Introduction
Colorectal cancer (CRC) is one of the most common
types of tumor and is the fourth leading cause of cancer mortality
worldwide (1). With economic
development and changes in lifestyle, the incidence and mortality
rates of CRC have rapidly increased in China. With the exception of
surgery, the majority of treatments for CRC, including traditional
chemo- and radiation therapies, are not fully efficacious in
treating the disease. The recent development of novel drugs
targeting CRC has improved the survival rate of patients with the
disease. Targeted drugs for the treatment of cancer have rapidly
developed. The human epidermal growth factor receptor 2 (HER-2)
signaling pathway plays an important role in tumor proliferation,
angiogenesis, differentiation and metastasis in CRC (2). HER-2-targeted drugs, including
Herceptin, have been developed and widely applied for the treatment
of breast cancer presenting with membranous HER-2 overexpression
(3,4).
The HER-2 oncogene is a member of the tyrosine
kinase family of receptors, which includes HER-1, also known as
epidermal growth factor receptor (EGFR), HER-2, HER-3 and HER-4.
HER-2 is located on chromosome 17q21 and encodes a 185 kDa
transmembrane protein. HER-2 activation initiates signal cascades,
including the mitogen-activated protein kinase and phosphoinositide
3-kinase/Akt signaling pathways, which are essential for cell
proliferation and differentiation. Thus, HER-2 overexpression leads
to the disordered proliferation and malignant transformation of
cells. HER-2 is overexpressed in numerous types of malignant
cancer, including breast, ovarian, gastric, lung, colorectal and
prostate cancers (5). Chen et
al (6) revealed that the
detection of HER-2 protein expression may be used to assess the
malignant biological behavior and prognosis of gastric cancer. The
European Committee has already approved chemotherapy combined with
Herceptin as a treatment for cases of gastric cancer presenting
with membranous HER-2 overexpression (7). Thus, ensuring that the expression
level of HER-2 in patients with gastric cancer is examined
accurately is of importance. However, conflicting data exist with
regard to the prevalence of HER-2 overexpression in CRC, with a
range between 2 and 47%, while the prevalence of HER-2 gene
amplification ranges between 2.5 and 7.4% (8–27).
Similarly, there is controversy in the published literature with
regard to the association between the survival rate and HER-2
overexpression in CRC (10–13,28).
Since there are few published studies investigating
HER-2 expression in CRC and genetic differences exist between
ethnic groups with regard to tumorigenesis, numerous topics require
further study. Thus, the present study investigated the frequency
of HER-2 overexpression and gene amplification in CRC, and whether
HER-2 overexpression and gene amplification were consistent. In
addition, associations between HER-2 overexpression with
clinicopathological parameters and the prognosis of CRC were
analyzed.
Materials and methods
Patients and tissue specimens
Clinicopathological data and paraffin-embedded
specimens were collected from 878 patients who underwent colorectal
resections at Dongfang Hospital (Fuzhou, China) between January
2006 and April 2012. The study was approved by the ethics committee
of Dongfang Hospital and written informed patient consent was
obtained from the patient or the patient’s family. Of the 878
patients, 541 were male and 337 were female, with ages ranging
between 17 and 85 years (median age, 51 years). A total of 396
tumors were located in the rectum, while 482 tumors were in the
colon. None of the patients had received preoperative neoadjuvant
chemotherapy or radiotherapy. A total of 100 paraneoplastic normal
tissue specimens of CRC were used as controls.
The conditions of the patients were assessed
according to the Tumor Node and Metastasis (TNM) Classification of
Malignant Tumors (29). TNM
classification revealed that 490 (55.8%) patients were at stages 0,
I or II, while 388 (44.2%) patients were at stages III or IV. One
tumor was classified as pTis, 148 tumors were pT1, 341 tumors were
pT2, 314 tumors were pT3 and 74 tumors were pT4. The World Health
Organization Classification of Tumors was used for histological
classification (30). A total of
761 (86.7%) tumors were classified as well and moderately
differentiated, while 117 (13.3%) were poorly differentiated. All
the specimens were routinely fixed in 10% formalin, embedded in
paraffin and verified pathologically prior to inclusion in the
present study. Follow-up was conducted at 6, 12, 18 and 24 months
following surgery and in one-year intervals thereafter. Patients
who succumbed within four weeks following radical surgery and those
who were >85-years-old were excluded from the current
analysis.
Immunohistochemical staining
HER-2 overexpression analysis was conducted on
4-μm-thick sections. Briefly, following deparaffinization and
rehydration, the tissue samples were incubated in a citrate buffer
solution at 90–95°C for 20 min. The slides were washed with
phosphate-buffered saline (PBS) for three times for 3 min.
Endogenous peroxidase activity was suppressed by a 10 min
incubation in methanol with 3% hydrogen peroxide. A primary
monoclonal rabbit antibody against the human HER-2 protein (Clone
SP3; Lab Vision Corporation, Fremont, CA, USA) was applied for 60
min at room temperature. Subsequently, a secondary goat anti-rabbit
antibody (Lab Vision Corporation) conjugated to horseradish
peroxidase was applied for 30 min at room temperature. The bound
antibody was visualized using a peroxidase chromogen substrate. The
sections were counterstained with hematoxylin and a coverslip was
applied. Paraffin slides of invasive breast carcinomas were used as
a positive control (these were obtained from the Department of
Pathology, Dongfang Hospital). For antibody-negative controls, the
primary antibodies were substituted with PBS.
Slides were examined separately by two independent
pathologists blinded to each others results. Discrepancies between
the investigators (<5% of cases) required a third joint
observation with a conclusive agreement. The HercepTest™
scoring system specific to gastric cancer was used to determine
tumor cell reactivity, as described by Hofmann et al
(31) in 2008. No reactivity or
membranous reactivity in <10% of the tumor cells was defined as
an immunohistochemistry (IHC) score of 0; faint/barely perceptible
partial membrane reactivity in >10% of the tumor cells was
defined as a score of 1+; weak to moderate complete or basolateral
membranous reactivity in >10% of the tumor cells was defined as
a score of 2+; strong complete or basolateral membranous reactivity
in >10% of the tumor cells was defined as a score of 3+. A score
of 0 or 1+ was considered negative, while a score of 2+ or 3+ was
considered positive. Cytoplasmic staining may have been present,
but was not included in the determination of positivity.
Fluorescence in situ hybridization
(FISH)
FISH analysis was applied to all IHC2+ and 3+
tumors, as well as to 20 randomly selected IHC0 and 1+ cases.
Paraffin slides of invasive breast cancers were used as a positive
control. FISH was conducted with a HER-2 DNA Probe kit (GP Medical
Technologies, Ltd., Beijing, China), according to the
manufacturer’s instructions. The commercially available
double-color FISH probe consisted of two probes: 17q11.2-q12
(labeled with a red signal) covering the whole HER-2 gene and the
control, centromeric chromosome 17p11.1-q11.1 (labeled with a green
signal).
The FISH-fixed glass slides with 4-μm-thick sections
were heated overnight at 65°C, deparaffinized in two 10-min changes
of xylene, rehydrated with two 3-min changes of 100% ethanol, one 3
min change of 85% ethanol and one 3 min change of 70% ethanol, and
immersed for 15 min in pure water at 90°C. The slides were
incubated (in a water bath) for 35 min in sodium sulfite acid at
50°C and washed in 2X sodium saline citrate (SSC; pH 7.2) at room
temperature. The slides were incubated for 12 min in proteinase K
solution at 37°C, washed in 2X SSC (pH 7.2) at room temperature,
dehydrated with 70, 85 and 100% ethanol and allowed to air-dry. To
denature the DNA, the slides were placed in 78.5°C preheated 70%
formamide/2X SSC for 8 min and dehydrated in a graded series of
ethanol concentrations that had been precooled to −20°C. Following
air-drying, 10 μl probe, which had been previously destructured at
75.5°C for 7 min, was applied onto each slide. A cover slip was
placed and sealed with rubber cement, and the slides were
hybridized at 42.8°C overnight. After 16–18 h hybridization, the
slides were washed in 46°C preheated post-hybridization buffer (2X
SSC/0.1% sodium dodecyl sulfate) for 5 min and rinsed in 70%
ethanol. Following air-drying (out of direct light), the slides
were counterstained with 10 μl
4′,6-diamidino-2-phenylindole/anti-fade solution and coverslips
were applied.
After 10 min, the slides were observed under a
fluorescence microscope (Olympus BX51; Olympus, Tokyo, Japan). A
total of 30 randomly selected tumor nuclei were evaluated in three
separate, distinct microscopic areas. Cases were classified as
negative/no amplification when the HER-2 gene (red signal) to
centromeric probe 17 (green signal) ratio was <1.8, while cases
with a ratio of >2.2 were classified as positive/amplification.
When the ratio was between 1.8 and 2.2, ≥100 randomly selected
tumor nuclei were evaluated. Furthermore, a cell was considered to
demonstrate amplification when a definite cluster or >10 red
signals for the HER-2 gene were identified, as described in a
previous FISH study (32). Cases
were defined as chromosome 17 polysomy when the green signal was
>2.25 in each nucleus when counting ≥30 tumor nuclei.
Statistical analysis
The χ2test was performed to analyze the
association between HER-2 overexpression and the
clinicopathological characteristics of CRC, and the correlation
between IHC and FISH. The probability of survival for the various
subgroups was calculated using the Kaplan-Meier method. All
statistical analyses were performed two-sided, where P<0.05 was
considered to indicate a statistically significant difference. SPSS
16.0 software (SPSS, Inc., Chicago, IL, USA) was used for
analysis.
Results
Overexpression of HER-2
A total of 102 cases (11.6%) out of the 878 patients
were demonstrated to have overexpressed HER-2 by IHC. Of these, 25
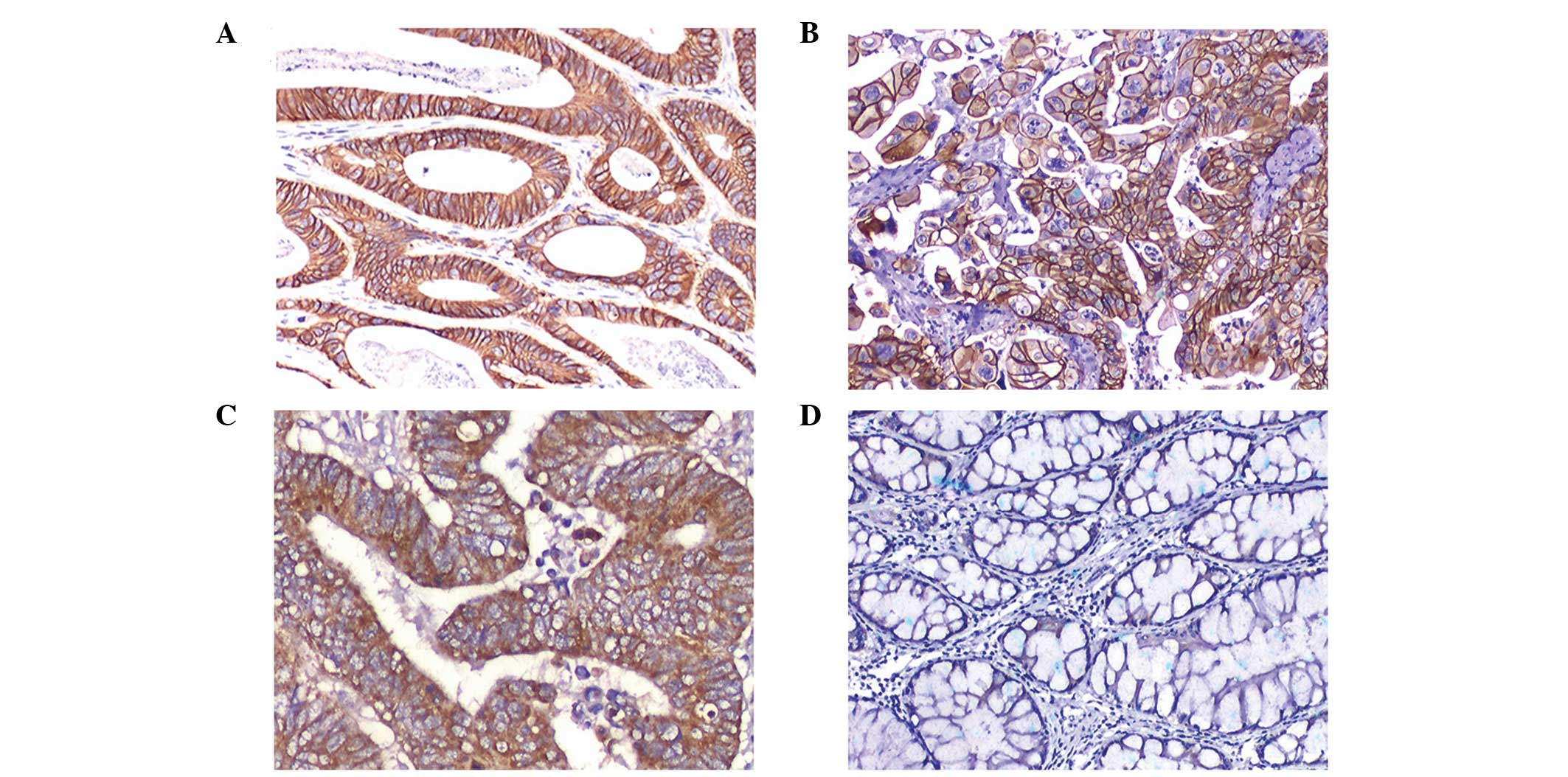
cases were strongly positive (3+; Figs. 1A and B) and 77 cases revealed
moderate staining (2+; Fig. 1C).
HER-2 overexpression was more frequent in 0, I and II stage cases
compared with stage III and IV cases (P<0.001). No association
was observed between HER-2 overexpression and gender, age, tumor
site, size, depth of invasion, lymph node metastases or distant
metastases (P>0.05; Table I).
Stromal and normal epithelial cells adjacent to the tumor tissue
were negative (Fig. 1D).
 | Table IAssociation between HER-2
overexpression (IHC3+ and 2+) and the clinicopathological
characteristics of CRC. |
Table I
Association between HER-2
overexpression (IHC3+ and 2+) and the clinicopathological
characteristics of CRC.
| Clinicopathological
characteristic | Cases, n | HER-2
overexpression, n (%) | P-value |
|---|
| Gender |
| Male | 541 | 64 (11.8) | 0.803 |
| Female | 337 | 38 (11.3) | |
| Age, years |
| <60 | 443 | 51 (11.5) | 0.922 |
| ≥60 | 435 | 51 (11.7) | |
| Tumor site |
| Colon | 482 | 50 (10.4) | 0.204 |
| Rectum | 396 | 52 (13.1) | |
| Tumor size, cm |
| <5 | 445 | 49 (11.0) | 0.570 |
| ≥5 | 433 | 53 (12.2) | |
| Depth of
invasion |
| Tis+T1 | 12 | 0 (0.0) | 0.514 |
| T2 | 174 | 20 (11.5) | |
| T3 | 648 | 79 (12.2) | |
| T4 | 44 | 3 (6.8) | |
| Classification |
| Well and
moderate | 761 | 94 (12.4) | 0.083 |
| Moderate and
poor | 117 | 8 (6.8) | |
| TNM stage |
| 0/I/II | 490 | 79 (16.1) | 0.000 |
| III/IV | 388 | 23 (5.9) | |
| Lymph node
metastases |
| N0 | 513 | 56 (10.9) | 0.611 |
| N1 | 229 | 27 (11.8) | |
| N2 | 136 | 19 (14.0) | |
| Distant
metastases |
| M0 | 804 | 92 (11.4) | 0.595 |
| M1 | 74 | 10 (13.5) | |
HER-2 gene amplification
Following FISH analysis, 24.5% (25/102) of the IHC3+
cases were shown to exhibit HER-2 gene amplification (Fig. 2A). By contrast, only 6.5% (5/77) of
IHC2+ cases (Fig. 2B), and none of
the randomly selected 20 cases with IHC0/1+, demonstrated HER-2
gene amplification (Fig. 2C). A
relatively high level of consistency was observed between IHC3+ and
IHC0/1+ with FISH (64 and 100%, respectively); however, there was a
low level of consistency with the results between IHC2+ and FISH
(6.5%; Table II).
 | Table IIConcordance analysis between HER-2
overexpression and amplification. |
Table II
Concordance analysis between HER-2
overexpression and amplification.
| | FISH, n (%) | |
|---|
| |
| |
|---|
| IHC status | Cases, n | Amplification | No
amplification | Concordance
(%) |
|---|
| IHC3+ | 25 | 16 (64.0) | 9 (36.0) | 64.0 |
| IHC2+ | 77 | 5 (6.5) | 72 (93.5) | 6.5 |
| IHC0/1+ | 20 | 0 (0.0) | 20 (100.0) | 0.0 |
| Positive
control | 10 | 10 (100.0) | 10 (0.0) | 100.0 |
Chromosome 17 polysomy and
non-polysomy
Chromosome 17 copy number analysis was applied to
all IHC2+ and 3+ cases. Two cases (8%) revealed chromosome 17
polysomy out of 25 IHC3+ cases, while only one case (1%) was
identified in the 77 IHC2+ cases. Among the 21 tumors with HER-2
gene amplification, only one case (5%) exhibited chromosome 17
polysomy, while two cases (2.5%) were observed in the 81 cases
without HER-2 gene amplification. In the FISH-positive cases, there
was one case (6.3%) of chromosome 17 polysomy in 16 IHC3+ cases and
no chromosome 17 polysomy observed in the five IHC2+ cases. With
regard to the FISH-negative cases, one case (11%) out of nine IHC3+
cases and one case (1%) out of the 72 IHC2+ cases had chromosome 17
polysomy (Table III).
 | Table IIIAssociation between chromosome 17
copy number and HER-2 overexpression/amplification. |
Table III
Association between chromosome 17
copy number and HER-2 overexpression/amplification.
| IHC/FISH
status | Cases, n | Chromosome 17 copy
number, n (%) |
|---|
|
|---|
| Polysomy | Non-polysomy |
|---|
| IHC3+ | 25 | 2 (8) | 23 (92) |
| IHC2+ | 77 | 1 (1) | 76 (99) |
| FISH+ | 21 | 1 (4.8) | 20 (95.2) |
| FISH- | 81 | 2 (2.5) | 79 (97.5) |
| IHC3+/FISH+ | 16 | 1 (6.3) | 15 (93.7) |
| IHC2+/FISH+ | 5 | 0 (0) | 0 (100) |
| IHC3+/FISH- | 9 | 1 (11) | 8 (89) |
| IHC2+ /FISH- | 72 | 1 (1) | 71 (99) |
Survival analysis
Follow-up was conducted on 349 cases, including 46
HER-2-positive (IHC3+ and 2+) and 303 HER-2-negative (IHC0 and 1+)
cases. Among the 349 cases, 202 were early-stage (0, I or II stage;
HER-2-positive, 25; HER-2-negative, 177) and 147 were advanced
stage cases (III or IV stage; HER-2-positive, 21; HER-2-negative,
126). The median follow-up duration was 28 months (range, 2–84
months) and 135 cases were followed for >3 years. The median
survival time was 84 months and the mean survival time was 60.9
months. The mean survival times of the HER-2-positive and -negative
groups were 64.9 and 59.5 months, respectively. The HER-2-positive
CRC patients exhibited higher three- and five-year survival rates
compared with HER-2-negative patients (77.7 vs. 68.8% and 77.7 vs.
61.4%, respectively); however, the difference was not statistically
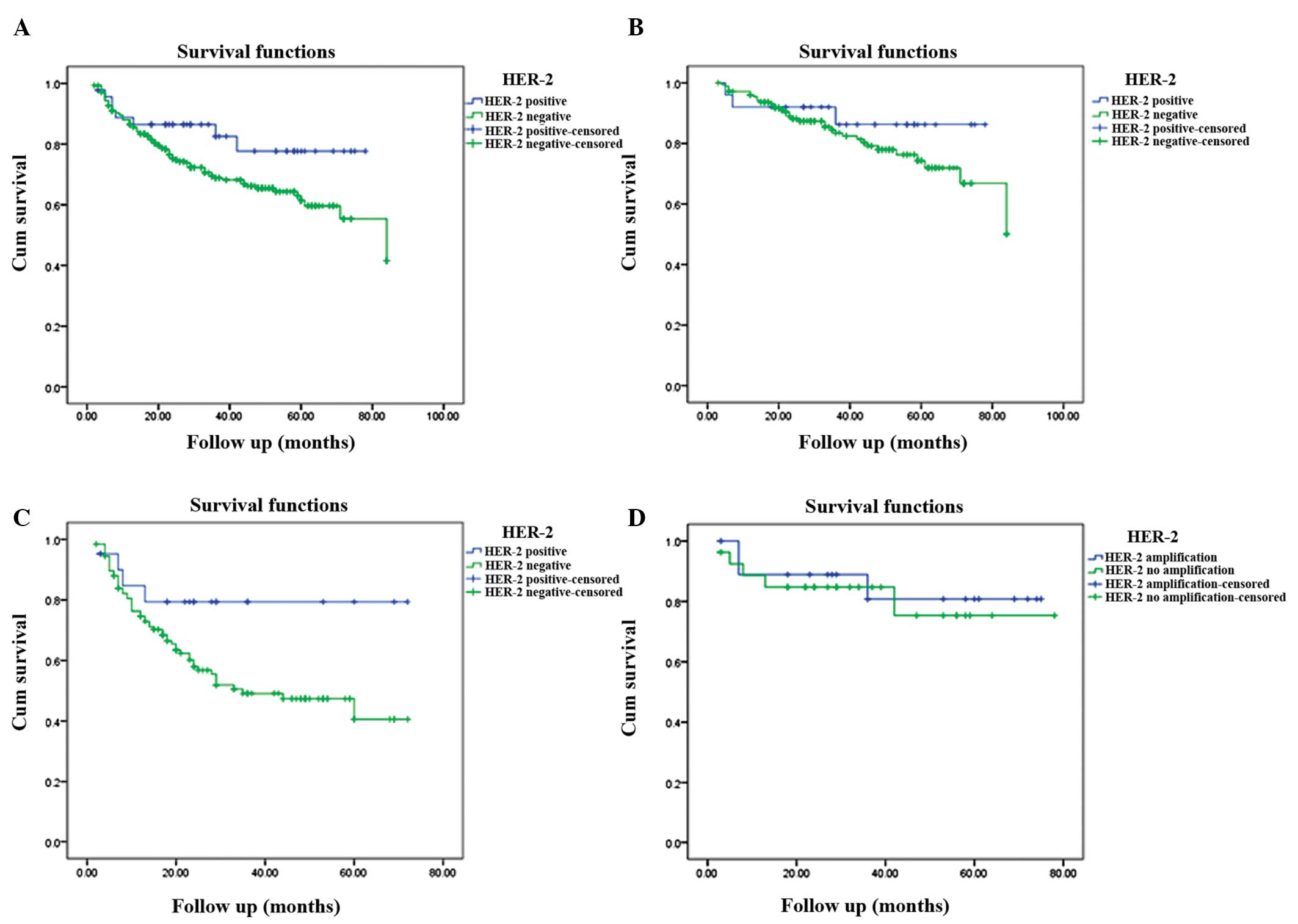
significant (P=0.082; Fig. 3).
HER-2-positive patients with early and advanced stage CRC revealed
higher survival rates compared with HER-2-negative cases at three
years (86.2 vs. 83.5% and 79.4 vs. 49.1%, respectively) and five
years (86.2 vs. 74.3% and 79.4 vs. 40.6%, respectively); however,
this difference was also not statistically significant (P=0.328 and
P=0.06, respectively; Fig 3). A
total of 20 HER-2 gene amplification and 26 gene non-amplification
cases were included in the 46 HER-2-positive cases. In the
HER-2-positive group, HER-2 gene amplification and
non-amplification exhibited a three-year survival rate of 80.8 vs.
84.7%, respectively, and five-year survival rate of 80.8 vs. 75.3%,
respectively (Fig 3); however,
this difference was not statistically significant (P=0.736). In
general, there was no association between HER-2 overexpression or
gene amplification and survival time.
Discussion
In the present study, HER-2 overexpression was
observed in 102 (11.6%) of the 878 Chinese CRC samples. Previous
studies have reported positive rates of HER-2 overexpression in CRC
ranging between 2 and 47.4%. The positive rates of HER-2
overexpression may have varied in these studies due to differences
in the IHC procedure, sample size and the scoring system employed.
Park et al (10) revealed
HER-2 overexpression in 47.4% of 137 patients with CRC, whereas
Antonacopoulou et al (16)
observed overexpression in 24.7% of 124 patients using IHC
performed on whole sections. Demirbas et al (11) demonstrated HER-2 overexpression in
9.6% of 104 patients with CRC using tissue microarray (TMA). The
results of these studies indicate that the expression of HER-2 in
CRC is associated with the prognosis and may constitute a potential
candidate for novel adjuvant therapies involving humanized
monoclonal antibodies, such as Herceptin. However, other studies
have demonstrated that the expression of HER-2 in CRC was not
associated with the prognosis, based on a subjunctive scoring
system of IHC. Kruszewski et al (20) reported HER-2 overexpression in 27%
of 202 CRC patients, while Kavanagh et al (12) observed overexpression in 11% of 132
patients using IHC performed on whole sections. Kim et al
(23) reported HER-2
overexpression in 0.5% of 185 patients with CRC, and Marx et
al (24) reported
overexpression in 2.7% of 1,851 patients using TMA. Furthermore, a
number of studies have demonstrated that HER-2 overexpression was
not associated with gender, age, histological tumor type, tumor
localization, grading, pT, pN, pM or survival (12,22,28).
Consequently, there are two hypotheses on the role
of HER-2 expression in CRC at present. Firstly, HER-2
overexpression may be an independent prognostic factor in CRC,
whilst secondly, expression of HER-2 in CRC is not associated with
prognosis. No associations between HER-2 overexpression and gender,
age, tumor site, size, depth of invasion, lymph node metastases or
distant metastases (P>0.05) were observed in the present study.
Furthermore, no statistically significant difference was observed
between HER-2 amplification and HER-2 non-amplification (P=0.736)
in the three- and five-year survival rates. Thus, the current data
were consistent with the latter hypothesis that HER-2
overexpression is not an independent prognostic factor of CRC.
However, the present study also revealed that HER-2 overexpression
was associated with the TNM stage. Early-stage cancers exhibited a
higher rate of HER-2 overexpression compared with advanced-stage
cancers (16.1 vs. 5.9%; P<0.001). However, this observation is
not consistent with those of previous studies where the HER-2
positivity rate of early-stage cancers was lower than that of
advanced-stage tumors (16,33),
or where the HER-2 positivity rate of cancers was shown not be
associated with the TNM stage (12,20,24,26,34).
A previous study demonstrated that Herceptin, an
anti-HER-2 monoclonal antibody, inhibits HCA-7 cell proliferation
in vitro and in vivo (35). As the first HER-2 dimerization
inhibitor, pertuzumab (a monoclonal antibody), also exhibits
antitumor activity on human colon cancer cells in vitro and
in vivo, in particular when combined with erlotinib
(36). A phase II trial revealed
that a low overexpression rate of HER-2 (8.0%) in advanced CRC
limits the application of Herceptin as a treatment for
advanced-stage CRC; however, partial responses were observed in
five of the seven evaluable patients (17). Annually, there are ~one million new
cases of CRC worldwide, indicating that of these, 100,000 cases may
overexpress HER-2, according to the HER-2 positivity rate of 11.6%
in the present study. HER-2 gene amplification is vital for
targeted tumor therapy, such as Herceptin for breast tumors.
However, not all HER-2-positive cases exhibit HER-2 gene
amplification.
In the current study, HER-2 gene amplification was
observed in 21% (21/102) of the tumors exhibiting HER-2
overexpression and in 2.4% of the total 878 cases of CRC. These
results were similar to those from previous studies where the HER-2
gene amplification rate ranged between 2.5 and 7.4% (11,19,24).
Liu et al (37) reported
that the rate of consistency between IHC and FISH was 70% for IHC3+
and 14% for IHC2+ in gastric cancer samples. In the present study,
a relatively high consistency rate was observed between IHC3+ and
IHC0/1+ with FISH (64 and 100%, respectively); however, there was a
low consistency result between IHC2+ and FISH (6.5%). Thus, the
concordance rate between IHC and FISH in CRC is analogous to that
observed in gastric cancer.
A number of studies have demonstrated that
chromosome 17 polysomy may be the main reason for HER-2
overexpression but not HER-2 gene amplification (37–39).
In the present study, only one case (11%) of chromosome 17 polysomy
was observed out of nine IHC3+ cases with no HER-2 gene
amplification. In addition, there was only one case of chromosome
17 polysomy in the 72 IHC2+ cases with no HER-2 gene amplification.
Thus, it was hypothesized that chromosome 17 polysomy may not be
the reason for HER-2 positivity without HER-2 gene amplification in
CRC. It may be that different mechanisms result in HER-2
overexpression, including transcriptional activation by other
genes, post-transcriptional events or a new genomic environment
associated with amplification (40–42).
However, it is considered that the inconsistency between HER-2
overexpression and gene amplification is associated with the two
methods, namely of immunohistochemical staining and fluorescence
in situ hybridization.
IHC is less expensive and time-consuming, easy to
store and perform, and requires a routinely available microscope.
However, the IHC techniques may be potentially affected by a number
of variables, including tissue fixation, processing, primary
antibody selection, detection systems and methods of antigen
retrieval. Furthermore, as the proposed scoring system for IHC is
subjective, interpretation may vary among observers. These factors,
in addition to the small study sample sizes, may also account for
the variable rates of HER-2 immunoreactivity, as well as the
conflicting results indicating that HER-2 is associated with
adverse clinical outcomes in certain studies, but not in others. At
present, FISH is regarded as the most effective method for the
detection of HER-2 amplification, as it is has high rates of
sensitivity and specificity. FISH is also advantageous as it can be
conducted with small tumor samples and with formalin-fixed and
paraffin-embedded tissue samples. This technique allows for the
direct visualization of gene amplification in the nuclei and
provides an objective count of genes and chromosomes on a
cell-by-cell basis. However, this method is expensive,
time-consuming, requires a fluorescent microscope and is difficult
to separate in situ and invasive carcinomas. Furthermore,
fluorescence fades rapidly; thus, a permanent record is not created
(32,43,44).
CRC involves changes in multiple oncogenes, tumor
suppressor genes and signal transduction pathways. Almost all
tumors with more than one locus are involved in tumorigenesis. EGFR
inhibitors have been widely used in oncotherapy. The identification
of the mutant kirsten rat sarcoma viral oncogene homolog (KRAS) as
a predictor of resistance to EGFR monoclonal antibodies created a
major change in the treatment of CRC (43–45).
As it is known drug resistance results in the failure of
chemotherapy and poor prognosis. However, it also remains a cause
for limiting the EGFR inhibitor long-term efficacy, with the
exception of the KRAS mutant that plays a vital role in predicting
EGFR monoclonal antibodies in CRC. EGFR inhibitor resistance is
associated with the mechanisms that follow signal pathway
activation of HER-2, VEGF and platelet-derived growth factor. In
other words, all of these are activated by circumventing EGFR
protein tyrosine kinase signaling pathway, activation.
Herreros-Villanueva et al (25) hypothesized that HER-2 gene
amplification may be one of the causes of insensitivity to
anti-EGFR therapies, including cetuximab. The study reported that
HER-2 gene amplification was observed in 26.3% of KRAS and v-raf
murine sarcoma viral oncogene homolog B (BRAF) wild type colorectal
carcinomas in Spanish patients. In addition, previous studies have
reported that KRAS and BRAF mutations are mutually exclusive in
CRC; if there are KRAS mutations, no BRAF mutations are present,
and vice versa (45,46). The KRAS mutation statuses in 280
samples selected from 878 patients with CRC were detected in a
previous study (47). The results
revealed that there were no cases of HER-2 gene amplification in
KRAS mutant types, with all HER-2 gene amplification occurring in
the KRAS wild type. Considering that there were no cases of the
KRAS mutation type in the patients with CRC, whether KRAS mutations
and HER-2 gene amplification are mutually exclusive remains to be
elucidated. Furthermore, the association between BRAF mutations and
HER-2 gene amplification requires further investigation, as well as
whether HER-2 monoclonal antibodies may be used to aid EGFR
inhibitor resistance in CRC.
As the standard treatment for breast and gastric
cancers, the premise of the success of Herceptin is concordance
between HER-2 expression and gene amplification. A previous study
revealed that lapatinib, an EGFR/HER-2 kinase inhibitor, combined
with Panobinostat, a histone deacetylase inhibitor, interacted
synergistically to inhibit the proliferation and colony formation
in all CRC cell lines tested. Compared with either agent alone,
there was no apparent increase in toxicity (48). A phase II trial revealed that
Herceptin exerted a therapeutic effect on CRC, although the low
overexpression rate of HER-2 (8.0%) in advanced CRC limited the
efficacy of the drug (17). Chen
et al (49) revealed that
there was no statistically significant difference in HER-2
expression between colorectal liver metastases and the
corresponding primary tumors. Thus, metastatic lesions may also be
suitable for anti-HER-2 therapy due to the homogenicity of HER-2
expression in CRC. These results indicate that HER-2 may be a
promising target as an adjuvant therapy for patients with CRC.
However, to determine the precise curative effect of anti-HER-2
therapy on CRC, multicenter or international cooperation is
required, through large clinical trials, to study the association
between the HER-2 and KRAS genes.
In conclusion, HER-2 overexpression and gene
amplification are present in CRC. With the exception of clinical
stages, no associations were observed between HER-2 overexpression
and other clinicopathological data in the present study. HER-2
overexpression and gene amplification did not correlate with
established prognostic indicators. However, IHC3+ and 2+ cases
should be further analyzed by FISH to assess the gene status of
HER-2 in CRC. Patients with HER-2 gene amplification may be
potential candidates for targeted therapy with Herceptin. However,
further studies are required to confirm these results.
References
|
1
|
Ferlay J, Shin H, Bray F, et al: GLOBOCAN
2008 v1.2, Cancer Incidence and Mortality Worldwide. IARC
CancerBase No. 10. [Internet]. International Agency for Research on
Cancer; Lyon, France: 2010, http://globocan.iarc.fruri.
Accessed October 7, 2012
|
|
2
|
Olayioye MA, Neve RM, Lane HA and Hynes
NE: The ErbB signalling network: receptor heterodimerisation in
development and cancer. EMBO J. 19:3159–3167. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smith I, Procter M, Dowsett M, et al; HERA
study team. 2-year follow-up of trastuzumab after adjuvant
chemotherapy in HER2-positive breast cancer: a randomised
controlled trial. Lancet. 369:29–36. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Slamon DJ, Leyland-Jones B, Shak S, et al:
Use of chemotherapy plus a monoclonal antibody against HER2 for
metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bernhard H1, Salazar L, Schiffman K, et
al: Vaccination against the HER-2/neu oncogenic protein. Endocr
Relat Cancer. 9:33–44. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen J, Li DS, Yu YH, et al: Clinical
significance of Her-2 protein expression in gastric cancer. Shi Jie
Hua Ren Xiao Hua Za Zhi. 18:1375–1379. 2010.(In Chinese).
|
|
7
|
Ford R, Schwartz L, Dancey J, et al:
Lessons learned from independent central review. Eur J Cancer.
45:268–274. 2009. View Article : Google Scholar
|
|
8
|
Takahari D, Yamada Y, Okita NT, et al:
Relationships of insulin-like growth factor-1 receptor and
epidermal growth factor receptor expression to clinical outcomes in
patients with colorectal cancer. Oncology. 76:42–48. 2009.
View Article : Google Scholar
|
|
9
|
Lim SW, Kim HR, Kim HY, et al:
Over-expression of Her-2 in colorectal cancer tissue, but not in
serum, constitutes an independent worse prognostic factor. Cell
Oncol (Dordr). 36:311–321. 2013. View Article : Google Scholar
|
|
10
|
Park DI, Kang MS, Oh SJ, et al: HER-2/neu
overexpression is an independent prognostic factor in colorectal
cancer. Int J Colorectal Dis. 22:491–497. 2007. View Article : Google Scholar
|
|
11
|
Demirbaş S, Sücüllü I, Yildirim S and
Celenk T: Influence of the c-erb B-2, nm23, bcl-2 and p53 protein
markers on colorectal cancer. Turk J Gastroentrol. 17:13–19.
2006.
|
|
12
|
Kavanagh DO, Chambers G, O’Grady L, et al:
Is overexpression of HER-2 a predictor of prognosis in colorectal
cancer? BMC Cancer. 9:12009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ismail HM, El-Baradie M, Moneer M, et al:
Clinico-pathological and prognostic significance of p53, Bcl-2 and
Her-2/neu protein markers in colorectal cancer using tissue
microarray. J Egypt Natl Canc Inst. 19:3–14. 2007.
|
|
14
|
Rossi HA, Liu Q, Banner B, et al: The
prognostic value of invariant chain (Ii) and Her-2/neu expression
in curatively resected colorectal cancer. Cancer J. 8:268–275.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Essapen S, Thomas H, Green M, et al: The
expression and prognostic significance of HER-2 in colorectal
cancer and its relationship with clinicopathological parameters.
Int J Oncol. 24:241–248. 2004.PubMed/NCBI
|
|
16
|
Antonacopoulou AG, Tsamandas AC, Petsas T,
et al: EGFR, HER-2 and COX-2 levels in colorectal cancer.
Histopathology. 53:698–706. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ramanathan RK, Hwang JJ, Zamboni WC, et
al: Low overexpression of HER-2/neu in advanced colorectal cancer
limits the usefulness of trastuzumab (Herceptin) and irinotecan as
therapy. A phase II trial. Cancer Invest. 22:858–865. 2004.
View Article : Google Scholar
|
|
18
|
Kapitanović S, Radosević S, Kapitanović M,
et al: The expression of p185(HER-2/neu) correlates with the stage
of disease and survival in colorectal cancer. Gastroenterology.
112:1103–1013. 1997. View Article : Google Scholar
|
|
19
|
Half E, Broaddus R, Danenberg KD, et al:
HER-2 receptor expression, localization, and activation in
colorectal cancer cell lines and human tumors. Int J Cancer.
108:540–548. 2004. View Article : Google Scholar
|
|
20
|
Kruszewski WJ, Rzepko R, Ciesielski M, et
al: Expression of HER-2 in colorectal cancer does not correlate
with prognosis. Dis Markers. 29:207–212. 2010. View Article : Google Scholar
|
|
21
|
Kountourakis P, Pavlakis K, Psyrri A, et
al: Clinicopathologic significance of EGFR and Her-2/neu in
colorectal adenocarcinomas. Cancer J. 12:229–236. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schuell B, Gruenberger T, Scheithauer W,
et al: HER 2/neu protein expression in colorectal cancer. BMC
Cancer. 6:1232006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim JY, Lim SJ and Park K:
Cyclooxygenase-2 and c-erbB-2 expression in colorectal carcinoma
assessed using tissue microarray. Appl Immunohistochem Mol Morphol.
12:67–70. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marx AH, Burandt EC, Choschzick M, et al:
Heterogenous high-level HER-2 amplification in a small subset of
colorectal cancers. Hum Pathol. 41:1577–1585. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Herreros-Villanueva M, Rodrigo M, Claver
M, et al: KRAS, BRAF, EGFR and HER2 gene status in a Spanish
population of colorectal cancer. Mol Biol Rep. 38:1315–1320. 2011.
View Article : Google Scholar
|
|
26
|
Pappas A, Lagoudianakis E, Seretis C, et
al: Clinical role of HER-2/neu expression in colorectal cancer. J
BUON. 18:98–104. 2013.PubMed/NCBI
|
|
27
|
Li Q, Wang D, Li J and Chen P:
Clinicopathological and prognostic significance of HER-2/neu and
VEGF expression in colon carcinomas. BMC Cancer. 11:2772011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Seo AN, Kwak Y, Kim DW, et al: HER2 Status
in colorectal cancer: its clinical significance and the
relationship between HER2 gene amplification and expression. PLoS
One. 9:e985282014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sobin LH and Fleming ID: TNM
classification of malignant tumors, fifth edition (1997). Union
Internationale Contre le Cancer and the American Joint Committee on
Cancer. Cancer. 80:1803–1804. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hamtilon SR and Aaltonen LA: World Health
Organization classification of tumours. Tumours of the oesophagus.
Pathology and genetics tumors of the digestive system. IARC Press;
Lyon: 2006
|
|
31
|
Hofmann M, Stoss O, Shi D, et al:
Assessment of a HER2 scoring system for gastric cancer: results
from a validation study. Histopathology. 52:797–805. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pauletti G, Dandekar S, Rong H, et al:
Assessment of methods for tissue-based detection of the HER-2/neu
alteration in human breast cancer: a direct comparison of
fluorescence in situ hybridization and immunohistochemistry. J Clin
Oncol. 18:3651–3664. 2000.PubMed/NCBI
|
|
33
|
Tavangar SM, Shariftabrizi A and Soroush
AR: Her-2/neu over-expression correlates with more advanced disease
in Iranian colorectal cancer patients. Med Sci Monit.
11:CR123–CR126. 2005.PubMed/NCBI
|
|
34
|
McKay JA, Loane JF, Ross VG, et al:
c-erbB-2 is not a major factor in the development of colorectal
cancer. Br J Cancer. 86:568–573. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mann M, Sheng H, Shao J, et al: Targeting
cyclooxygenase 2 and HER-2/neu pathways inhibits colorectal
carcinoma growth. Gastroenterology. 120:1713–1719. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pohl M, Stricker I, Schoeneck A, et al:
Antitumor activity of the HER2 dimerization inhibitor pertuzumab on
human colon cancer cells in vitro and in vivo. J Cancer Res Clin
Oncol. 135:1377–1386. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu W, Zhong S, Chen J and Yu Y: HER-2/neu
overexpression is an independent prognostic factor for
intestinal-type and early-stage gastric cancer patients. J Clin
Gastroenterol. 46:e31–e37. 2012. View Article : Google Scholar
|
|
38
|
Rossi E, Ubiali A, Cadei M, et al:
HER-2/neu in breast cancer: a comparative study between histology,
immunohistochemistry, and molecular technique (FISH). Appl
Immunohistochem Mol Morphol. 14:127–131. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chibon F, de Mascarel I, Sierankowski G,
et al: Prediction of HER2 gene status in HER2 2+ invasive breast
cancer: a study of 108 cases comparing ASCO/CAP and FDA
recommendations. Mod Pathol. 22:403–409. 2009. View Article : Google Scholar
|
|
40
|
Bartlett JM, Going JJ, Mallon EA, et al:
Evaluating HER2 amplification and overexpression in breast cancer.
J Pathol. 195:422–428. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Downs-Kelly E, Yoder BJ, Stoler M, et al:
The influence of polysomy 17 on HER2 gene and protein expression in
adenocarcinoma of the breast: a fluorescent in situ hybridization,
immunohistochemical, and isotopic mRNA in situ hybridization study.
Am J Surg Pathol. 29:1221–1227. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dal Lago L, Durbecq V, Desmedt C, et al:
Correction for chromosome-17 is critical for the determination of
true Her-2/neu gene amplification status in breast cancer. Mol
Cancer Ther. 5:2572–2579. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Winston JS, Ramanaryanan J and Levine E:
HER-2/neu evaluation in breast cancer are we there yet? Am J Clin
Pathol. 121(Suppl): S33–S49. 2004.PubMed/NCBI
|
|
44
|
Seidal T, Balaton AJ and Battifora H:
Interpretation and quantification of immunostains. Am J Surg
Pathol. 25:1204–1207. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Amado RG, Wolf M, Peeters M, et al:
Wild-type KRAS is required for panitumumab efficacy in patients
with metastatic colorectal cancer. J Clin Oncol. 26:1626–1634.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Di Nicolantonio F, Martini M, Molinari F,
Sartore-Bianchi A, et al: Wild-type BRAF is required for response
to panitumumab or cetuximab in metastatic colorectal cancer. J Clin
Oncol. 26:5705–5712. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu W, Wang L, Yu YH, et al: Detection of
k-ras gene mutations in Chinese patients with colorectal cancer.
World Chinese Journal of Digestology. 19:1367–1374. 2011.
|
|
48
|
LaBonte MJ, Wilson PM, Fazzone W, et al:
The dual EGFR/HER2 inhibitor lapatinib synergistically enhances the
antitumor activity of the histone deacetylase inhibitor
panobinostat in colorectal cancer models. Cancer Res. 71:3635–3648.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen J, Li Q, Wang C, Wu J and Zhao G:
Prognostic significance of c-erbB-2 and vascular endothelial growth
factor in colorectal liver metastases. Ann Surg Oncol.
17:1555–1563. 2010. View Article : Google Scholar : PubMed/NCBI
|