Introduction
Pulmonary vascular remodeling is a significant
pathological factor for pulmonary arterial hypertension (1), resulting in increased pulmonary
vascular resistance and reduced elasticity. The overproliferation
of pulmonary arterial smooth muscle cells is the predominant
feature of pulmonary vascular remodeling, which induces thickening
of the pulmonary arterial wall, stenosis of the lumina, and
muscularization of the pulmonary arteries (2). Previous studies (3,4) have
indicated that cigarette smoke induces pulmonary vascular
remodeling through direct affects on the lung vessels. However, the
potential mechanism remains unclear. Basic fibroblast growth factor
(bFGF) has been reported to play an important role in the
regulation of fibroblasts, airway smooth muscle cells, and
endothelial cells through the autocrine and paracrine systems
(5). Nevertheless, to the best of
our knowledge, no study has been performed to investigate whether
it is involved in the remodeling of lung vessels in rats exposed to
cigarette smoke (6).
Cellular proliferation and cell numbers are
regulated by the cell cycle, which involves a series of cyclins
(7). Cyclin D1 has been shown to
play a crucial role in the G1/S transition; for example, Liu and
Templeton (8) reported that
crocetin inhibited the G1/S transition through the suppression of
cyclin D1 in vascular smooth muscle cells. Our previous study
(9) indicated that cyclins D1 and
E are the rate-limiting activators of the G1/S transition, and that
cyclin D1 may play a specialized role in facilitating emergence
from quiescence. In the present study, the aim was to investigate
the effect of the duration of cigarette smoke exposure on the
expression of bFGF and cyclin D1 in the pulmonary vessels in rats,
based on which their roles in pulmonary vascular remodeling were
investigated.
Materials and methods
Animals
A total of 24 male Wistar rats (body weight, 150–200
g; age, 6 weeks) were randomly divided into four groups: Control
(n=6), tobacco smoke-exposed I (n=6), tobacco smoke-exposed II
(n=6) and tobacco smoke-exposed III (n=6). For the tobacco
smoke-exposed groups, the animals were placed in a ventilated
smoking chamber and exposed to the smoke produced by 20 cigarettes
(nicotine, 1.0 mg per cigarette; tar, 13 mg per cigarette) for 60
min, twice a day for 2 weeks (group I), 4 weeks (group II) and 8
weeks (group III). The control group was exposed to fresh air with
no contact with smoke. This study was approved by the Ethics
Committee of the First Affiliated Hospital of Anhui Medical
University (Hefei, China).
Sample preparation
The animals were anesthetized with chloral hydrate
(10%) and blood (1 ml) was extracted from the abdominal aorta for
blood gas analysis prior to sacrifice. The animals were then
sacrificed by exsanguination. The chest cavity was opened and the
right lung was removed, following which the aortic smooth muscles
of the right lobe were separated. Subsequently, the tissues were
frozen at −80° for further study. The left lung was perfused with
4% paraform through the trachea until the pleura was flat. The left
main bronchus was resected following ligation. Subsequently, the
tissues were fixed using 4% paraform and embedded with
paraffin.
Determination of pulmonary vessel wall
thickness
Sections were embedded with paraffin and cut to a
thickness of 6 μm. Then the sections were stained with hematoxylin
and eosin (H&E) as previously described (10). Five pulmonary arteries with a
diameter of 50–150 μm were selected using an Olympus BX51 light
microscope (Olympus Corporation, Tokyo, Japan). The slides were
scanned using an scanner (Canon LIDE110; Canon Inc., Tokyo, Japan).
The scanned images were processed using HMIAS-2000 high definition
color medical image analysis software (Qianping Co. Ltd., Wuhan,
China) to determine the thickness of the pulmonary blood vessels
and the pulmonary vessel wall thickness (10). As described in a previous study
(9), the pulmonary vessel wall
thickness was expressed as the percentage of the external diameter:
2 × measured wall thickness/external diameter × 100.
Immunohistochemistry
Immunohistochemistry was conducted using the
Histostain-SP immunohistochemical staining kit purchased from
Beijing Zhongshan Golden Bridge Biological Technology Co., Ltd.
(Beijing, China) following the manufacturer’s instructions. In
brief, the paraffin sections were incubated overnight at 4°C with
mouse anti-rat α-smooth muscle (SM) actin monoclonal antibody
(1:200 dilution). The immunohistochemical staining of bFGF and
cyclin D1 was conducted in a similar manner as that for α-SM actin,
with the exception that a corresponding primary antibody was used.
Subsequently, the sections were stained with diaminobenzidine (DAB)
and counterstained with hematoxylin (11). The muscularization degree of the
arteries was determined according to the staining results (those
stained in a buffy color in the intracytoplasm were considered as
positive results). The ratio of muscularized arteries was presented
as the number of muscularized arteries to the total amount of
arterioles.
Quantitative polymerase chain reaction
(qPCR) analysis of the expression of bFGF and cyclin D1 mRNA
Total mRNA was extracted using TRIzol®
reagent purchased from Invitrogen Co., Ltd (Shanghai, China)
according to the manufacturer’s instructions. cDNA was synthesized
using a reverse transcriptase kit (ReverTra Ace-α-, FSK-101;
Toyobo, Osaka, Japan), following the manufacturer’s instructions.
qPCR was performed using the SYBR® Green Realtime PCR
Master Mix (QPK-201, Toyobo) and a Lightcycler® 480
instrument (Roche, Basel, Switzerland). The cDNA fragments were
denatured at 95.8°C for 15 sec, annealed at 58.8°C for 15 sec and
extended at 72.8°C for 45 sec for 40 cycles. Each sample was
examined in triplicate and the mRNA level was normalized by
β-actin. The primers used were: for bFGF, 5′-CGTTTGTGCCTATTGTTCTT
GTT-3′ and 5′-TGATCCATTGCTTTACCGTCTAC-3′; for cyclin D1,
5′-TGTTCGTGGCCTCTAAGATGAAG-3′ and 5′-GGAAGTGTTCGATGAAATCGTG-3′; and
for β-actin, 5′-GACTACCTCATGAAGATCCTG-3′ and
5′-CATAGAGGTCTTTACGGATGT-3′: The amplification results for qPCR was
calculated as 2(−ΔΔCt), where ΔΔCt = (Ct gene of
interest − Ct control) − (Ct control − Ct control).
Western blot analysis
For the determination of bFGF and cyclin D1, western
blot analysis was performed as previously described (9). Briefly, the tissues were homogenized
in radioimmunoprecipitation assay lysis buffer containing protease
and phosphatase inhibitors. Proteins were separated by
electrophoresis on a 10% SDS-PAGE gel and transferred to a Hybond-P
polyvinylidene difluoride membrane. Subsequently, the membrane was
blocked in 5% non-fat milk and incubated with a primary antibody
(rabbit anti-bFGF or rabbit anti-cyclin D1 antibody; 1:1,000
dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
overnight at 4°C, followed by incubation with the
peroxidase-conjugated secondary antibody [rabbit anti-goat
immunoglobulin G (IgG) or goat anti-rabbit IgG; 1:3,000 dilution;
Santa Cruz Biotechnology, Inc.] for 1 h at room temperature.
Subsequent to washing with phosphate-buffered saline, the bound
primary antibody was visualized and exposed to X-ray film. The same
membrane was probed for β-actin as the loading control. The
analyses of cyclin D1 and bFGF were conducted in a similar manner,
with the exception of the primary and secondary antibodies. The
relative density of bFGF and cyclin D1 to that of β-actin was
analyzed with Quantity One software (Bio-Rad, Hercules, CA,
USA).
Statistical analysis
All data are presented as the mean ± standard error
of the mean. SPSS software, version 12.0 (SPSS, Inc., Chicago, IL,
USA) was used for the statistical analyses. χ2 test was
performed for inter-group comparisons, and analyzed with one-way
analysis of variance. Pearson correlation analysis was used to
analyze the correlations between the percentage wall thickness
(%WT), and bFGF and cyclin D1 mRNA and protein in the pulmonary
artery smooth muscle cells. P<0.05 was considered to indicate a
statistically significant result in all statistical analyses.
Results
Partial pressure of oxygen in arterial
blood (PaO2)
Table I summarizes
the PaO2, %WT and the muscularization of the pulmonary
arteries. No statistical difference was noted in the
PaO2 among the four groups (P>0.05).
 | Table IPaO2, %WT and the
muscularization of the pulmonary arteries. |
Table I
PaO2, %WT and the
muscularization of the pulmonary arteries.
| Group | PaO2
(mmHg) | %WT | Muscularization
(%) |
|---|
| Control | 91±4.5 | 20.5±1.8 | 4.1±1.2 |
| Smoke-exposed I | 92±4.6 | 31.6±1.4a | 6.2±1.8 |
| Smoke-exposed II | 91±3.7 | 39.3±2.1a,b | 17.5±2.6a,b |
| Smoke-exposed
III | 93±4.1 | 50.7±1.5a–c | 28.3±4.5a–c |
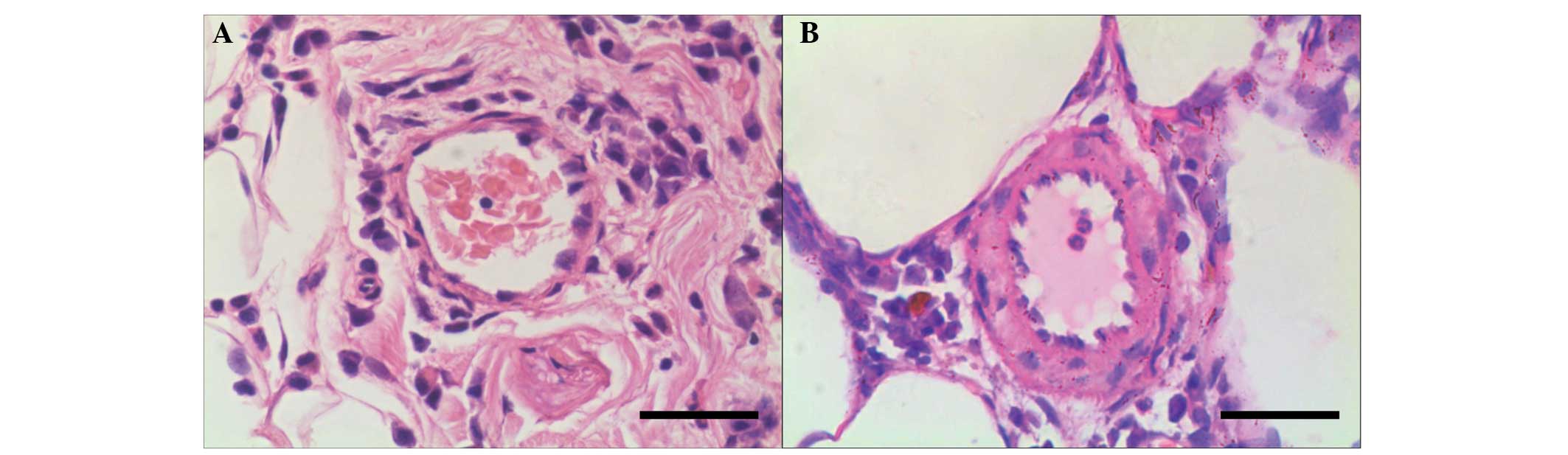
H&E staining and %WT
No abnormality was noted in the anatomical structure
of the lung in the control group (Fig.
1A). Gradual thickening of the pulmonary vascular wall was
noted. No significant signs of emphysema, such as anatomical
disorder of the alveolus and dilatation of alveolar space, were
observed. Vessel wall thickness exhibited a significant increase in
the smoke exposure group as compared with the control group
(Fig. 1B). Compared with the
control group, the %WT showed significant increases in the three
smoking exposure groups. Statistically significant differences were
noted in the %WT subsequent to the animals being exposed to smoke
for two, four and eight weeks, respectively (Table I).
Pulmonary arteriole muscularization
With regard to the muscularization of the pulmonary
arteriole, notable increases were identified in the smoke exposure
groups compared with that of the control group (P<0.05).
Enhanced pulmonary arteriole muscularization was noted with the
increase of the exposure duration (Table I).
Immunohistochemistry, qPCR and western
blotting
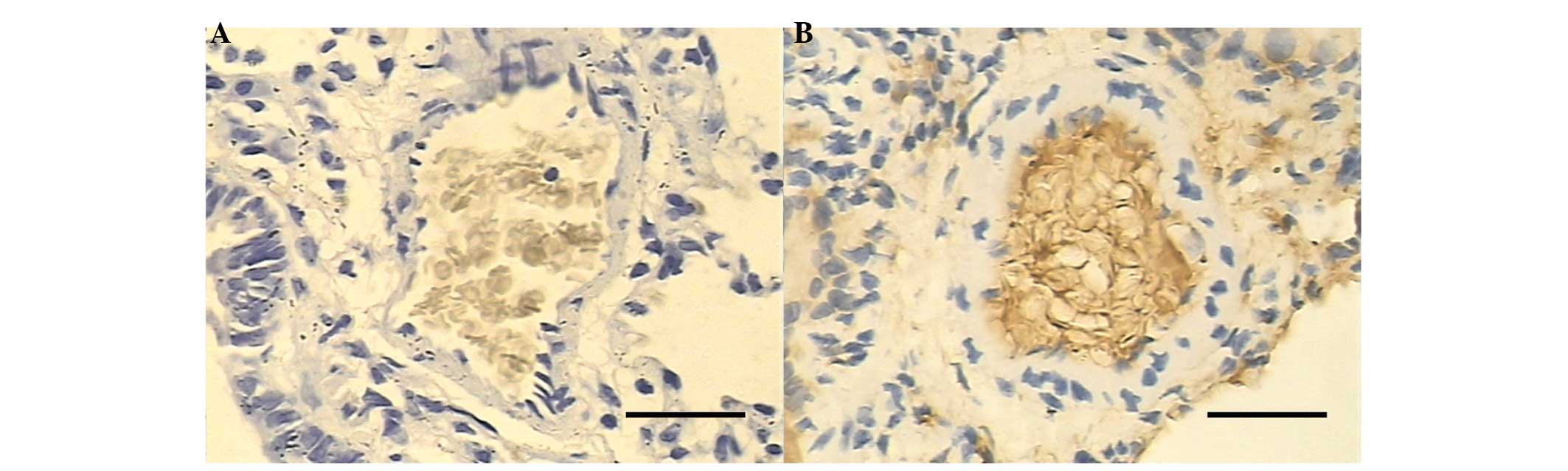
No positive staining of bFGF was identified in the
pulmonary arterial smooth muscle cells in the control group
(Fig. 2A). Low staining of bFGF
was noted in the pulmonary arterial smooth muscle cells of the
smoke-exposed group I. Enhanced staining of bFGF was noted in the
smoke-exposed groups II and III compared with that of the
smoke-exposed group I (Fig. 2B).
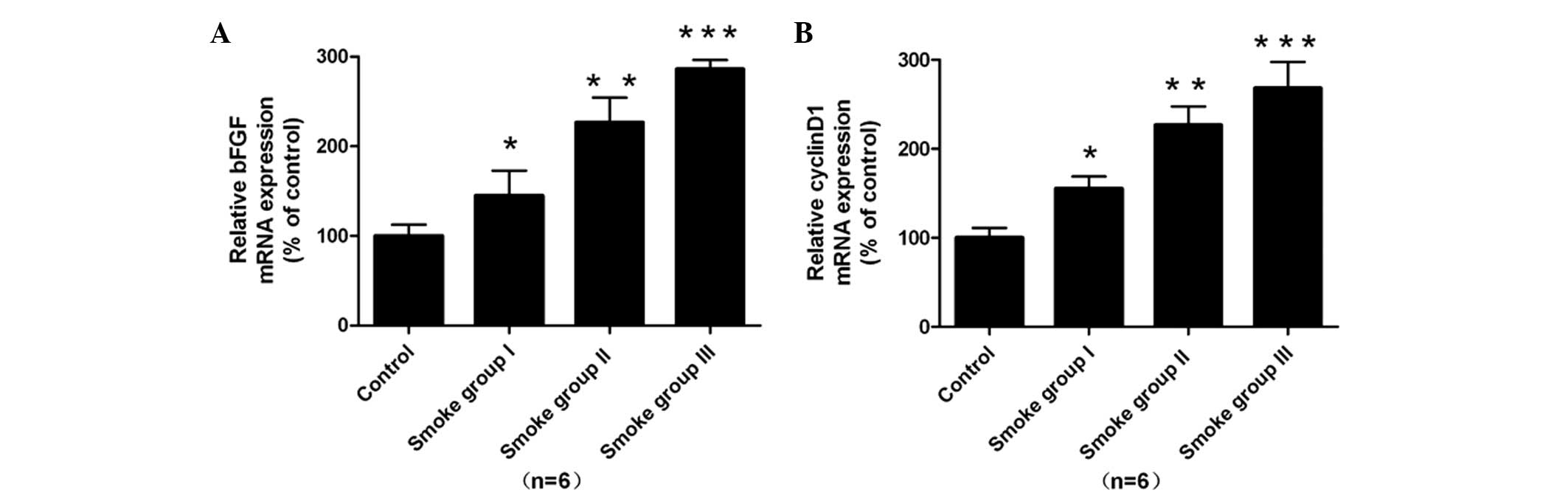
With regard to the expression of mRNA, upregulation of bFGF mRNA
was noted in the smooth muscle cells in the smoke-exposed groups
compared with that of the normal control (P<0.05, Fig. 3A).
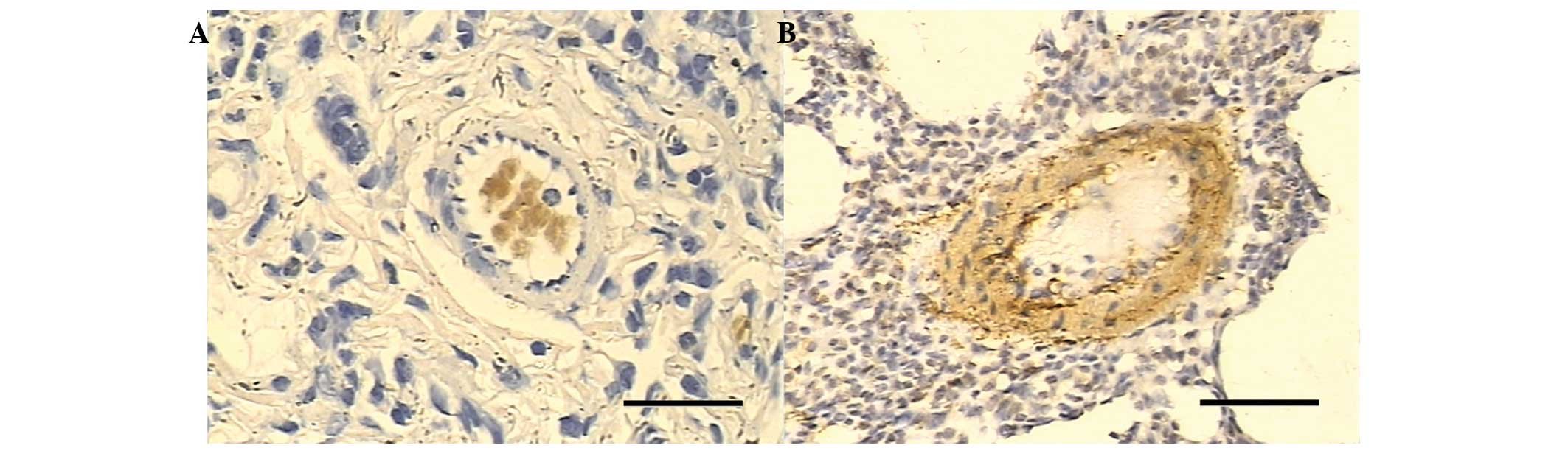
With regard to the positive immunohistochemical
staining of cyclin D1, no staining was noted in the control group
(Fig. 4A), while the smoke-exposed
groups I and II showed moderate increases compared with that of the
control group. Enhanced staining of cyclin D1 was observed in the
smoke-exposed group III (Fig. 4B).
Furthermore, enhanced expression of cyclin D1 mRNA was observed in
the smoke-exposed groups compared with that in the normal control
(P<0.05, Fig. 3B). With regard
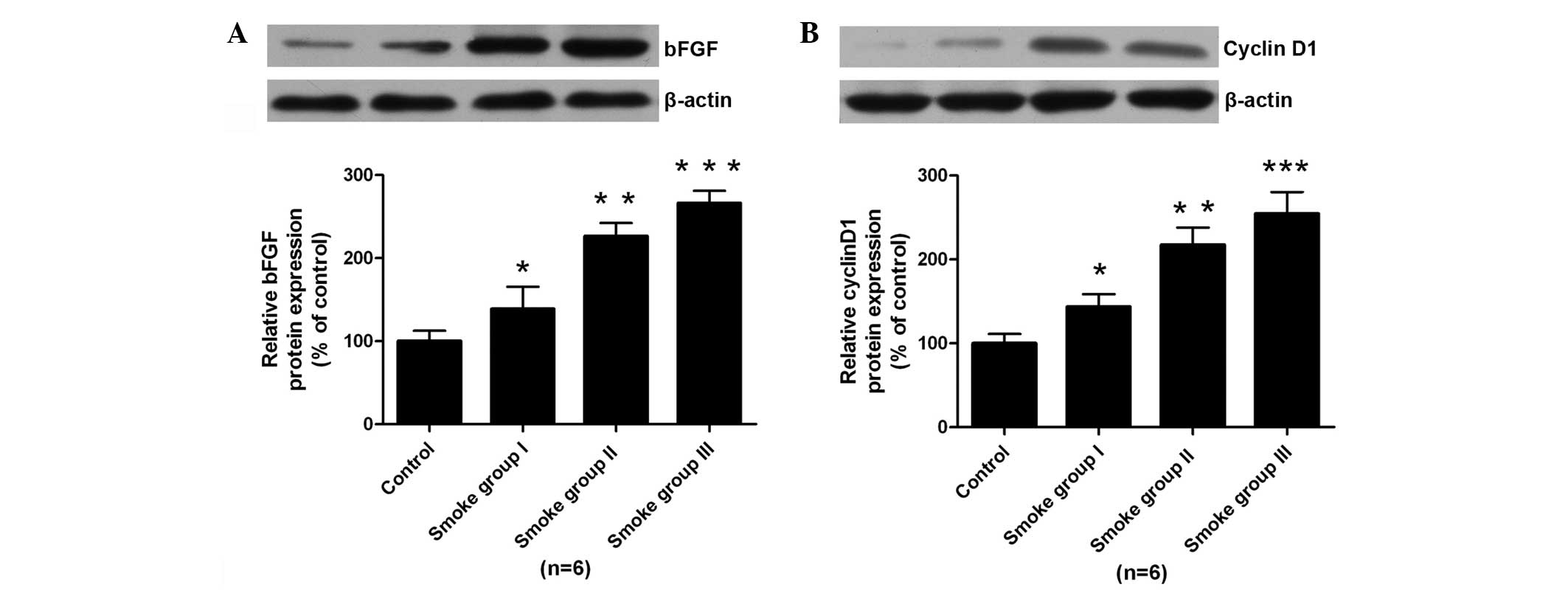
to the protein expression levels of bFGF and cyclin D1 determined
by western blotting, upregulation of bFGF and cyclin D1 was noted
in the smoke-exposed groups compared with that of control group
(P<0.05, Fig. 5).
Correlation analysis
Pearson’s correlation analysis indicated that the
%WT was positively associated with the expression of bFGF mRNA
(r=0.907, P<0.01) and bFGF protein (r=0.912, P<0.01) in the
pulmonary arterial smooth muscle cells. In addition, the %WT was
positively with the expression of cyclin D1 mRNA (r=0.918,
P<0.01) and cyclin D1 protein (r=0.906, P<0.01). Furthermore,
the mRNA expression of bFGF and cyclin D1 was positively associated
with that of bFGF protein (r=0.914, P<0.01) and cyclin D1
protein (r=0.922, P<0.01), respectively.
Discussion
Pulmonary hypertension denotes a disorder of the
pulmonary arterioles that is characterized by elevated pulmonary
artery pressure, right-heart failure, persistent vasoconstriction,
thickening of the pulmonary vascular wall, vascular remodeling and
a progressive increase of pulmonary vascular resistance (12,13).
Generally, the pathological foundation of pulmonary hypertension
includes muscularization of the pulmonary arteries induced by the
overproliferation and migration of pulmonary smooth muscle cells,
intima-media thickening of the arteriole and neointimal formation.
Smoking has been considered as a great threat to public health as
it is a risk factor for a variety of diseases. Cigarettes contain
>4,000 chemical compounds, and ≥400 toxic substances including
nicotine, tar, acrolein, carbon monoxide, hydrogen cyanide,
ammonia, aromatic compounds, arsenic, mercury and chromium. The
pulmonary vascular structure in smokers has been extensively
investigated, and has shown that cigarette smoke is able to induce
the remodeling of pulmonary vessels (14). In the present study, the percentage
of pulmonary arteriole muscularization showed a marked increase
following exposure to cigarette smoke for four and eight weeks
compared with that in the control group, which demonstrated that
pulmonary vascular remodeling was induced following exposure to
cigarette smoke. As no reduction in PaO2 was observed,
it may be speculated that cigarette smoke was involved in the
pulmonary vascular remodeling through direct interaction with the
pulmonary vasculature. The present study was consistent with a
previous study (15).
Previous studies have indicated that the
upregulation of the gene expression and protein production of
vascular cell proliferative agents, endothelin and vascular
endothelial growth factor is associated with the pulmonary vascular
remodeling induced by cigarette smoke (16,17).
bFGF is a potent regulator of various cellular functions, including
mitosis, cell proliferation, differentiation, survival, cellular
adhesion, migration, motility and apoptosis, vasculogenesis and
blood vessel remodeling (18–20).
In the present study, the expression of bFGF was identified in the
pulmonary arteries following exposure to cigarette smoke. Also, the
upregulation of bFGF mRNA and protein was noted. Muscularization of
the pulmonary artery and %WT were found to be positively correlated
with the expression of bFGF mRNA and protein. These results
indicate that bFGF plays a crucial role in the pulmonary arterial
remodeling induced by cigarette smoke, which is consistent with the
findings of previous studies (21,22).
The present results showed that upregulation of
cyclin D1 mRNA and protein occurred in the smooth muscle cells of
the pulmonary artery in rats exposed to cigarette smoke.
Additionally, the expression of cyclin D1 mRNA and protein was
found to be positively correlated with the muscularization of the
pulmonary artery and %WT. In addition, the expression levels of
cyclin D1 mRNA and protein were positively correlated with those of
bFGF. Based on these results, it may be speculated that the smoke
exposure induced the upregulation of bFGF, which resulted in the
upregulation of cyclin D1 subsequently. Following this,
proliferation of the smooth muscle cells in the pulmonary artery
was triggered due to the upregulation of cyclin D1, resulting in
pulmonary vascular remodeling.
In conclusion, the present study demonstrates that
the upregulation of bFGF and cyclin D1 occurs in the smooth muscle
cells of the pulmonary arteries following exposure to cigarette
smoke and is associated with the duration of cigarette smoke
exposure. The expression levels of bFGF mRNA and protein were found
to be positively correlated with those of cyclin D1, which
indicates that these two proteins play a crucial role in the
remodeling of the pulmonary artery. The present study may provide
helpful information for the identification of treatment targets for
pulmonary hypertension. Further studies are required to investigate
the potential roles of bFGF and cyclin D1 in the process of
pulmonary arterial remodeling.
Acknowledgements
This study was supported by funds from the Natural
Science Foundation of China (nos. 81300041 and 81100038), the
Natural Science Foundation of the Anhui Higher Education
Institutions of China (no. KJ2012Z184), the academic backbone of
the excellent young and middle-age people of Anhui Medical
University (2013) and the First Affiliated Hospital of Anhui
Medical University for Fostering Talents of the National Natural
Science Foundation of China (no. 2011KJ05).
References
|
1
|
Humbert M, Sitbon O and Simonneau G:
Treatment of pulmonary arterial hypertension. N Engl J Med.
351:1425–1436. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tuder RM: Pathology of pulmonary arterial
hypertension. Semin Respir Crit Care Med. 30:376–385. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wright JL, Farmer SG and Churg A: A
neutrophil elastase inhibitor reduces cigarette smoke-induced
remodelling of lung vessels. Eur Respir J. 22:77–81. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seimetz M, Parajuli N, Pichl A, et al:
Inducible NOS inhibition reverses tobacco-smoke-induced emphysema
and pulmonary hypertension in mice. Cell. 147:293–305. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ribatti D, Vacca A, Rusnati M and Presta
M: The discovery of basic fibroblast growth factor/fibroblast
growth factor-2 and its role in haematological malignancies.
Cytokine Growth Factor Rev. 18:327–334. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang JH, Han KY and Azar DT: Wound
healing fibroblasts modulate corneal angiogenic privilege:
interplay of basic fibroblast growth factor and matrix
metalloproteinases in corneal angiogenesis. Jpn J Ophthalmol.
54:199–205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Handayani R, Rice L, Cui YH, et al: Soy
isoflavones alter expression of genes associated with cancer
progression, including interleukin-8, in androgen-independent PC-3
human prostate cancer cells. J Nutr. 136:75–82. 2006.
|
|
8
|
Liu Y and Templeton DM: Iron-loaded
cardiac myocytes stimulate cardiac myofibroblast DNA synthesis. Mol
Cell Biochem. 281:77–85. 2006. View Article : Google Scholar
|
|
9
|
Wang R, Xu YJ, Liu XS, Zeng DX and Xiang
M: Knockdown of connective tissue growth factor by plasmid-based
short hairpin RNA prevented pulmonary vascular remodeling in
cigarette smoke-exposed rats. Arch Biochem Biophys. 508:93–100.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carraway MS, Ghio AJ, Suliman HB, Carter
JD, Whorton AR and Piantadosi CA: Carbon monoxide promotes hypoxic
pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol.
282:L693–L702. 2002.PubMed/NCBI
|
|
11
|
Quinlan TR, Li D, Laubach VE, Shesely EG,
Zhou N and Johns RA: eNOS-deficient mice show reduced pulmonary
vascular proliferation and remodeling to chronic hypoxia. Am J
Physiol Lung Cell Mol Physiol. 279:L641–L650. 2000.PubMed/NCBI
|
|
12
|
Mandegar M, Fung YCB, Huang W, Remillard
CV, Rubin LJ and Yuan JXJ: Cellular and molecular mechanisms of
pulmonary vascular remodeling: role in the development of pulmonary
hypertension. Microvasc Res. 68:75–103. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pidgeon GP, Tamosiuniene R, Chen G, et al:
Intravascular thrombosis after hypoxia-induced pulmonary
hypertension - Regulation by cyclooxygenase-2. Circulation.
110:2701–2707. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wright JL, Levy RD and Churg A: Pulmonary
hypertension in chronic obstructive pulmonary disease: current
theories of pathogenesis and their implications for treatment.
Thorax. 60:605–609. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamato H, Sun JP, Churg A and Wright JL:
Guinea pig pulmonary hypertension caused by cigarette smoke cannot
be explained by capillary bed destruction. J Appl Physiol.
82:1644–1653. 1997.PubMed/NCBI
|
|
16
|
Wright JL, Tai H and Churg A: Vasoactive
mediators and pulmonary hypertension after cigarette smoke exposure
in the guinea pig. J Appl Physiol. 100:672–678. 2006. View Article : Google Scholar
|
|
17
|
Wang R, Xu YJ, Liu XS, Zeng DX and Xiang
M: CCN2 promotes cigarette smoke-induced proliferation of rat
pulmonary artery smooth muscle cells through upregulating cyclin D1
expression. J Cell Biochem. 113:349–359. 2012. View Article : Google Scholar
|
|
18
|
Friehs I, Moran AM, Stamm C, et al:
Promoting angiogenesis protects severely hypertrophied hearts from
ischemic injury. Ann Thorac Surg. 77:2004–2011. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Detillieux KA, Sheikh F, Kardami E and
Cattini PA: Biological activities of fibroblast growth factor-2 in
the adult myocardium. Cardiovasc Res. 57:8–19. 2003. View Article : Google Scholar
|
|
20
|
Garbern JC, Minami E, Stayton PS and Murry
CE: Delivery of basic fibroblast growth factor with a
pH-responsive, injectable hydrogel to improve angiogenesis in
infarcted myocardium. Biomaterials. 32:2407–2416. 2011. View Article : Google Scholar :
|
|
21
|
Benisty JI, McLaughlin VV, Landzberg MJ,
et al: Elevated basic fibroblast growth factor levels in patients
with pulmonary arterial hypertension. Chest. 126:1255–1261. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arcot SS, Fagerland JA, Lipke DW,
Gillespie MN and Olson JW: Basic fibroblast growth factor
alterations during development of monocrotaline-induced pulmonary
hypertension in rats. Growth Factors. 12:121–130. 1995. View Article : Google Scholar : PubMed/NCBI
|