Introduction
Urothelial cancer of the bladder is the fourth most
common malignancy diagnosed in the USA. It is estimated that
~74,690 patients were diagnosed in 2014 (1,2).
Among the cases of urothelial cancer of the bladder, ~75% are
superficial bladder cancer with low-grade, noninvasive or
superficial tumors confined to the mucosa. Patients with
superficial bladder cancer are at high risk of relapse following
surgery with concomitant radiotherapy and chemotherapy (3,4). The
goal of treatment for these types of cancer is reducing tumor
recurrence and preventing tumor progression, which would require
additional aggressive therapies. Intravesical immunotherapy with
Bacillus Calmette-Guérin (BCG) is an effective adjuvant therapy for
superficial bladder cancer (5).
The anticancer effect of BCG in the treatment of bladder cancer
involves a complex local immune response, including the activation
of B, T and natural killer cells induced by multiple cytokines, for
example interleukin (IL)-1, -6 and -8 and granulocyte-macrophage
colony-stimulating factor (6,7).
Although a clinical evaluation of the effectiveness
of BCG indicates that it can induce robust immune responses against
tumor antigens in patients with bladder cancer, the clinical
benefits of BCG have been limited. A previous study identified
cells of myeloid origin that are potent suppressors of tumor
immunity and therefore represent a significant obstacle against
tumor immunotherapy (8).
Myeloid-derived suppressor cells (MDSCs) have been shown to
accumulate at tumor sites as well as in the blood, lymph nodes and
bone marrow in the majority of patients and experimental animals
with cancer, and inhibit both adaptive and innate immunity
(9,10). In mice, MDSCs are uniformly
characterized by the expression of cell surface molecules detected
by antibodies against Gr1 and cluster of differentiation (CD)11b.
MDSCs act to suppress antitumor immunity through a number of
diverse mechanisms. T-cell activation is suppressed by the
production of reactive oxygen species and arginase, the nitration
of the T-cell receptor and the induction of regulatory T cells
(Tregs). Innate immunity is impaired by the increase in the
production of IL-10 by MDSCs, the downregulation of
macrophage-produced IL-12 and the inhibition of natural killer cell
cytotoxicity (11). In the present
study, the antitumor immune suppressive activity of intravesically
administered BCG in an immunocompetent mouse model was
investigated. Ideally, new therapeutic strategies should be tested
rigorously in a relevant animal model.
Materials and methods
Animals
A total of 21 eight-week-old female C3H/HeN mice
were obtained from Guangdong Provincial Research Center for
Laboratory Animal Medicine (Foshan, China). The mice were
maintained at the Animal Center of Southern Medical University
(Guangzhou, China) in a specific pathogen-free environment with
food and water provided ad libitum. The animals were housed
and handled in accordance with the Southern Medical University
Animal Research Committee Guidelines.
Cell line and reagents
The murine bladder cancer cell line MBT-2 was
provided by the American Type Culture Collection (Rockville, MD,
USA). The MBT-2 cells were maintained in RPMI-1640 medium
supplemented with 10% fetal bovine serum. The cells were cultured
at 37°C in a 5% CO2 atmosphere and routinely passaged by
trypsin-EDTA treatment in 100-cm2 flasks containing BCG
(81 mg; Connaught substrain, ImmuCyst, Nihou Kayaku, Inc., Tokyo,
Japan), and phosphate-buffered saline (PBS) for in vivo
studies.
In vivo effects in the murine bladder
cancer models
To establish the orthotopic bladder cancer tumors,
the mice were anesthetized by the intraperitoneal (i.p.)
administration of ketamine/xylazine solution at dose of 0.1 ml/10 g
body weight (K113; Sigma-Aldrich Japan G.K., Tokyo, Japan). A
24-gauge Teflon intravenous catheter was subsequently inserted
through the urethra into the bladder using an inert lubricant. In
order to prepare the bladder for tumor implantation, a brief acid
exposure, followed by alkaline neutralization, promoted a chemical
lesion on the bladder wall, performed by the intravesical
instillation of 8 μl 1 MOI silver nitrate. This led to the
formation of an adequate and controlled diffuse bladder wall
lesion. After 15 sec, the content was washed out by transurethral
infusion of PBS. The first catheter was removed and a new 24-gauge
catheter was inserted in the urethra for intravesical instillation
of MBT-2 cells.
MBT-2 cells stably expressing luciferase (MBT-2-Luc;
luciferase L4899 obtained from Sigma-Aldrich Japan G.K.) were
generated by transfecting MBT-2 cells with the pGL3-Luc plasmid
using a TransIT®-3T3 transfection kit (Mirus Bio LLC,
Madison, WI, USA). Cells that stably expressed luciferase were
obtained by selection with 500 μg/m1 of G418 for two weeks.
Following G418 selection, growth medium from MBT-2-Luc was tested
for luciferase activity to confirm the expression and secretion of
luciferase into the cell medium. Luciferase-transfected MBT-2 cells
(5×104 cells mixed with 0.1 ml PBS) were instilled and
retained for 1.5 h by stitches. Every 10 days, tumor imaging was
performed following i.p. administration of luciferin using
bioluminescence technology (Xenogen IVIS200 system; Xenogen
Coproration, Hopkinton, MA, USA). Prior to the initiation of the
treatment, the mice were randomly divided into three groups [seven
mice per group; control (PBS), low-dose BCG and high-dose BCG]
according to the tumor imaging results, as determined by the
luciferase expression. BCG (1×105 CFU/100 μl or
1×107 CFU/100 μl) was administered intravesically once
weekly for three weeks. The mice received i.p. injections of
luciferin, and the luciferase expression in the tumors was measured
by the Xenogen IVIS200 System. The body weights of the mice were
measured once a week. All mice were sacrificed on day 60 after each
treatment, and one mouse was selected from each group for a
histological analysis. The mice were killed using CO2
for euthanasia and according to the guidelines for the euthanasia
of animals (Edition, 2013).
Flow cytometry
For the flow cytometry, 100-μl aliquots of blood
collected from the mouse bladder cancer models were mixed with 4 μl
2 mmol/l EDTA and incubated with fluorescein isothiocyanate-labeled
anti-mouse CD11b and CD4 monoclonal antibodies (mAbs) and
phycoerythrin-labeled anti-mouse Gr-1 and Forkhead box P3 (Foxp3)
mAbs for 1 h at 4°C, prior to washing twice with PBS. The cells
were resuspended in 250 μl PBS and analyzed with a BD FACSCalibur™
flow cytometer (BD Biosciences, Bedford, MA, USA), using a
lymphocyte gating strategy.
Histology
One mouse from each group was sacrificed for
histological analysis on day 60 after each treatment. The tissues
were removed, fixed in formalin, embedded in paraffin and
sectioned. The 5-μm sections were stained with hematoxylin and
eosin and examined for histological changes using an Olympus IX71
microscope (Olympus, Tokyo, Japan).
Statistical analysis
The data are presented as the mean ± standard error
of the mean. An unpaired Student’s t-test was performed to analyze
the difference between any two groups. Differences were considered
to be significant if P<0.05.
Results
Antitumor effect of low-/high-dose BCG
treatment in the C3H/HeN mouse orthotopic bladder cancer model
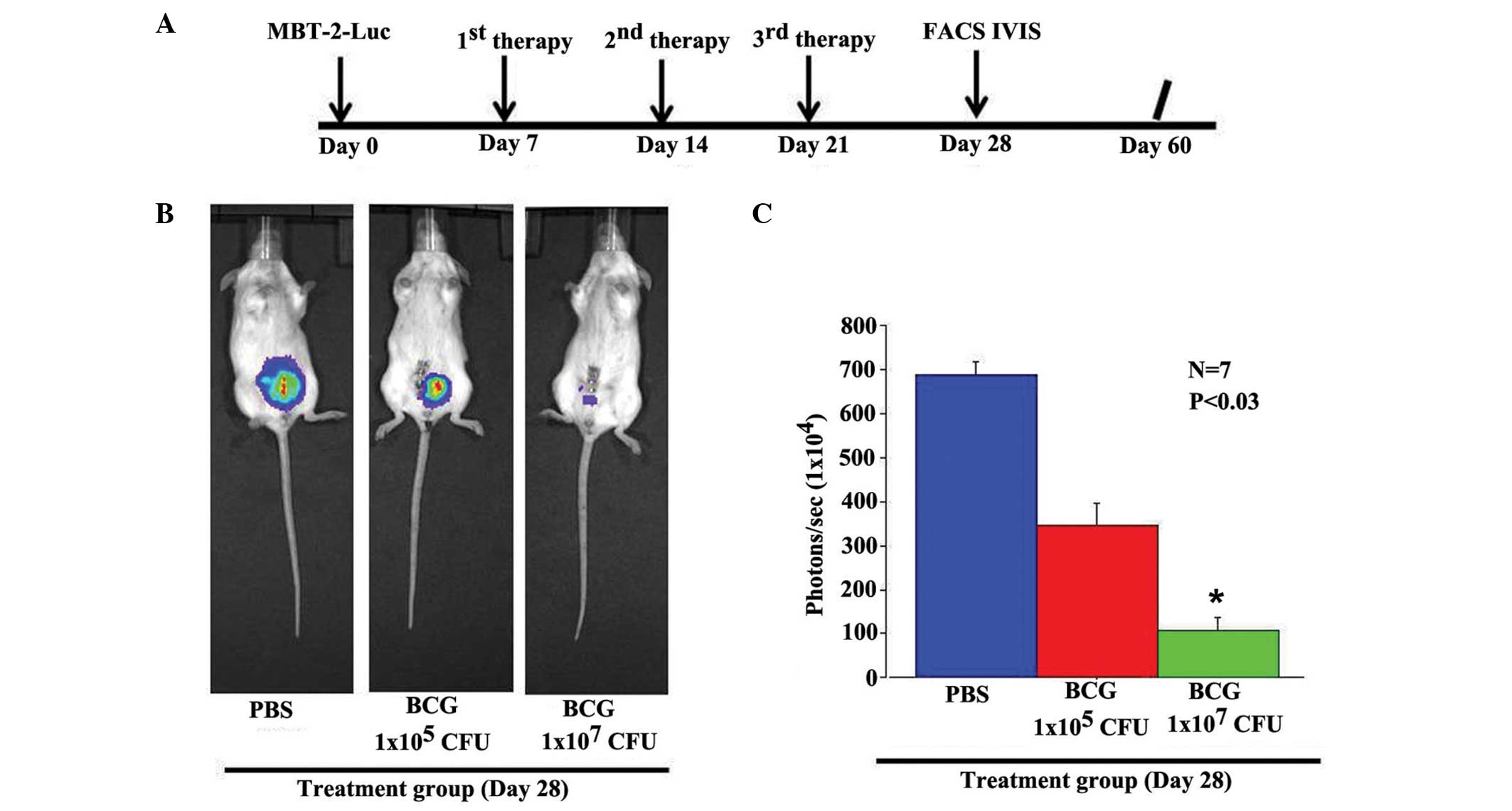
The therapeutic efficacy of BCG on the growth of
MBT-2 cells was assessed in vivo. Luciferase-expressing
MBT-2 cells were injected orthotopically in immunocompetent mice.
Mice bearing orthotopic bladder cancer were treated intravesically
on days 7, 14 and 21 post-cancer implantation with PBS, low-dose
BCG (1×105 CFU/100 μl) or high-dose BCG
(1×107 CFU/100 μl) (Fig.
1A). After 60 days of treatment, the PBS-treated mice did not
show a significant growth-inhibitory effect, as assessed by the
Xenogen IVIS200 System (Fig. 1B and
C). Low-dose BCG administration induced a 50% reduction of
tumor growth. However, the high-dose BCG induced a synergistic
inhibitory effect that was greater than that induced by the
administration of the low-dose BCG. The differences in the levels
of luciferase expression correlated with the tumor area.
BCG affects the activation of
CD11b+/Gr-1+ cells and Tregs in the
peripheral blood of murine models of MBT-2 tumors
The potential mechanisms underlying the antitumor
effect elicited by low-/high-dose BCG were next explored. In this
experiment, the peripheral blood samples obtained from mouse models
of MBT-2 tumors were assessed. Blood was collected prior to
initiating the treatment and at day 28 after the treatment, and the
CD11b+/Gr-1+ cells and
CD4+/Foxp3+ Tregs were quantified by
fluorescence-activated cell sorting (FACS) analysis. As shown in
Fig. 2A and B, the
CD11b+/Gr-1+ cells and
CD4+/Foxp3+ Tregs were observed in all the
mice prior to treatment. After four weeks of treatment, the
CD11b+/Gr-1+ cell population in the
low-dose-treatment group was higher than that in the mice treated
with the high-dose BCG. However, the largest
CD11b+/Gr-1+ cell population was observed in
the mice of the control group. The size of the population of
CD4+/Foxp3+ Tregs was also different between
the treatment and non-treatment groups. The largest
CD4+/Foxp3+ Treg population was observed in
the control group and the smallest appeared in the high-dose BCG
group (Fig. 2A and B). These
experiments were repeated three times, and the values shown in the
figure are the average of the three experiments. At day 60 after
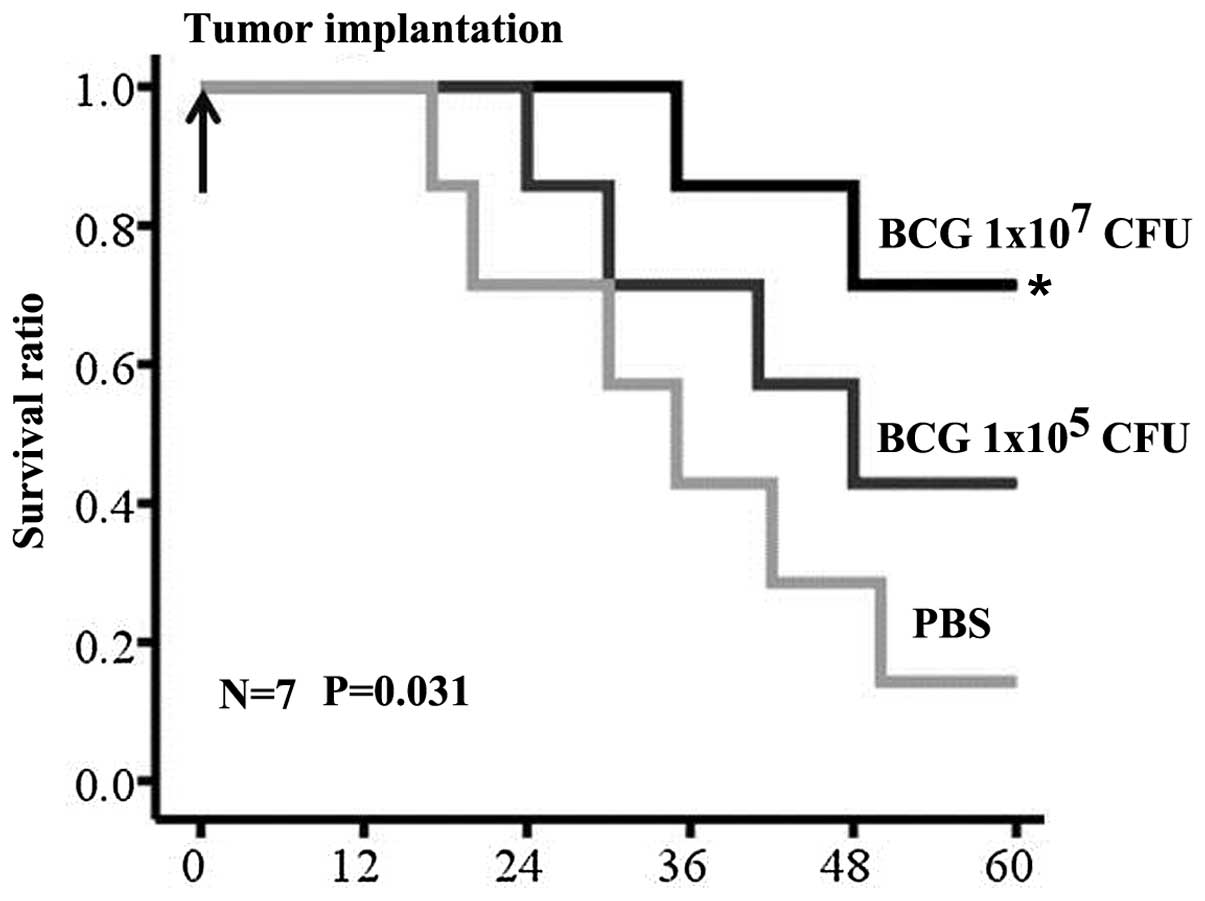
MBT-2 implantation, all the mice in the control group had died,
with the exception of one mouse. Two of the seven low-dose
BCG-treated mice survived, and five of the seven mice survived in
the high-dose BCG group. Significant differences in survival time
existed between the control and treatment groups (Fig. 3) (P<0.05).
Tumor-specific cytological changes
In order to investigate the mechanism and effect of
BCG on bladder tumors and other body tissues, the mice were
sacrificed on day 60 after treatment initiation. A
histopathological analysis revealed extensive tumor tissue
degeneration in the orthotopic bladder cancer of mice in the high-
and low-dose BCG groups (Fig. 4A and
B), but tumor tissue degeneration was not observed in the
control group. These findings provided evidence for the tumor
volume reduction or growth inhibition observed following each
treatment. However, an analysis of liver sections from the mice
treated with BCG demonstrated no histological evidence of
hepatocellular damage.
Discussion
It has been reported that ~80% of bladder cancers
are superficial at the time of diagnosis, and a high rate of local
recurrence and progression occurs following transurethral resection
of bladder tumor (TUR-Bt) (12).
The high rate of recurrence (70%) and the progression rate (45% for
grade three), as well as the unpredictability of progression
patterns, have led to the widespread use of intravesical adjuvant
therapy following TUR-Bt. Intravesical immunotherapy with BCG is an
effective adjuvant therapy for high-grade, non-muscle-invasive
bladder cancers (13,14).
In the present study, it was investigated whether
BCG treatment induced a change in immune cells in a bladder cancer
murine model. Our data demonstrated that the population of MDSCs
(CD11b+/Gr-1+) was decreased in murine models
of orthotopic bladder cancer receiving BCG treatment. A
dose-dependent antitumor and survival effect was shown in the
orthotopic models receiving BCG treatment. High-dose BCG treatment
exhibited significantly enhanced tumor inhibition and survival
benefits versus the control group. Low-dose BCG did not demonstrate
a significant difference in tumor inhibition and survival versus
the control group.
To examine the anticancer immunomodulation in each
mouse, the ratio of peripheral CD11b+/Gr-1+
MDSCs was measured by FACS analysis. The population of MDSCs was
significantly downregulated following the high-dose BCG therapy
compared with the low-dose therapy. Therefore, the in vivo
synergistic effect of high-dose BCG induced the downregulation of
peripheral CD11b+/Gr-1+ MDSCs that was
observed in the bladder cancer orthotopic model. This immunological
synergistic effect in the suppression of
CD11b+/Gr-1+ MDSCs may explain the robust
antitumor therapeutic effects of the high-dose BCG therapy.
Numerous studies have shown that MDSCs represent a
heterogeneous population of variably matured myeloid cells, which
mediate the suppression of antitumor immune responses (15,16).
In mice with tumors, CD11b+/Gr-1+ MDSCs can
accumulate during cancer progression and inhibit antitumor T-cell
responses. Two mechanisms are used by MDSCs to downregulate the
activation T cells: MDSC-mediated downregulation of L-selectin and
MDSC sequestration of cysteine, an amino acid that the T cells are
unable to synthesize de novo and that they require for
activation.
In recent years, a number of bases (17) and clinical trials (18,19)
have shown that BCG antigens can be presented at the cell surfaces
of urothelial and antigen-presenting cells in major
histocompatibility complex class II, thus stimulating
CD4+ T cells and inducing a primarily T-helper type 1
(Th1) immune response (6,20). Within the tumor microenvironment,
the immunosuppressive effects of Tregs may prevent the initiation
of antitumor immune responses by interferon-γ-producing
CD4+ Th1 and CD8+ T cells; however, these
effects may not be capable of overcoming the already established
cycle of IL-6- and signal transducer and activator of transcription
3-mediated inflammation (21). In
the present study, the change in CD4+/Foxp3+
Tregs also exhibited the same result: Following the BCG treatment,
the population of CD4+/Foxp3+ Tregs was
decreased in the blood. These findings may assist in the
clarification of the mechanism underlying the synergistic antitumor
immunological response elicited by BCG.
In conclusion, the present study in an orthotopic
mice bladder cancer model indicates that the antitumor and
tumor-immunology efficacy of BCG is dose-dependent. The activation
of MDSCs also suggests dose-dependence. These findings are notable
in terms of the clinical evaluation of this therapy for patients
with bladder cancer. The outcomes of this study also provide
important implications regarding antitumor immune responses in
human cancer.
Acknowledgements
This study was supported by the Pearl River Nova
Program of Guangzhou (no. 2013J2200044) and the National Natural
Science Foundation of China (no. 81101559).
References
|
1
|
DeSantis CE, Lin CC, Mariotto AB, et al:
Cancer treatment and survivorship statistics, 2014. CA Cancer J
Clin. 64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Horinaga M, Fukuyama R, Iida M, et al:
Enhanced antitumor effect of coincident intravesical gemcitabine
plus BCG therapy in an orthotopic bladder cancer model. Urology.
76:1267e1–e6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burke J: Virustherapy for bladder cancer.
Cytokine Growth Factor Rev. 21:99–102. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jinesh GG and Kamat AM: Redirecting
neutrophils against bladder cancer cells by BCG and Smac mimetic
combination. Oncoimmunology. 1:1161–1162. 2012. View Article : Google Scholar
|
|
6
|
Askeland EJ, Newton MR, O’Donnell MA, et
al: Bladder cancer immunotherapy: BCG and beyond. Adv Urol.
2012:1819872012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Razack AH: Bacillus Calmette-Guerin and
bladder cancer. Asian J Surg. 30:302–309. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ring S, Karakhanova S, Johnson T, et al:
Gap junctions between regulatory T cells and dendritic cells
prevent sensitization of CD8(+) T cells. J Allergy Clin Immunol.
125:237–246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gabrilovich DI and Nagaraj S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Palucka K and Banchereau J: Cancer
immunotherapy via dendritic cells. Nat Rev Cancer. 12:265–277.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gabrilovich DI and Nagaraj S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ro JY, Staerkel GA and Ayala AG: Cytologic
and histologic features of superficial bladder cancer. Urol Clin
North Am. 19:435–453. 1992.PubMed/NCBI
|
|
13
|
Ping SY, Wu CL and Yu DS: Sunitinib can
enhance BCG mediated cytotoxicity to transitional cell carcinoma
through apoptosis pathway. Urol Oncol. 30:652–659. 2012. View Article : Google Scholar
|
|
14
|
Chan ES, Patel AR, Smith AK, et al:
Optimizing orthotopic bladder tumor implantation in a syngeneic
mouse model. J Urol. 182:2926–2931. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sica A and Bronte V: Altered macrophage
differentiation and immune dysfunction in tumor development. J Clin
Invest. 117:1155–1166. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ostrand-Rosenberg S and Sinha P:
Myeloid-derived suppressor cells: linking inflammation and cancer.
J Immunol. 182:4499–4506. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gan C, Mostafid H, Khan MS and Lewis DJ:
BCG immunotherapy for bladder cancer - the effects of substrain
differences. Nat Rev Urol. 10:580–588. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Babjuk M, Burger M, Zigeuner R, et al: EAU
guidelines on non-muscle-invasive urothelial carcinoma of the
bladder: update 2013. Eur Urol. 64:639–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ehdaie B, Sylvester R and Herr HW:
Maintenance bacillus Calmette-Guérin treatment of
non-muscle-invasive bladder cancer: a critical evaluation of the
evidence. Eur Urol. 64:579–585. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zuiverloon TC, Nieuweboer AJ, Vékony H, et
al: Markers predicting response to bacillus Calmette-Guérin
immunotherapy in high-risk bladder cancer patients: a systematic
review. Eur Urol. 61:128–145. 2012. View Article : Google Scholar
|
|
21
|
Zamarron BF and Chen W: Dual roles of
immune cells and their factors in cancer development and
progression. Int J Biol Sci. 7:651–658. 2011. View Article : Google Scholar : PubMed/NCBI
|