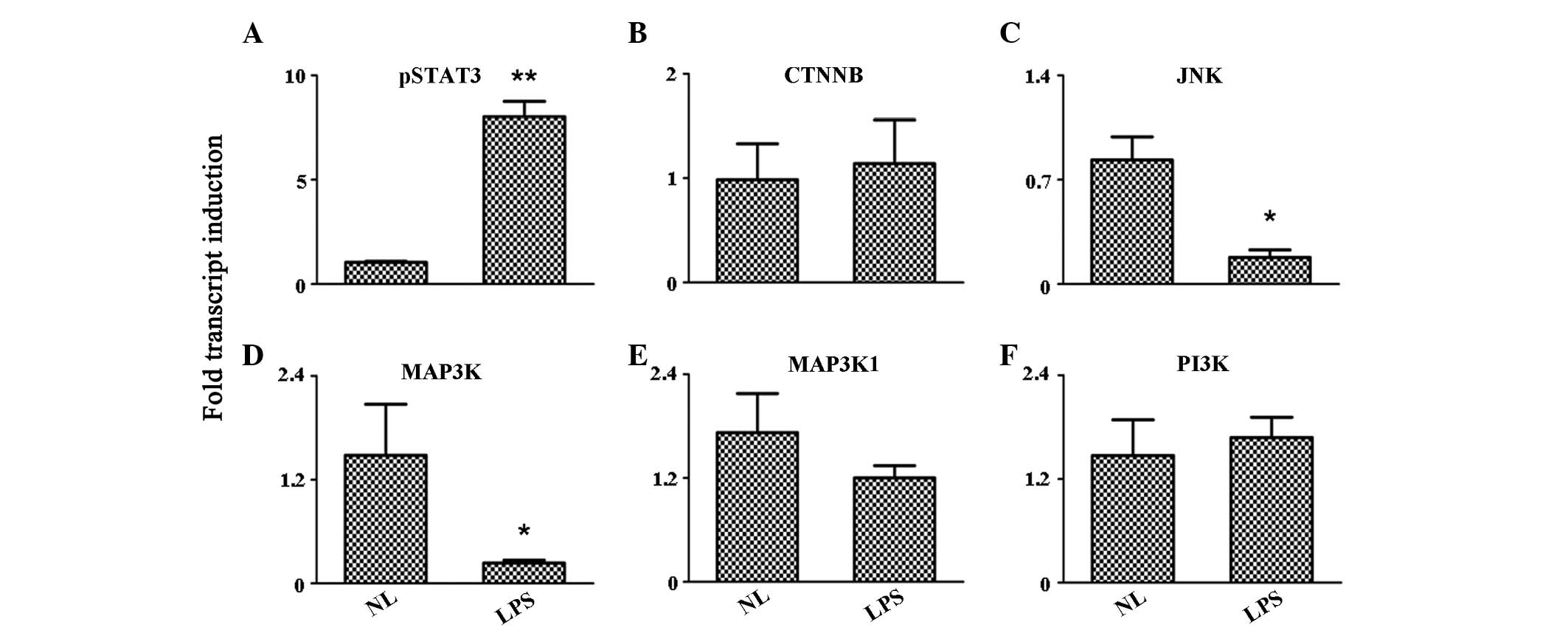 | Figure 3Expression level variation of the
protein kinases, (A) pSTAT3, (B) CTNNB, (C) JNK, (D) MAP3K, (E)
MAP3K1 and (F) P13K, as detected by quantitative reverse
transcription polymerase chain reaction. *P<0.05 and
**P<0.001, vs. control group. pSTAT3, phosphorylated
signal transducer and activator of transcription 3; CTNNB,
cadherin-associated protein β; JNK, c-Jun N-terminal kinase; MAP3K,
mitogen-activated protein kinase kinase kinase; PI3K,
phosphatidylinositol-4,5-bisphosphate 3-kinase; NL, normal PBMCs;
LPS, lipopolysaccharide-treated PBMCs; PBMCs, peripheral blood
mononuclear cells. |
LPS induces the secretion of
proinflammatory molecules
Supernatants collected from the LPS-treated and
untreated PBMCs were analyzed to determine the secretion levels of
major immune molecules using an antibody chip. A total of 20
proteins, including α-fetoprotein, albumin, E-selectin,
intercellular adhesion molecule-1, IFN-α, IFN-γ, IL-10, IL-12,
IL-18, IL-1β, IL-4, IL-5, IL-6, IL-8, monocyte chemoattractant
protein (MCP)-1, MCP-3, MIP-1α, Notch-1, TGF-β and VEGF, were
selected for analysis. The results revealed that only six of the
aforementioned proteins were affected by TLR4 activation (Fig 4). Among these, the secretion levels
of IL-1β, IL-6 and MIP-1α were significantly enhanced (P<0.001),
while IL-8 secretion was moderately increased (P<0.05). In
addition, the secretion levels of MCP-1 and MCP-3 were
inhibited.
 | Figure 4Secretion levels of (A) IL-1β, (B)
IL-6, (C) IL-8, (D) MCP-1, (E) MCP-3 and (F) MIP-1α in the
supernatant. *P<0.05 and **P<0.001, vs.
control group. IL, interleukin; MCP, monocyte chemoattractant
protein; MIP, macrophage inflammatory protein; NL, normal control
PBMCs; LPS, lipopolysaccharide-treated PBMCs; PBMCs, peripheral
blood mononuclear cells. |
Discussion
Vertebrates have evolved systems of immune defense
to eliminate invading pathogens. The innate immune system is the
first line of the host defense against microorganisms and
recognizes the conserved components of pathogens via pattern
recognition receptors (PRRs) (9).
Among the 11 human TLRs, TLR3, TLR7, TLR8 and TLR9 are expressed on
the surface of endosomes, while TLR1, TLR2, TLR4, TLR5, TLR6, TLR10
and TLR11 are located on the cellular surface (10). TLR4 plays an important role in the
protection against fungi by recognizing LPS components. Stimulation
with a TLR4 agonist induces proinflammatory signals, as well as the
production of type I IFNs (11).
In the present study, PBMCs were isolated from
healthy volunteers and stimulated by the TLR4 agonist, LPS. At 4 h
after the treatment, the supernatant and cellular RNA were isolated
and assayed using an antibody chip and RT-qPCR, respectively. Using
these methods, the immune molecules involved in the immunological
signature of PBMCs can be identified and the TLR4 signaling pathway
can be further understood. The molecules selected in the present
study exhibit a number of important biological functions in
vivo. In addition to increasing the expression levels of IL-8
and IL-6 (12), TLR4 agonist
stimulation was found to influence numerous immunomodulatory
factors. Among the cytokines examined, the expression levels of
IL-1β, IL-6, IL-8, IL-15, IFN-β, IFN-γ, MIP-3α and MIP-3β increased
markedly. During chemokine detection, the expression levels of
CCL22, CCL24, CCL26, CXCL2 and CXCL6 were upregulated upon LPS
stimulation. In addition, the expression levels of several growth
factors, including NF-κB, PTEN, TNF-α and TGF-β, were enhanced.
Detecting the expression levels of protein kinases, which play
important roles in TLR-mediated biological functions, was important
in this study. The expression level of pSTAT3 was found to increase
in LPS-treated PBMCs, while the expression levels of CTNNB, MAP3K1
and PI3K remained unchanged in the treated and untreated groups.
However, the expression of JNK was inhibited following LPS
treatment. These results indicated that the activation of TLR4 had
varying effects on the different kinase pathways. The cytokine
levels were detected in the supernatants of LPS-treated PBMCs, and
the secretion levels of IL-1β, IL-6, IL-8 and MIP-1α were found to
be enhanced, indicating the important role of TLR4 in shaping the
immune status of PBMCs.
Compared with previous studies, the present study
analyzed a greater number of immune molecules that may be important
in TLR-related functions. However, a limitation of the study was
that the variation in the expression levels of immune molecules was
only detected at a gene level. Future studies should analyze the
association between these candidate immune molecules and
TLR-mediated functions and should investigate the MEprotein
expression levels.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 81101261).
References
|
1
|
Takeuchi O and Akira S: Pattern
recognition receptors and inflammation. Cell. 140:805–820. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hou B, Reizis B and DeFranco AL: Toll-like
receptors activate innate and adaptive immunity by using dendritic
cell-intrinsic and -extrinsic mechanisms. Immunity. 29:272–282.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miyake K: Innate immune sensing of
pathogens and danger signals by cell surface Toll-like receptors.
Semin Immunol. 19:3–10. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Blasius AL and Beutler B: Intracellular
Toll-like receptors. Immunity. 32:305–315. 2010. View Article : Google Scholar
|
|
5
|
Yamamato M, Sato S, Mori K, Hoshino K,
Takeuchi O, Takeda K and Akira S: Cutting edge: a novel Toll/IL-1
receptor domain-containing adapter that preferentially activates
the IFN-beta promoter in the Toll-like receptor signaling. J
Immunol. 169:6668–6672. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ghosh TK, Mickelson DJ, Solberg JC, Lipson
KE, Inglefield JR and Alkan SS: TLR-TLR cross talk in human PBMC
resulting in synergistic and antagonistic regulation of type-1 and
2 interferons, IL-12 and TNF-alpha. Int Immunopharmacol.
7:1111–1121. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaisho T, Takeuchi O, Kawai T, Hoshino K
and Akira S: Endotoxin-induced maturation of MyD88-deficient
dendritic cells. J Immunol. 166:5688–5694. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Activation of the TLR1/2 pathway induces
the shaping of the immune response status of peripheral blood
leukocytes. Exp Ther Med. 7:1708–1712. 2014.PubMed/NCBI
|
|
9
|
Beutler B: Inferences, questions and
possibilities in Toll-like receptor signalling. Nature.
430:257–263. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kopp E and Medzhitov R: Recognition of
microbial infection by Toll-like receptors. Curr Opin Immunol.
15:396–401. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shan JY, Ji WZ, Li HT, Tuxun T, Lin RY and
Wen H: TLR2 and TLR4 expression in peripheral blood mononuclear
cells of patients with chronic cystic echinococcosis and its
relationship with IL-10. Parasite Immunol. 33:692–696. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaisho T and Akira S: Toll-like receptor
function and signaling. J Allergy Clin Immunol. 117:979–987. 2006.
View Article : Google Scholar : PubMed/NCBI
|


















